Aggregation-Induced Emission-Fluorescent-Microsphere-Based Lateral Flow Immunoassay for Highly Sensitive Detection of Capsaicinoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Hapten and Preparation of mAb
2.3. Preparation of AIEFM-mAb and AuNP-mAb
2.3.1. Preparation of AIEFM-mAb
2.3.2. Preparation of AuNP-mAb
2.4. Fabrication and Optimization of LFIA Test Strips
2.5. Oil Sample Preparation
2.6. Validation of AIEFM-LFIA
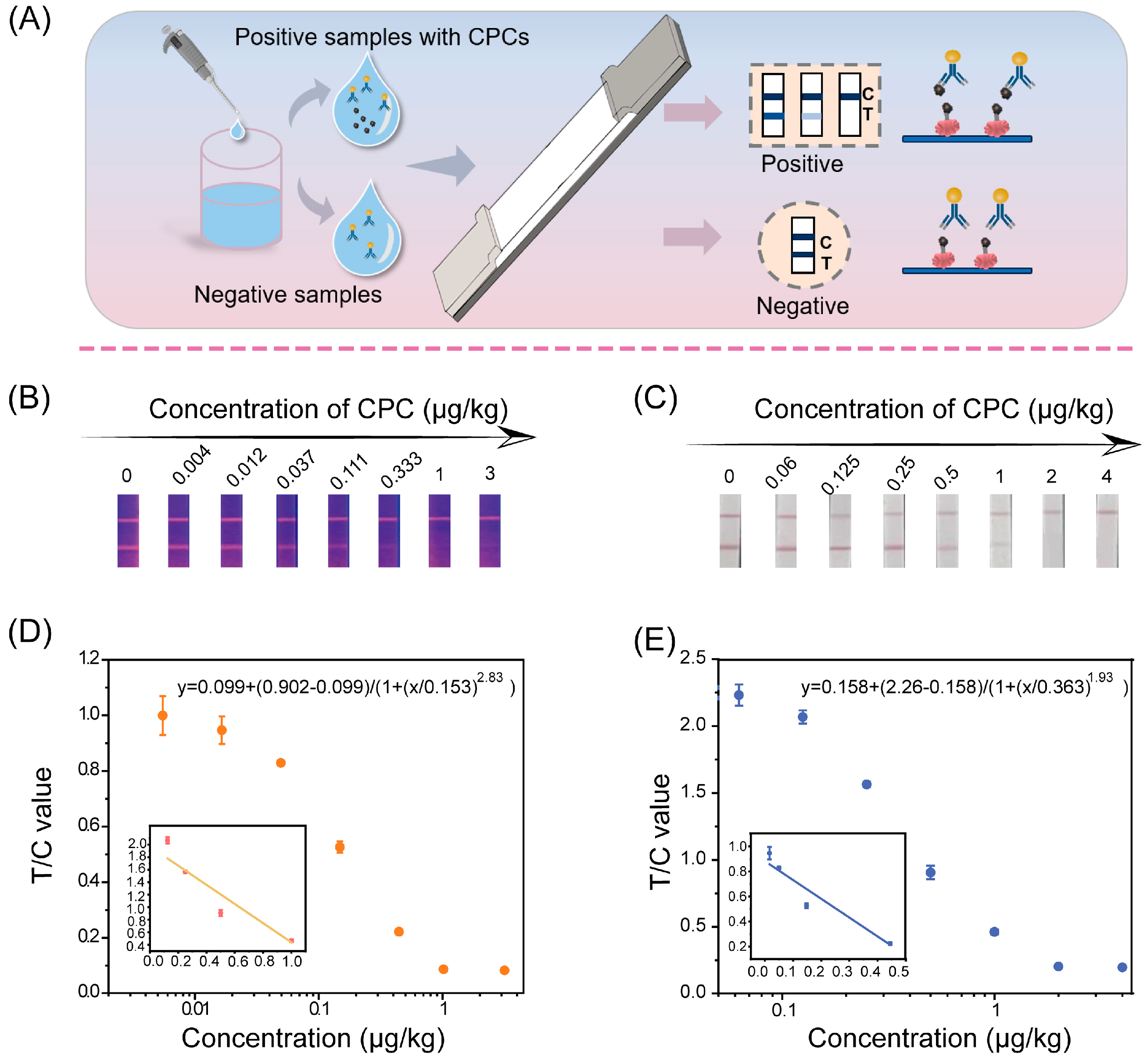
2.6.1. Sensitivity
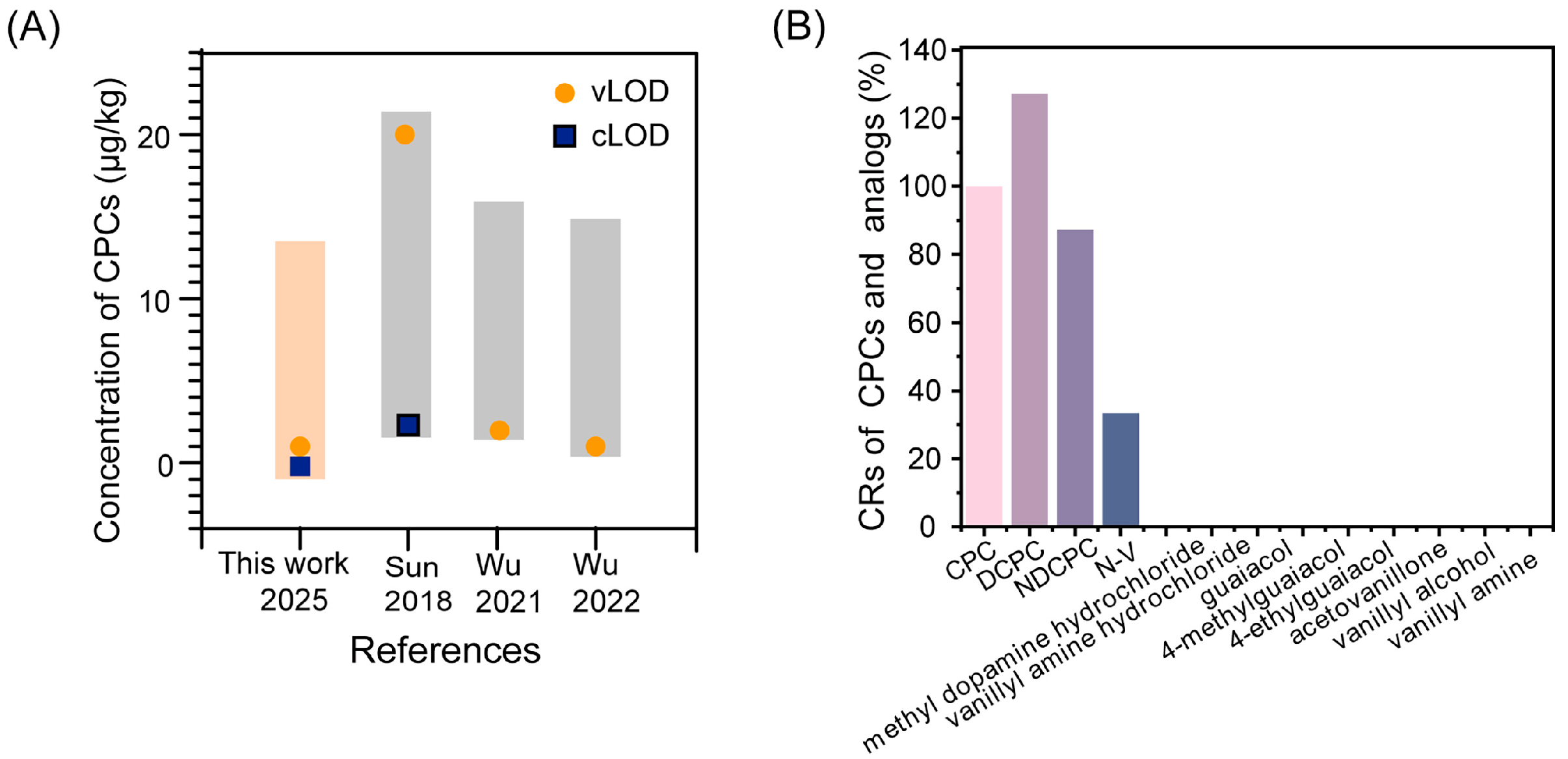
2.6.2. Specificity of Assay
2.6.3. Accuracy
2.6.4. Reliability
3. Results
3.1. Preparation and Identification of Hapten and mAb
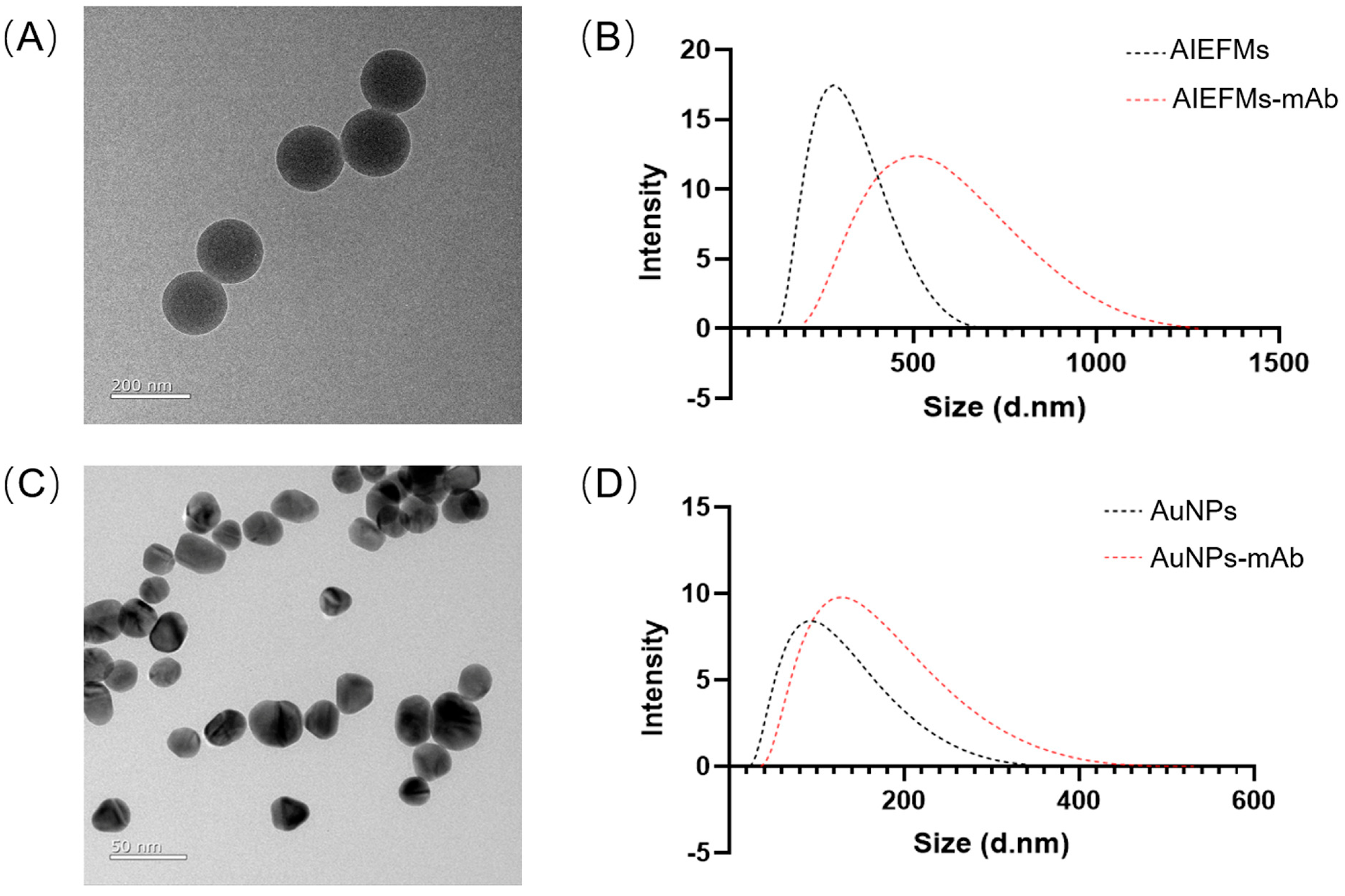
3.2. Characterization of AIEFMs, AIEFM-mAb, AuNPs, and AuNP-mAb
3.3. Detection Mechanism of AIEFM-LFIA
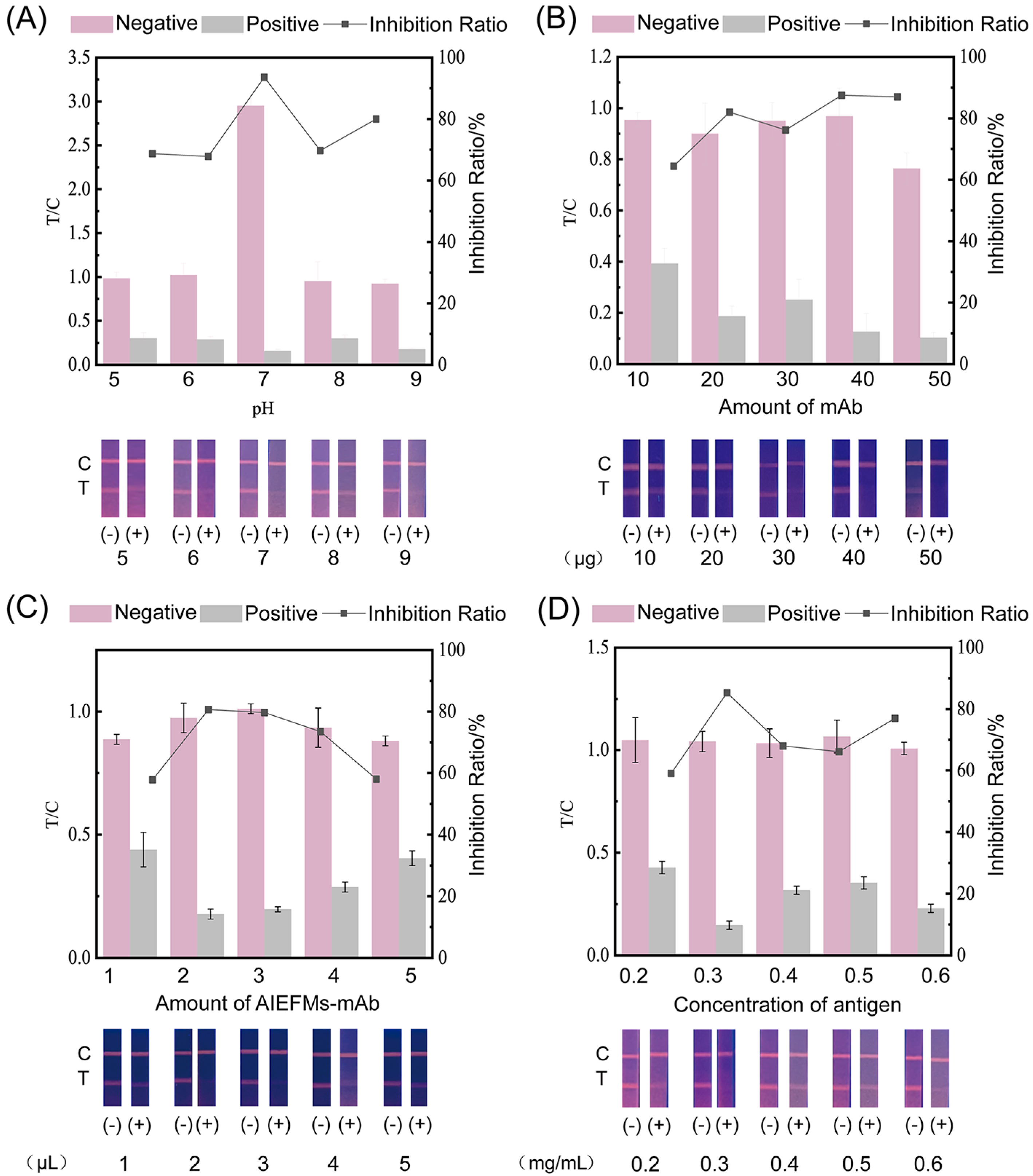
3.4. Optimization of AIEFM-LFIA
3.5. Detection Performance of AIEFM-LFIA and AuNP-LFIA in Oil Samples
3.6. The Accuracy and Precision of the AIEFM-LFIA
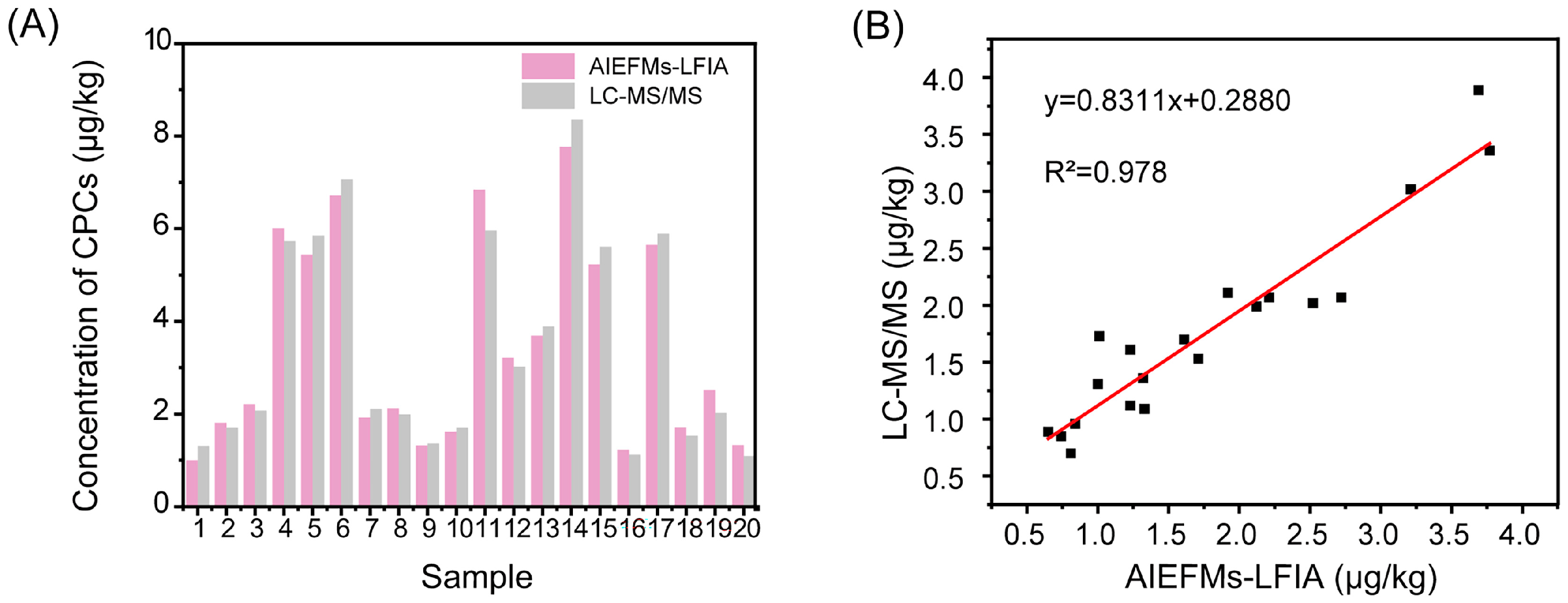
3.7. The Stability and Reliability of the AIEFM-LFIA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPCs | Capsaicinoids |
| AIEFMs | Aggregation-induced emission fluorescent microspheres |
| LFIA | Lateral flow immunoassay |
| AuNPs | Gold nanoparticle |
| LOD | Limit of detection |
| R2 | Correlation coefficient |
| CPC | Capsaicin |
| DCPC | Dihydrocapsaicin |
| NDCPC | Nordihydrocapsaicin |
| HPLC | High-performance liquid chromatography |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| N-V | N-vanillylnonanamide |
| BSA | Bovine serum albumin |
| MES | Morpholinoethanesulfonic acid |
| EDC | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride |
| NHS | N-hydroxysuccinimide |
| mAb | Monoclonal antibody |
