Isolation and Identification of Aspergillus spp. from Rotted Walnuts and Inhibition Mechanism of Aspergillus flavus via Cinnamon Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Locations and Sampling
2.3. Morphological Identification
2.4. DNA Isolation, PCR Amplification, and Sequence Analysis
2.5. Pathogenicity Testing
2.6. Measuring the Antifungal Effectiveness of CEO
2.6.1. Assay for the Inhibition of Mycelial Radical Growth
2.6.2. MIC and Minimum Fungicidal Concentration (MFC)
2.7. Sterilization Mechanisms
2.7.1. Treatment of A. flavus Mycelium with CEO
2.7.2. Cell Membrane Permeability
2.7.3. Cell Membrane Integrity
2.7.4. Malondialdehyde (MDA) Content
2.7.5. SOD Content
2.8. Statistical Analyses
3. Results
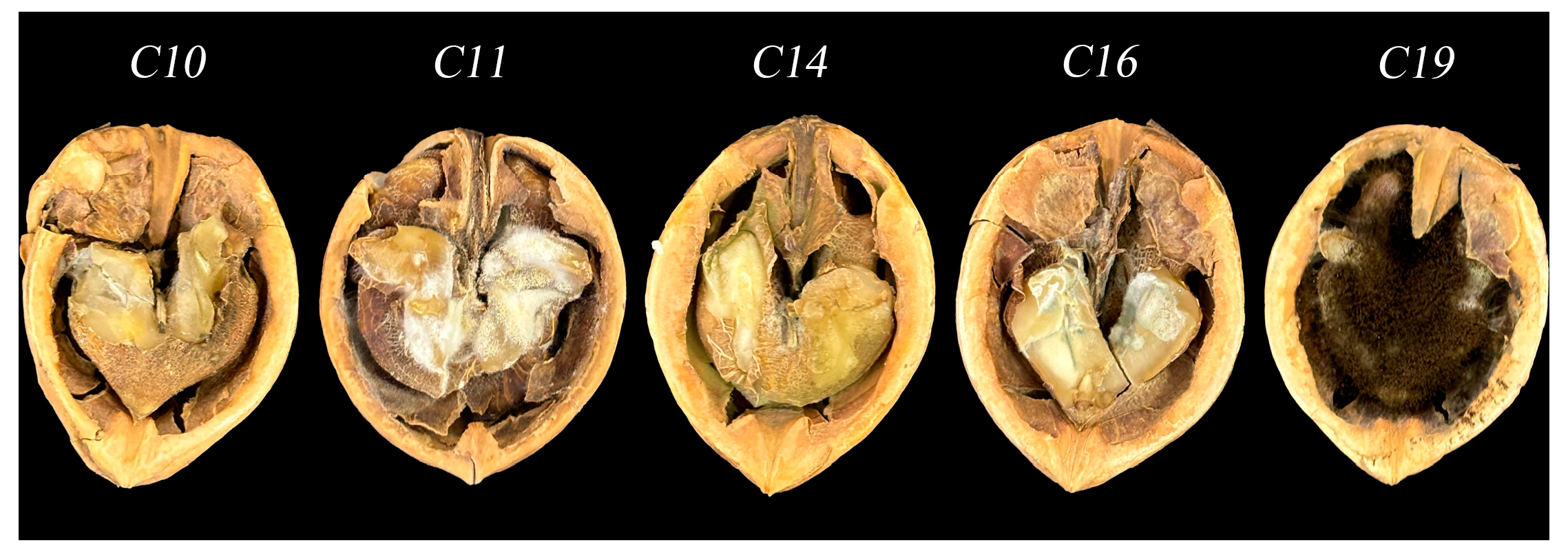
3.1. Microscopic Analysis of Aspergillus Species in Contaminated Walnut Samples
3.2. Identification of Fungal Isolates via ITS Sequence Analysis
3.3. Pathogenicity Testing
3.4. Frequency of Aspergillus spp. in Moldy Walnut Samples
3.5. Antifungal Activities of CEO
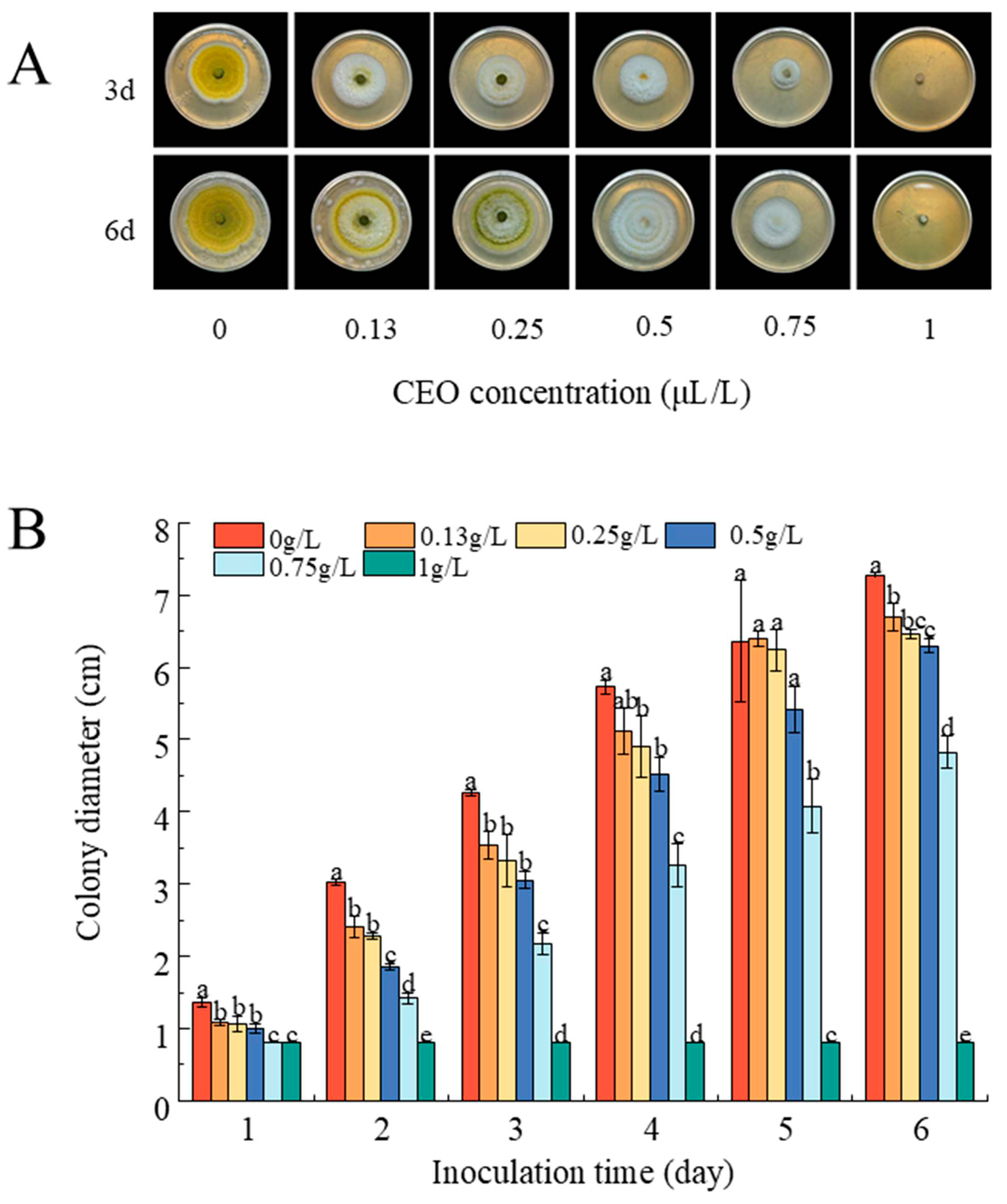
3.5.1. CEO Inhibits Mycelial Growth
3.5.2. MIC and MFC
3.6. Effect of CEO on A. flavus Cell Membrane Permeability
3.7. Impact of CEO on the Integrity of Microbial Cell Membranes
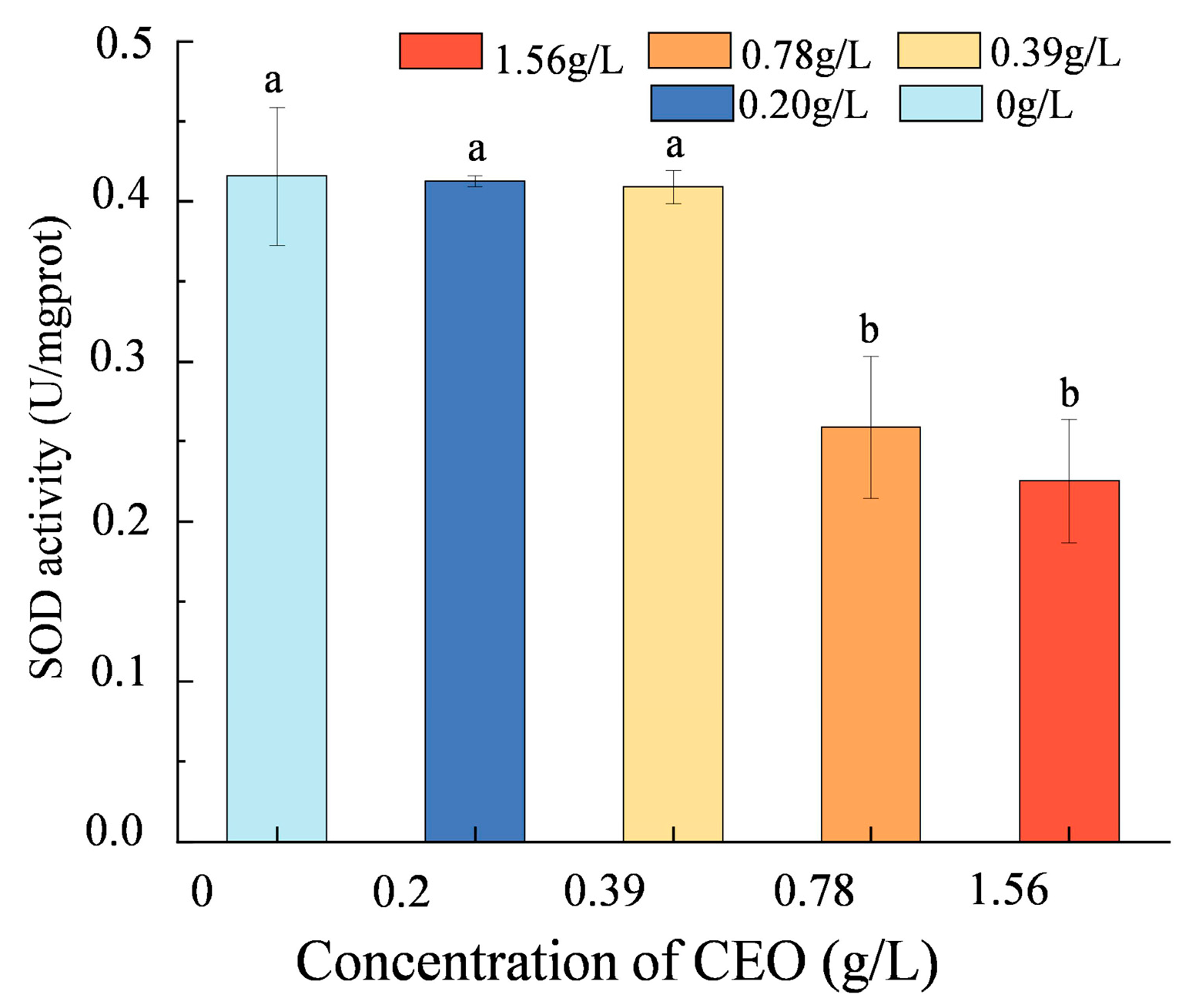
3.8. Oxidative Stress Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabadan, A.; Pardo, J.E.; Pardo-Gimenez, A.; Alvarez-Orti, M. Effect of Genotype and Crop Year on the Nutritional Value of Walnut Virgin Oil and Defatted Flour. Sci. Total Environ. 2018, 634, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Magige, E.A.; Fan, P.Z.; Wambulwa, M.C.; Milne, R.; Wu, Z.Y.; Luo, Y.H.; Khan, R.; Wu, H.Y.; Qi, H.L.; Zhu, G.F.; et al. Genetic Diversity and Structure of Persian Walnut (Juglans regia L.) in Pakistan: Implications for Conservation. Plants 2022, 11, 1652. