Antioxidant Peptides from Tiger Nut (Cyperus esculentus L.): Chemical Analysis and Cytoprotective Functions on HepG2 and Caco-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Experimental Optimization of TNP
Preparation of TNP
2.3. Basic Functional Properties
2.3.1. Preparation of Tiger Nut Protein
2.3.2. Water and Oil-Holding Capacity
2.3.3. Solubility
2.3.4. Foaming Properties
2.3.5. Emulsifying Properties
2.4. SDS-PAGE
2.5. Antioxidant Activity Measurements
2.6. Amino Acid Analysis
2.7. Isolation and Purification of TNP
2.8. Cytoprotective Effects on HepG2 and Caco-2 Cells Damage Induced by H2O2
2.8.1. Cytotoxicity Assay
2.8.2. H2O2-Induced Oxidative Stress Assay and Indicator Measurement
2.8.3. Microscopic Observation of Morphological Change
2.9. Statistical Analysis
3. Results
3.1. Preparation of the TNP
3.2. Basic Functional Properties Research Results
3.2.1. Water/Oil-Holding Capacity and Solubility
3.2.2. Foaming and Emulsifying Properties
3.3. Antioxidant Activity Analysis
3.3.1. Antioxidant Activity of Crude Peptides
3.3.2. Antioxidant Activity of TNPs by Isolation and Purification
3.4. Cytoprotective Functions on HepG2 and Caco-2 Cells
3.4.1. Cell Cytotoxicity
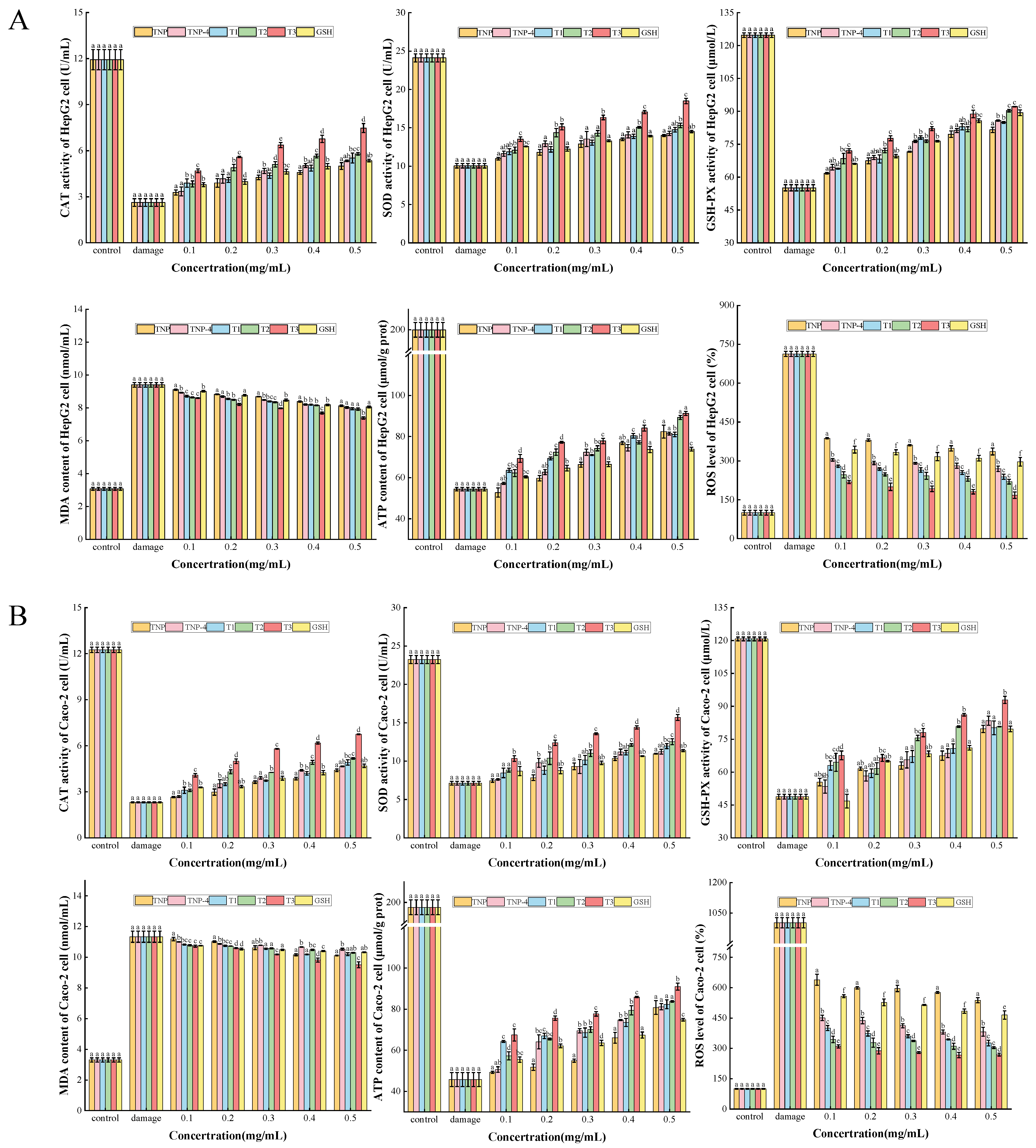
3.4.2. Cytoprotective Effects on Cell Damage Induced by H2O2
Protection of TNPs Against Oxidative Stress in Cells
Morphological Observation
CAT, SOD, GSH-PX Activities, and MDA, ATP, ROS Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TNP | tiger nut peptide |
| ROS | reactive oxygen species |
| H2O2 | hydrogen peroxide |
| DH | degree of hydrolysis |
| pI | isoelectric point |
| DPPH• | 1,1-diphenyl-2-picroyl |
| ABTS•+ | 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid |
| •OH | hydroxyl radical |
| FRAP | hiazoline-6-sulfonic acid |
| RSA | radical scavenging ability |
| WHC | water-holding capacity |
| OHC | oil-holding capacity |