| TEM | Transmission electron microscopy |
| cLOD | Calculated LOD |
| vLOD | Visual LOD |
| CR% | Cross-reactivity |
| DLS | Dynamic light scattering |
| CVs | Coefficients of variation |
References
- Kemsawasd, V.; Jayasena, V.; Karnpanit, W. Incidents and potential adverse health effects of serious food fraud cases originated in Asia. Foods 2023, 12, 3522. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Q.; Wu, W.; Zhang, D.; Mo, L.; Liu, J.; Yang, X. Food fraud vulnerability assessment in the edible vegetable oil supply chain: A perspective of Chinese enterprises. Food Control 2022, 138, 109005. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Liu, L.; Li, Y.; Ren, N.; Kannan, K. Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J. Agric. Food Chem. 2012, 60, 6913–6919. [Google Scholar] [CrossRef]
- Lu, F.; Wu, X. China food safety hits the “gutter”. Food Control 2014, 41, 134–138. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Zhao, Y. Detection of polycyclic aromatic hydrocarbons from cooking oil using ultra-thin layer chromatography and surface enhanced Raman spectroscopy. J. Mater. Chem. B 2015, 3, 1898–1906. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers- A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 488–505. [Google Scholar] [CrossRef]
- Su, M.; Jiang, Q.; Guo, J.; Zhu, Y.; Cheng, S.; Yu, T.; Du, S.; Jiang, Y.; Liu, H. Quality alert from direct discrimination of polycyclic aromatic hydrocarbons in edible oil by liquid-interfacial surface-enhanced Raman spectroscopy. LWT 2021, 143, 111143. [Google Scholar] [CrossRef]