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Zhao, D.; Wang, K.; Jia, M.; Wang, J. Morphological and Compositional Analysis of Two Walnut (Juglans regia L.) Cultivars Growing in China. Plant Foods Hum. Nutr. 2020, 75, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Zeb, A. Walnuts Ameliorated Hepatic Inflammation and Toxicity Induced by Thermally Oxidised High-Fat Diet in Mice. J. Funct. Foods 2024, 114, 106080. [Google Scholar] [CrossRef]
- Awad, A.B.; Fink, C.S. Phytosterols as Anticancer Dietary Components: Evidence and Mechanism of Action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.; Ma, H. Production, Bioactivities and Bioavailability of Bioactive Peptides Derived from Walnut Origin by-Products: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 8032–8047. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and Antiproliferative Activities of Common Edible Nut Seeds. LWT—Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Hellany, H.; Assaf, J.C.; Matta, J.; Khalil, M.I. Fungal Isolation, Detection, and Quantification of Aflatoxins in Nuts Sold in the Lebanese Market. Processes 2024, 12, 1018. [Google Scholar] [CrossRef]
- Qiao, L.; Jiao, Y.; Li, X.; Zhang, Y.; Lu, L.; Zhang, X.; Liu, X. Herbal Smoke Fumigation for Controlling Penicillium Crustosum in Fresh Walnuts. Food Res. Int. 2023, 167, 112709. [Google Scholar] [CrossRef]
- Wei, Y.; Li, L.; Liu, Y.; Xiang, S.; Zhang, H.; Yi, L.; Shang, Y.; Xu, W. Identification Techniques and Detection Methods of Edible Fungi Species. Food Chem. 2022, 374, 131803. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Sasani, E.; Rezaie, S.; Soltan Dallal, M.M.; Mahmoudi, S.; Ahmadi, A.; Ghaffari, M.; Aala, F.; Khodavaisy, S. Molecular Identification of Aflatoxigenic Aspergillus Species in Dried Nuts and Grains Collected from Tehran, Iran. J. Environ. Health