| FC | foaming capacity |
| FS | foaming stability |
| SDS-PAGE | sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| CAT | catalase |
| GSH | glutathione |
| GSH-PX | glutathione peroxidase |
| ATP | adenosine triphosphate |
| MEM | minimum essential medium |
| FBS | fetal bovine serum |
| MTT | 3- (4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
| DMSO | dimethyl sulfoxide |
| AA | amino acid |
References
- Ruan, J.; Chen, J.; Zeng, J.; Yang, Z.; Wang, C.; Hong, Z.; Zuo, Z. The protective effects of Nile tilapia (Oreochromis niloticus) scale collagen hydrolysate against oxidative stress induced by tributyltin in HepG2 cells. Environ. Sci. Pollut. Res. 2019, 26, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, G.; Li, H.; Zhang, T.; Sun, D.; Lu, W.P.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; et al. Preparation and identification of novel antioxidant peptides from camel bone protein. Food Chem. 2023, 424, 136253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gui, M.; Fan, W.; Gao, L.; Bu, X. Response surface methodology optimization on extraction and antioxidant activity evaluation of antioxidant peptide from enzymatic hydrolysates of sturgeon bone. LWT 2024, 198, 116042. [Google Scholar] [CrossRef]
- Magalhães, I.S.; Guimarães, A.D.B.; Tribst, A.A.L.; de Oliveira, E.B.; Júnior, B.R.D.C.L. Ultrasound-assisted enzymatic hydrolysis of goat milk casein: Effects on hydrolysis kinetics and on the solubility and antioxidant activity of hydrolysates. Food Res. Int. 2022, 157, 111310. [Google Scholar] [CrossRef]
- Tian, S.; Du, K.; Yan, F.; Li, Y. Microwave-assisted enzymatic hydrolysis of wheat germ albumin to prepare polypeptides and influence on physical and chemical properties. Food Chem. 2022, 374, 131707. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Xie, H.; Liu, Z.; Rakariyatham, K.; Yu, C.; Shahidi, F.; Zhou, D. Antioxidant activity and functional properties of Alcalase-hydrolyzed scallop protein hydrolysate and its role in the inhibition of cytotoxicity in vitro. Food Chem. 2021, 344, 128566. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, F.; Yan, Y.; Zeng, Y.; Li, Y.; Zhou, R. Purification and Identification of Peptides from Hydrilla verticillata (Linn. f.) Royle with Cytoprotective and Antioxidative Effect against H2O2-Treated HepG2 Cells. J. Agric. Food Chem. 2024, 72, 4170–4183. [Google Scholar] [CrossRef]
- Tonolo, F.; Coletta, S.; Fiorese, F.; Grinzato, A.; Albanesi, M.; Folda, A.; Ferro, S.; De Mario, A.; Piazza, I.; Mammucari, C.; et al. Sunflower seed-derived bioactive peptides show antioxidant and anti-inflammatory activity: From in silico simulation to the animal model. Food Chem. 2024, 439, 138124. [Google Scholar] [CrossRef]
- Zhu, L.; Xiong, H.; Huang, X.; Guyonnet, V.; Ma, M.; Chen, X.; Zheng, Y.; Wang, L.; Hu, G. Identification and molecular mechanisms of novel antioxidant peptides from two sources of eggshell membrane hydrolysates showing cytoprotection against oxidative stress: A combined in silico and in vitro study. Food Res. Int. 2022, 157, 111266. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutri. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Singh, J.; Gani, A. Exploration of bioactive peptides from various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies. Food Chem. 2022, 373, 131395. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Husain, Q.; Azam, A. Exploring the antioxidant effects of peptides from almond proteins using PAni-Ag-GONC conjugated trypsin by improving enzyme stability & applications. Int. J. Biol. Macromol. 2020, 158, 150–158. [Google Scholar]