- Teng, C.; Wu, S.; Sun, Y.; Gong, G. Determination of parent and oxygenated polycyclic aromatic hydrocarbons (PAHs) in waste cooking oil and oil deodorizer distillate by GC–QQQ–MS. J. AOAC Int. 2019, 102, 1884–1891. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Yang, H.; Ma, J.; Zheng, X.; Zhang, J.; Xu, D.; Zhang, X.; Zhou, Y. Dual-readout colorimetric immunoassay based on halogenated indophenol for rapid detection of aflatoxin B1. Microchem. J. 2024, 197, 109741. [Google Scholar] [CrossRef]
- Jiang, H.; He, Y.; Chen, Q. Determination of acid value during edible oil storage using a portable NIR spectroscopy system combined with variable selection algorithms based on an MPA-based strategy. J. Sci. Food Agric. 2021, 101, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Pulassery, S.; Abraham, B.; Ajikumar, N.; Munnilath, A.; Yoosaf, K. Rapid iodine value estimation using a handheld Raman spectrometer for on-site, reagent-free authentication of edible oils. ACS Omega 2022, 7, 9164–9171. [Google Scholar] [CrossRef] [PubMed]
- Ottaway, J.; Chance Carter, J.; Adams, K.; Camancho, J.; Lavine, B.; Booksh, K. Comparison of spectroscopic techniques for determining the peroxide value of 19 classes of naturally aged, plant-based edible oils. Appl. Spectrosc. 2021, 75, 781–794. [Google Scholar] [CrossRef]
- Daood, H.; Halasz, G.; Palotás, G.; Palotás, G.; Bodai, Z.; Helyes, L. HPLC determination of capsaicinoids with cross-linked C18 column and buffer-free eluent. J. Chromatogr. Sci. 2015, 53, 135–143. [Google Scholar] [CrossRef]
- Biradar, K.; Singh, J.; Pillai, S.; Crosby, K.; Patil, B. Separation of nordihydrocapsiate from capsiate and major capsaicinoid analogues using ultra high performance liquid chromatography. Food Chem. 2022, 382, 132585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wen, Z.; Liu, Z.; Zhang, Y.; Zhou, Y.; Feng, X. Capsaicinoids in food: An update on pretreatment and analysis methods since 2010. Crit. Rev. Anal. Chem. 2024, 54, 73–92. [Google Scholar] [CrossRef]