Sci. Eng. 2021, 19, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in Harvested Fruits and Vegetables: Insights in Producing Fungi, Biological Role, Conducive Conditions, and Tools to Manage Postharvest Contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus Species and Mycotoxins: Occurrence and Importance in Major Food Commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, L.; Ma, Q.; Ji, C. Novel Strategies for Degradation of Aflatoxins in Food and Feed: A Review. Food Res. Int. 2021, 140, 109878. [Google Scholar] [CrossRef] [PubMed]
- Ngolong Ngea, G.L.; Yang, Q.; Castoria, R.; Zhang, X.; Routledge, M.N.; Zhang, H. Recent Trends in Detecting, Controlling, and Detoxifying of Patulin Mycotoxin Using Biotechnology Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2447–2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, K.; Han, M.; Zhang, W.; Li, X.; Ma, D.; Wang, F.; Pang, M.; Qi, J. Isolation and Identification of Endophytic Fungi in Walnut. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 012138. [Google Scholar] [CrossRef]
- Cai, J.; Yan, R.; Shi, J.; Chen, J.; Long, M.; Wu, W.; Kuca, K. Antifungal and Mycotoxin Detoxification Ability of Essential Oils: A Review. Phytother. Res. 2022, 36, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Randrianarivelo, R.; Sarter, S.; Odoux, E.; Brat, P.; Lebrun, M.; Romestand, B.; Menut, C.; Andrianoelisoa, H.; Raherimandimby, M.; Danthu, P. Composition and Antimicrobial Activity of Essential Oils of Cinnamosma Fragrans. Food Chem. 2009, 114, 680–684. [Google Scholar] [CrossRef]
- Alam, A.; Ansari, M.J.; Alqarni, M.H.; Salkini, M.A.; Raish, M. Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum cassia L. Essential Oil. Plants 2023, 12, 834. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Tvrdá, E.; Terentjeva, M.; Žiarovská, J.; Kunová, S.; Savitskaya, T.; Grinshpan, D.; Štefániková, J.; et al. Antimicrobial and Antioxidant Activities of Cinnamomum cassia Essential Oil and Its Application in Food Preservation. Open Chem. 2021, 19, 214–227. [Google Scholar] [CrossRef]