- Ji, N.; Sun, C.; Zhao, Y.; Xiong, L.; Sun, Q. Purification and identification of antioxidant peptides from peanut protein isolate hydrolysates using UHR-Q-TOF mass spectrometer. Food Chem. 2014, 161, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, Y.; Xue, W.; Xia, Y.; Liang, G. Novel antioxidant peptides from soybean protein by employ computational and experimental methods and their mechanisms of oxidative stress resistance. J. Mol. Struct. 2024, 1318, 139284. [Google Scholar] [CrossRef]
- Quan, Y.; Ren, H.; Liu, S.; Zhao, X.; Hao, J. Identification and molecular mechanism of novel antioxidant peptides from tiger nut (Cyperus esculentus L.). Food Biosci. 2024, 63, 105738. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, M.; Sun, J. Novel antioxidant peptides in fermented pork sausage: Purification, characterization, and cytoprotective functions on Caco-2 cells. Food Chem. 2023, 426, 136566. [Google Scholar] [CrossRef]
- Igbokwe, C.J.; Feng, Y.; Louis, H.; Benjamin, I.; Quaisie, J.; Duan, Y.; Tuly, J.A.; Cai, M.; Zhang, H. Novel antioxidant peptides identified from coix seed by molecular docking, quantum chemical calculations and invitro study in HepG2 cells. Food Chem. 2024, 440, 138234. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products-A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, F.; Ma, Y.; Huo, N.; Guo, Y.; Yu, Y. Protein from tiger nut meal extracted by deep eutectic solvent and alkali-soluble acid precipitation: A comparative study on structure, function, and nutrition. Food Chem. 2024, 452, 139608. [Google Scholar] [CrossRef]
- Rebezov, M.; Usman Khan, M.; Bouyahya, A.; Imran, M.; Tufail, T.; Loretts, O.; Neverova, O.; Artyukhova, S.; Kuznetsova, E.; Ermolaev, V.; et al. Nutritional and technical aspect of tiger nut and its micro-constituents: An overview. Food Rev. Int. 2023, 39, 3262–3282. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, L.; Wang, G.; Zhang, A.; Wang, X.; Jiang, L. Ultrasonication effects on physicochemical and emulsifying properties of Cyperus esculentus seed (tiger nut) proteins. LWT 2021, 142, 110979. [Google Scholar] [CrossRef]