- Liu, S.; Lin, X.; Yang, Z.; Wen, B.; Zhang, F.; Zhang, Y.; Li, J. Label-free SERS strategy for rapid detection of capsaicin for identification of waste oils. Talanta 2022, 245, 123488. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, D.; Cao, W.; Shi, H.; Jiang, Y. Fast simultaneous determination of capsaicin, dihydrocapsaicin and nonivamide for detecting adulteration in edible and crude vegetable oils by UPLC-MS/MS. Food Addit. Contam. Part A 2018, 35, 1447–1452. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, H.; Li, D.; Zhao, Q. Determination of capsaicinoids by magnetic solid phase extraction coupled with UPLC-MS/MS for screening of gutter oil. J. Chromatogr. B 2020, 1158, 122344. [Google Scholar] [CrossRef]
- Aymard, C.; Kanso, H.; Serrano, M.; Pagán, R.; Noguer, T.; Istamboulie, G. Development of a new dual electrochemical immunosensor for a rapid and sensitive detection of enrofloxacin in meat samples. Food Chem. 2022, 370, 131016. [Google Scholar] [CrossRef]
- Li, L.; Hou, R.; Li, H.; Han, S.; Liang, J.; Si, Y.; Peng, D. Preparation and interaction mechanism analysis of single-chain fragment variables against phenylethanolamine A. Anim. Dis. 2024, 4, 8. [Google Scholar] [CrossRef]
- Yang, Z.; Li, F.; Zhang, M.; Li, Y.; Zhao, Q.; Wang, C.; Xu, L.; Liu, Y.; Li, W.; Zhu, Y. Rapid production of monoclonal antibodies from single mouse B cells against FMDV. Anim. Dis. 2024, 4, 28. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, J.; Ma, F.; Li, P.; Zhang, L.; Zhang, W.; Ding, X.; Zhang, Q. Quantitative determination of major capsaicinoids in serum by ELISA and time-resolved fluorescent immunoassay based on monoclonal antibodies. Biosens. Bioelectron. 2016, 81, 229–235. [Google Scholar] [CrossRef]