- Minozzo, M.; de Souza, M.A.; Bernardi, J.L.; Puton, B.M.S.; Valduga, E.; Steffens, C.; Paroul, N.; Cansian, R.L. Antifungal Activity and Aroma Persistence of Free and Encapsulated Cinnamomum cassia Essential Oil in Maize. Int. J. Food Microbiol. 2023, 394, 110178. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, A.; Hu, A.; Li, J.; Zen, Z.; Liu, Y.; Tang, S.; Li, C. Preparation and Characterization of Cinnamon Essential Oil Nanocapsules and Comparison of Volatile Components and Antibacterial Ability of Cinnamon Essential Oil before and after Encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, M.; Luo, J.; Zhang, H.; Lv, J.; Zhou, F.; Ru, Q.; Jin, Z.; Yang, S.; Yang, M. Inhibitory Mechanism and Application of Cinnamon Essential Oil against Aspergillus Flavus. LWT 2024, 201, 116267. [Google Scholar] [CrossRef]
- Bakr, J.G.; Khalid, S.A.; Khafaga, N.I.M.; Yassien, N.A.; Zaki, H.M.B.A. Impact of Using Cinnamon (Cinnamomum verum) Essential Oil and Its Pectin-Chitosan Nano-Emulsion on Survival of Aspergillus Flavus and Total Aflatoxin Inhibition in Beef Burger Patties. Food Control 2024, 159, 110294. [Google Scholar] [CrossRef]
- Bavaro, S.L.; Susca, A.; Frisvad, J.C.; Tufariello, M.; Chytiri, A.; Perrone, G.; Mita, G.; Logrieco, A.F.; Bleve, G. Isolation, Characterization, and Selection of Molds Associated to Fermented Black Table Olives. Front. Microbiol. 2017, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Tu, X.-F.; Thakur, K.; Hu, F.; Li, X.-L.; Zhang, Y.-S.; Zhang, J.-G.; Wei, Z.-J. Comparison of Antifungal Activity of Essential Oils from Different Plants against Three Fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and Antimicrobial Activity of Essential Oils Isolated from Egyptian Plants against Plant Pathogenic Bacteria and Fungi. Ind. Crops Prod. 2014, 52, 776–782. [Google Scholar] [CrossRef]