- Vega-Morales, T.; Mateos-Díaz, C.; Pérez-Machín, R.; Wiebe, J.; Gericke, N.P.; Alarcón, C.; López-Romero, J.M. Chemical composition of industrially and laboratory processed Cyperus esculentus rhizomes. Food Chem. 2019, 297, 124896. [Google Scholar] [CrossRef] [PubMed]
- Satir, G. The effects of fermentation with water kefir grains on two varieties of tigernut (Cyperus esculentus L.) milk. LWT 2022, 171, 114164. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, X.; Lu, X.; Zhang, T.; Cai, R.; Zheng, H.; Gao, F. Nutritional, structural and functional properties of protein fractions from tiger nut (Cyperus esculentus L.) seed meal. J. Food Meas. Charact. 2024, 18, 9867–9878. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Pérez-Santín, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Liu, F.F.; Li, Y.Q.; Wang, C.Y.; Liang, Y.; Zhao, X.Z.; He, J.X.; Mo, H.Z. Physicochemical, functional and antioxidant properties of mung bean protein enzymatic hydrolysates. Food Chem. 2022, 393, 133397. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, C.; Niu, C.; Wang, J.; Zheng, F.; Li, Q. Identification and virtual screening of novel antioxidant peptides from brewing by-products and their cytoprotective effects against H2O2-induced oxidative stress. Food Biosci. 2024, 58, 103686. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, R.; Rajput, Y.S.; Mann, B.; Gandhi, K. Distinction between glycomacropeptide and β-lactoglobulin with ‘stains all’dye on tricine SDS-PAGE gels. Food Chem. 2021, 340, 127923. [Google Scholar] [CrossRef]