- Sun, J.; Liu, L.; Song, S.; Cui, G.; Kuang, H. Development of an immunochromatographic strip assay for three major capsaicinoids based on an ultrasensitive monoclonal antibody. Food Agric. Immunol. 2018, 29, 930–940. [Google Scholar] [CrossRef]
- Bai, Y.; Jiang, H.; Zhang, Y.; Dou, L.; Liu, M.; Yu, W.; Wen, K.; Shen, J.; Ke, Y.; Yu, X.; et al. Hydrophobic moiety of capsaicinoids haptens enhancing antibody performance in immunoassay: Evidence from computational chemistry and molecular recognition. J. Agric. Food Chem. 2021, 69, 9957–9967. [Google Scholar] [CrossRef]
- Wu, Q.; Yao, L.; Qin, P.; Xu, J.; Sun, X.; Yao, B.; Ren, F.; Chen, W. Time-resolved fluorescent lateral flow strip for easy and rapid quality control of edible oil. Food Chem. 2021, 357, 129739. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, J.; Yu, J.; Zhuang, J.; Ma, F.; Tan, J.; Shen, Z. A monoclonal antibody for identifying capsaicin congeners in illegal cooking oil and its applications. Talanta 2022, 250, 123686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, F.; Zha, C.; Yang, Q.; Zhang, Q.; Zhang, W.; Li, P.; Sun, X. Effect of hapten structures on development of novel antibody against capsaicin and dihydrocapsaicin. Chin. J. Anal. Chem. 2022, 50, 100134. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Yang, Q.; Wei, L.; Yuan, B.; Pang, C.; Zhang, Y.; Sun, X.; Guo, Y. A simple and rapid homogeneous fluorescence polarization immunoassay for rapid identification of gutter cooking oil by detecting capsaicinoids. Anal. Bioanal. Chem. 2022, 414, 6127–6137. [Google Scholar] [CrossRef]