- Risslegger, B.; Lass-Florl, C.; Blum, G.; Lackner, M. Evaluation of a Modified Eucast Fragmented-Mycelium Inoculum Method for in Vitro Susceptibility Testing of Dermatophytes and the Activity of Novel Antifungal Agents. Antimicrob. Agents Chemother. 2015, 59, 3675–3682. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, M.; Deng, D. The Synergistic Antimicrobial Effects and Mechanism of Cinnamon Essential Oil and High Voltage Electrostatic Field and Their Application in Minced Pork. Food Control 2024, 163, 110475. [Google Scholar] [CrossRef]
- Geiser, D.M.; Klich, M.A.; Frisvad, J.C.; Peterson, S.W.; Varga, J.; Samson, R.A. The Current Status of Species Recognition and Identification in Aspergillus. Stud. Mycol. 2007, 59, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-Harvest Fungal Ecology: Impact of Fungal Growth and Mycotoxin Accumulation in Stored Grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, Y.; Chen, Y.; Wang, Y.; Gu, Q.; Song, D. Antifungal Activity and Mechanism of Litsea Cubeba (Lour.) Persoon Essential Oil against the Waxberry Spoilage Fungi Penicillium Oxalicum and Its Potential Application. Int. J. Food Microbiol. 2024, 411, 110512. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Huang, C.; Chen, Q.; Zou, Y.; Zhang, J. Nitric Oxide Alleviates Heat Stress-Induced Oxidative Damage in Pleurotus Eryngii Var. Tuoliensis. Fungal Genet. Biol. 2012, 49, 15–20. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, L.; Jiang, D.; Wang, M.; Liu, H.; Yu, H.; Yao, W. Transcriptomic Analysis of Inhibition by Eugenol of Ochratoxin A Biosynthesis and Growth of Aspergillus Carbonarius. Food Control 2022, 135, 108788. [Google Scholar] [CrossRef]