- Xiao, L.; Liang, Y.; Liu, G.; Lin, F.; Wen, X. Identification of antioxidant peptides after digestion and absorption of isinglass by serum peptidomics and cellular antioxidant activity analysis. Food Funct. 2023, 14, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Othmeni, I.; Karoui, R.; Blecker, C. Impact of pH on the structure, interfacial and foaming properties of pea protein isolate: Investigation of the structure-Function relationship. Int. J. Biol. Macromol. 2024, 278, 134818. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Cai, Y.; Liu, T.; Long, Z.; Huang, L.; Deng, X.; Zhao, Q.; Zhao, M. Effects of pretreatments on the structure and functional properties of okara protein. Food Hydrocoll. 2019, 90, 394–402. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, B.; Xie, F.; Hu, M.; Sun, Y.; Han, L.; Li, L.; Zhang, S.; Li, Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 2021, 113, 106391. [Google Scholar] [CrossRef]
- Rahman, M.S.; Choi, Y.H.; Choi, Y.S.; Alam, M.B.; Lee, S.H.; Yoo, J.C. A novel antioxidant peptide, purified from Bacillus amyloliquefaciens, showed strong antioxidant potential via Nrf-2 mediated heme oxygenase-1 expression. Food Chem. 2018, 239, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Pouzo, L.B.; Descalzo, A.M.; Zaritzky, N.E.; Rossetti, L.; Pavan, E. Antioxidant status, lipid and color stability of aged beef from grazing steers supplemented with corn grain and increasing levels of flaxseed. Meat Sci. 2016, 111, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger nut (Cyperus esculentus L.): Nutrition, processing, function and applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef]
- Ren, L.K.; Yang, Y.; Ma, C.M.; Fan, J.; Bian, X.; Liu, B.X.; Wang, D.F.; Zhu, P.Y.; Fu, Y.; Zhang, N. Identification and in silico analysis of novel antioxidant peptides in broken rice protein hydrolysate and its cytoprotective effect against H2O2-induced 2BS cell model. Food Res. Int. 2022, 162, 112108. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food protein-derived antioxidant peptides: Molecular mechanism, stability and bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Tao, H.; Wang, S.; Xing, B.; Wang, Z.; Liu, K.; Shao, Q.; Gao, F. Identification and functional validation of two novel antioxidant peptides in saffron. Antioxidants 2024, 13, 378. [Google Scholar] [CrossRef] [PubMed]

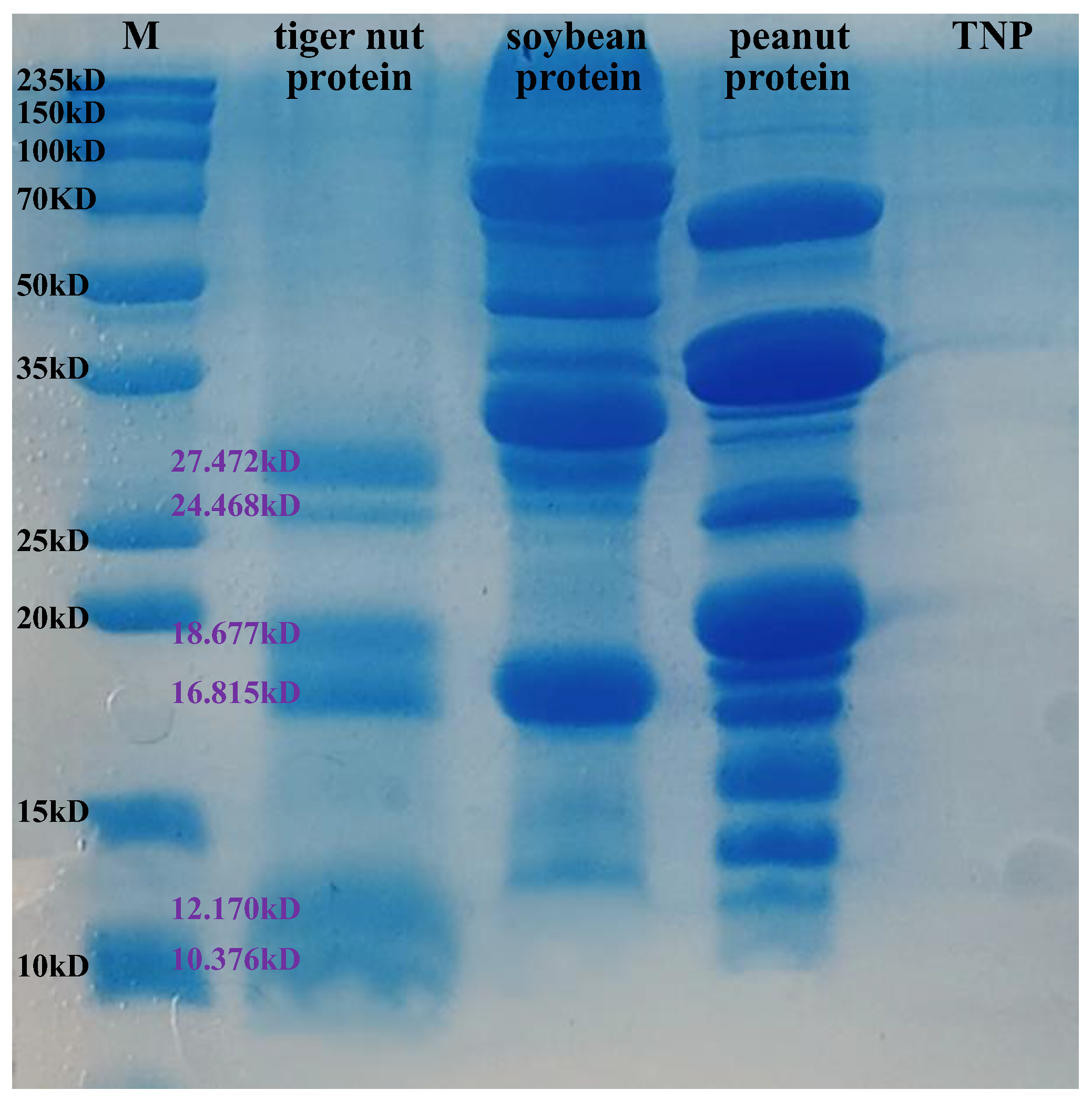
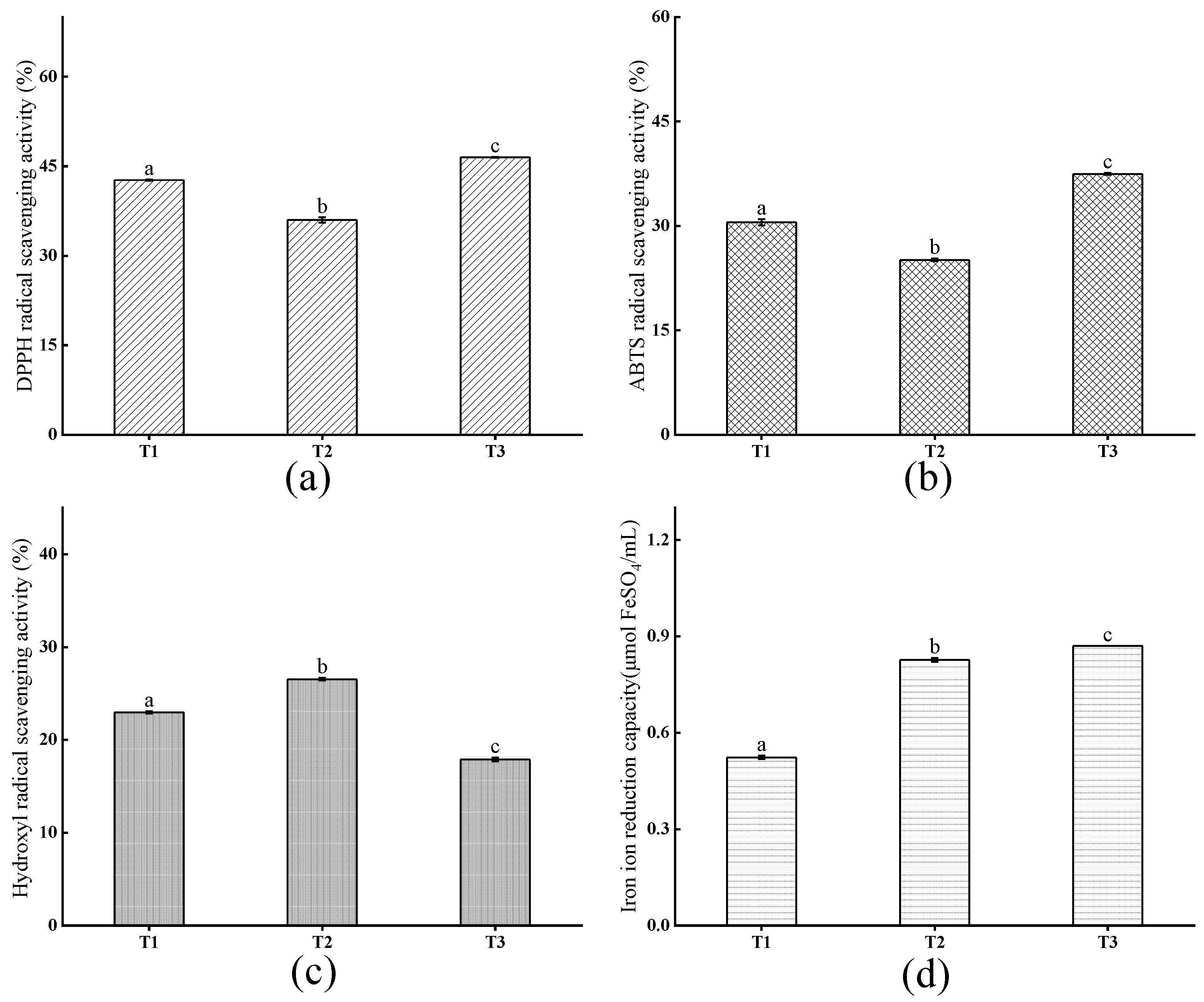
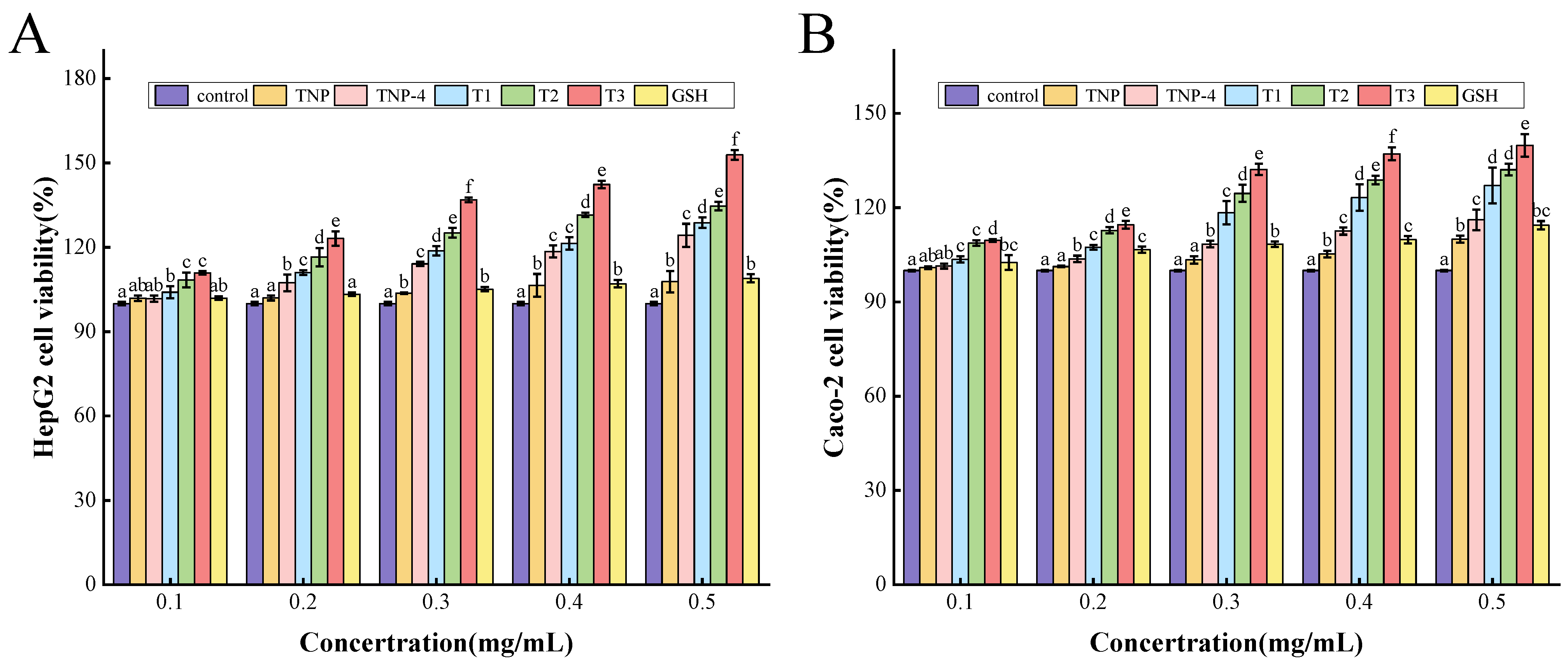
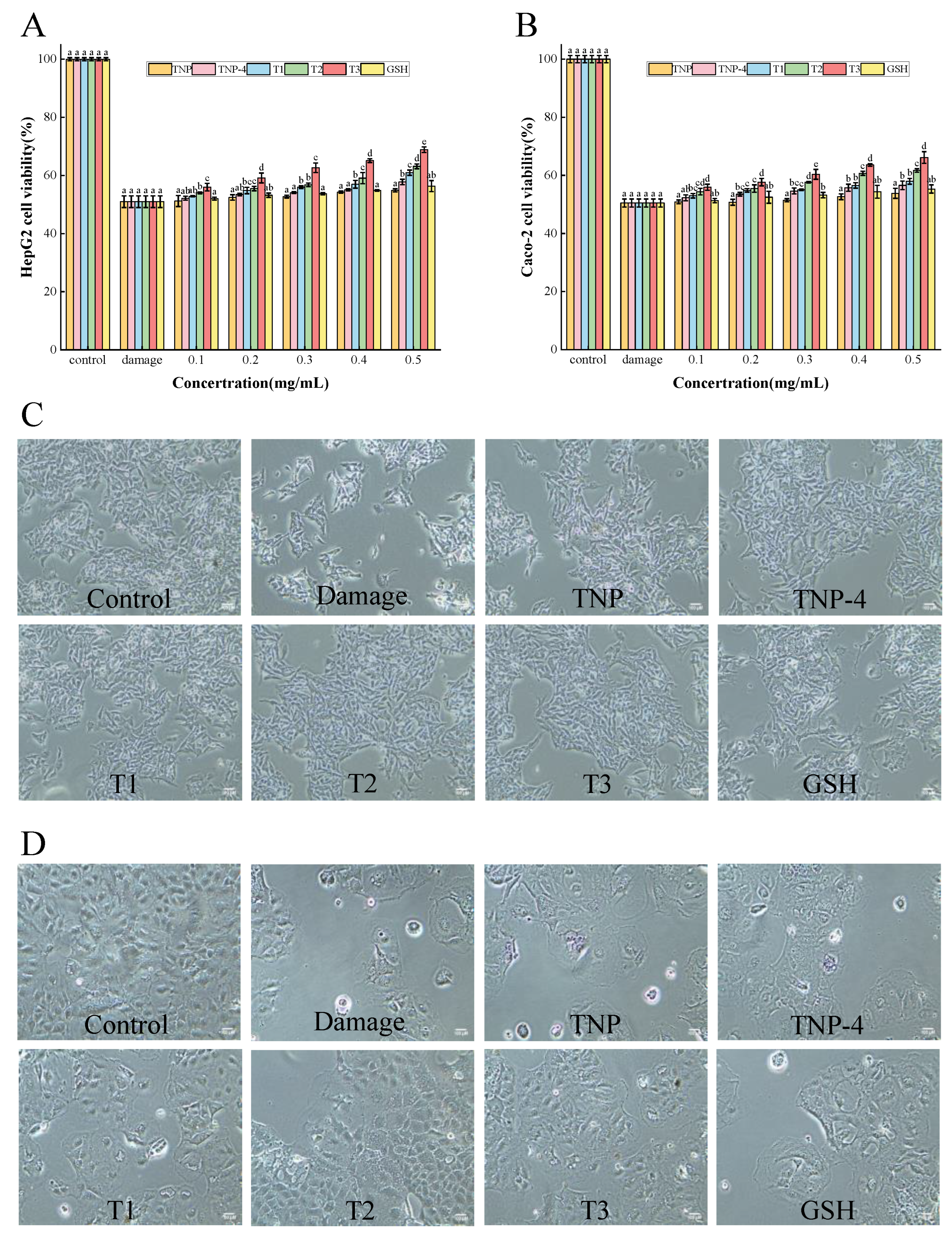
| Function | Tiger Nut Protein | TNP |
|---|---|---|
| WHC (g/g) | 2.86 ± 0.01 a | — |
| OHC (g/g) | 4.18 ± 0.01 a | 1.99 ± 0.01 b |
| Solubility (%) | 6.08 ± 0.12 a | 31.92 ± 2.25 b |
| FC (%) | 31.67 ± 2.89 a | 15.00 ± 0.00 b |
| FS (%) | 63.49 ± 5.50 a | 80.00 ± 0.00 b |
| Emulsibility (%) | 50.98 ± 0.00 a | 41.32 ± 0.00 b |
| Emulsion stability (%) | 76.92 ± 0.01 a | 60.00 ± 0.00 b |
| Amino Acids (g/100 g) | Tiger Nut Protein | TNP | Soybean Peptides | Peanut Peptides |
|---|---|---|---|---|
| Aspartic (Asp) | 6.62 ± 0.08 a | 9.02 ± 0.13 b | 12.17 ± 0.29 d | 12.71 ± 0.17 c |
| Threonine (Thr) | 4.82 ± 0.16 a | 4.65 ± 0.26 a | 3.99 ± 0.18 c | 2.59 ± 0.13 b |
| Serine (Ser) | 4.38 ± 0.40 a | 4.05 ± 0.45 a | 4.22 ± 0.86 a | 4.30 ± 0.65 a |
| Glutamic (Glu) | 18.48 ± 0.91 a | 17.40 ± 0.64 a | 18.97 ± 0.70 ab | 20.73 ± 0.75 b |
| Glycine (Gly) | 5.75 ± 0.04 a | 3.05 ± 0.06 b | 4.29 ± 0.25 c | 6.08 ± 0.13 a |
| Alanine (Ala) | 5.61 ± 0.08 a | 7.47 ± 0.13 b | 4.67 ± 0.25 c | 4.26 ± 0.16 c |
| Cysteine (Cys) | 1.54 ± 0.12 a | 0.86 ± 0.06 b | 1.16 ± 0.11 bc | 1.23 ± 0.17 ac |
| Valine (Val) | 6.06 ± 0.24 a | 6.74 ± 0.39 a | 5.10 ± 0.25 c | 4.21 ± 0.23 b |
| Methionine (Met) | 1.65 ± 0.04 a | 2.00 ± 0.00 a | 1.12 ± 0.23 b | 0.98 ± 0.29 b |
| Isoleucine (Ile) | 4.52 ± 0.52 a | 4.74 ± 0.26 a | 4.71 ± 0.27 a | 3.54 ± 0.20 b |
| Leucine (Leu) | 8.44 ± 0.04 a | 8.47 ± 0.00 a | 8.11 ± 0.20 a | 7.02 ± 0.19 b |