- Yuan, D.; Li, S.; Zhang, L.; Ma, F.; Wang, H.; Zhang, Q.; Li, P. Rapid and sensitive quantification of capsaicinoids for edible oil adulteration by immunomagnetic solid-phase extraction coupled with time-resolved fluorescent immunochromatographic assay. Food Chem. 2023, 404, 134552. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Zhang, J.; Xu, H.; Ren, K.; Yu, C.; Zhang, Q.; Li, F.; Yang, Q. Reverse design of haptens based on antigen spatial conformation to prepare anti-capsaicinoids&gingerols antibodies for monitoring of gutter cooking oil. Food Chem. X 2024, 22, 101273. [Google Scholar]
- Jin, Z.; Sheng, W.; Sun, M.; Bai, D.; Ren, L.; Wang, S.; Wang, Z.; Tang, X.; Ya, T. Preparation of a capsaicinoids broad spectrum antibody and its application in non-enzyme immunoassay based on DMSNs@PDA@Pt. J. Hazard. Mater. 2024, 466, 133670. [Google Scholar] [CrossRef]
- Ma, G.; Li, X.; Cai, J.; Wang, X. Carbon dots-based fluorescent probe for detection of foodborne pathogens and its potential with microfluidics. Food Chem. 2024, 451, 139385. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yang, J.; Li, Q.; Yang, X.; Fang, R.; Zhang, Y.; Chen, L.; Chen, F.; Tian, Y.; Shen, Y.; et al. Development of highly sensitive immunochromatography using time-resolved fluorescence microspheres for amantadine detection. J. Agric. Food Chem. 2024, 72, 21794–21803. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.; Nguyen, D.; Pham, T.; Pham, B.; Nguyen, T.; Pham, T.; Sharma, S.; Pham, D.; Gangavarapu, R.; Pham, T. A highly sensitive fluorescence nanosensor for determination of amikacin antibiotics using composites of carbon quantum dots and gold nanoparticles. Spectrochim. Acta Part. A 2024, 305, 123466. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Chen, X.; Liu, Z.; Chen, G.; Wei, X.; Li, X.; Leng, Y.; Xiong, Y.; Huang, X. Ultrasensitive lateral flow immunoassay for fumonisin B1 detection using highly luminescent aggregation-induced emission microbeads. Toxins 2023, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhou, H.; Chen, X.; Wu, J.; Su, Y.; Huang, X.; Xiong, Y. Janus plasmonic-aggregation induced emission nanobeads as high-performance colorimetric–fluorescent probe of immunochromatographic assay for the ultrasensitive detection of staphylococcal enterotoxin B in milk. Biosens. Bioelectron. 2024, 261, 116458. [Google Scholar] [CrossRef]
- Wu, W.; Shen, M.; Liu, X.; Shen, L.; Ke, X.; Li, W. Highly sensitive fluorescence-linked immunosorbent assay based on aggregation-induced emission luminogens incorporated nanobeads. Biosens. Bioelectron. 2020, 150, 111912. [Google Scholar] [CrossRef]
- Hu, L.; Chen, Z.; Li, T.; Ye, X.; Luo, Q.; Lai, W. Comparison of oriented and non-oriented antibody conjugation with AIE fluorescence microsphere for the immunochromatographic detection of enrofloxacin. Food Chem. 2023, 429, 136816. [Google Scholar] [CrossRef]
- Hao, Y.; Huo, B.; Wang, F.; Xie, Q.; Liang, W.; Jia, L.; Guo, H.; Wu, Y.; Wang, Q. Water-based green deep eutectic solvent: Application in liquid-liquid microextraction of trace bisphenol A in edible oils. Talanta 2025, 286, 127511. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Li, J.; Xie, Y. Detection of tert-butylhydroquinone in edible oils using an electrochemical sensor based on a nickel-aluminum layered double hydroxide@carbon spheres-derived carbon composite. Foods 2024, 13, 3431. [Google Scholar] [CrossRef]