| Tyrosine (Tyr) | 2.36 ± 0.16 a | 2.46 ± 0.26 a | 3.36 ± 0.14 b | 3.42 ± 0.04 b |
| Phenylalanine (Phe) | 4.01 ± 0.12 a | 4.56 ± 0.39 a | 5.31 ± 0.12 b | 5.48 ± 0.09 b |
| Lysine (Lys) | 6.34 ± 0.08 a | 4.51 ± 0.06 b | 6.78 ± 0.12 d | 3.72 ± 0.03 c |
| Histidine (His) | 3.42 ± 0.08 a | 2.78 ± 0.06 b | 2.88 ± 0.14 b | 2.44 ± 0.01 c |
| Arginine (Arg) | 8.25 ± 0.16 a | 10.30 ± 0.26 b | 7.61 ± 0.02 d | 12.63 ± 0.06 c |
| Proline (Pro) | 7.74 ± 0.32 a | 6.93 ± 0.26 b | 5.57 ± 0.05 d | 4.64 ± 0.03 c |
| Acidic amino acids (NCAA) | 25.1 ± 0.99 a | 26.42 ± 0.77 a | 33.44 ± 0.58 b | 31.13 ± 0.41 c |
| Basic Amino Acids (PCA) | 18.01 ± 0.32 a | 17.59 ± 0.26 ab | 18.79 ± 0.01 c | 17.26 ± 0.25 b |
| Hydrophobic amino acids (HAA) | 39.58 ± 0.99 a | 41.77 ± 1.36 a | 31.35 ± 0.64 b | 35.75 ± 0.66 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, Y.; Chen, L.; Fan, M.; Zhao, X.; Hao, J. Antioxidant Peptides from Tiger Nut (Cyperus esculentus L.): Chemical Analysis and Cytoprotective Functions on HepG2 and Caco-2 Cells. Foods 2025, 14, 349. https://doi.org/10.3390/foods14030349
Quan Y, Chen L, Fan M, Zhao X, Hao J. Antioxidant Peptides from Tiger Nut (Cyperus esculentus L.): Chemical Analysis and Cytoprotective Functions on HepG2 and Caco-2 Cells. Foods. 2025; 14(3):349. https://doi.org/10.3390/foods14030349
Chicago/Turabian StyleQuan, Yu, Lin Chen, Meiqi Fan, Xia Zhao, and Jianxiong Hao. 2025. "Antioxidant Peptides from Tiger Nut (Cyperus esculentus L.): Chemical Analysis and Cytoprotective Functions on HepG2 and Caco-2 Cells" Foods 14, no. 3: 349. https://doi.org/10.3390/foods14030349
APA StyleQuan, Y., Chen, L., Fan, M., Zhao, X., & Hao, J. (2025). Antioxidant Peptides from Tiger Nut (Cyperus esculentus L.): Chemical Analysis and Cytoprotective Functions on HepG2 and Caco-2 Cells. Foods, 14(3), 349. https://doi.org/10.3390/foods14030349