- Di Nardo, F.; Anfossi, L.; Ozella, L.; Saccani, A.; Giovannoli, C.; Spano, G.; Baggiani, C. Validation of a qualitative immunochromatographic test for the noninvasive assessment of stress in dogs. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1028, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, L.; Yang, Y.; Hu, L.; Lai, W. Fluorescent microsphere immunochromatographic assays for detecting bone alkaline phosphatase based on biolayer interferometry-selected antibody. RSC Adv. 2017, 7, 32952–32959. [Google Scholar] [CrossRef]


| Analyte | Amount Spiked (μg/kg) | Recovery (%) | CV (%) |
|---|---|---|---|
| CPC | 1.00 | 92.0 | 4.0 |
| 1.60 | 88.0 | 2.3 | |
| 2.40 | 91.0 | 6.8 | |
| DCPC | 1.00 | 90.0 | 2.6 |
| 1.60 | 106.0 | 2.1 | |
| 2.40 | 103.0 | 4.2 | |
| NDCPC | 1.00 | 81.0 | 3.4 |
| 1.60 | 106.0 | 7.9 | |
| 2.40 | 87.0 | 4.7 | |
| N-V | 1.00 | 75.0 | 8.3 |
| 1.60 | 99.0 | 6.3 | |
| 2.40 | 88.0 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Han, X.; Yang, Y.; Wang, Z.; Qiu, F. Aggregation-Induced Emission-Fluorescent-Microsphere-Based Lateral Flow Immunoassay for Highly Sensitive Detection of Capsaicinoids. Foods 2025, 14, 3634. https://doi.org/10.3390/foods14213634
Bai Y, Han X, Yang Y, Wang Z, Qiu F. Aggregation-Induced Emission-Fluorescent-Microsphere-Based Lateral Flow Immunoassay for Highly Sensitive Detection of Capsaicinoids. Foods. 2025; 14(21):3634. https://doi.org/10.3390/foods14213634
Chicago/Turabian StyleBai, Yuchen, Xinyue Han, Yang Yang, Zhanhui Wang, and Fubin Qiu. 2025. "Aggregation-Induced Emission-Fluorescent-Microsphere-Based Lateral Flow Immunoassay for Highly Sensitive Detection of Capsaicinoids" Foods 14, no. 21: 3634. https://doi.org/10.3390/foods14213634
APA StyleBai, Y., Han, X., Yang, Y., Wang, Z., & Qiu, F. (2025). Aggregation-Induced Emission-Fluorescent-Microsphere-Based Lateral Flow Immunoassay for Highly Sensitive Detection of Capsaicinoids. Foods, 14(21), 3634. https://doi.org/10.3390/foods14213634