- Ko Ko, T.W.; Stephenson, S.L.; Bahkali, A.H.; Hyde, K.D. From Morphology to Molecular Biology: Can We Use Sequence Data to Identify Fungal Endophytes? Fungal Divers. 2011, 50, 113–120. [Google Scholar] [CrossRef]
- Abbas, M.; Naz, S.A.; Shafique, M.; Jabeen, N.; Abbas, S. Fungal Contamination in Dried Fruits and Nuts: A Possible Source of Mycoses and Mycotoxicoses. Pak. J. Bot. 2019, 51, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A. Mycobiota and Mycotoxins of Nuts and Some Dried Fruits from Saudi Arabia. J. Am. Sci. 2012, 8, 525–534. [Google Scholar]
- Lee, H.; Kim, N.; Ryu, J.-H.; Kim, H. Inactivation of Aspergillus Flavus on Green Coffee Beans by Treatments with Organic Acid Vapor. Food Control 2024, 160, 110322. [Google Scholar] [CrossRef]
- Adkison, C.; Richmond, K.; Lingga, N.; Bikoba, V.; Mitcham, E. Optimizing Walnut Storage Conditions: Effects of Relative Humidity, Temperature, and Shelling on Quality after Storage. HortSci. 2021, 56, 1244–1250. [Google Scholar] [CrossRef]
- Bhabhra, R.; Askew, D.S. Thermotolerance and Virulence of Aspergillus Fumigatus: Role of the Fungal Nucleolus. Med. Mycol. 2005, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Wang, S.; Xiao, D.; Li, H.; Li, C.; Xiao, Z.; Fang, F.; Liu, M.; Wang, J. Characterization, Antibacterial and Snakehead Fish (Channa Argus) Preservation Effects of Active Films Infused with Compounded Cinnamon and Thyme Essential Oils. Food Packag. Shelf Life 2024, 46, 101353. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martin-Belloso, O. Antimicrobial Activity of Essential Oils on Salmonella Enteritidis, Escherichia Coli, and Listeria Innocua in Fruit Juices. J. Food Prot. 2006, 69, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Jung, M.; Lee, S.-C.; Huh, M.-J.; Seo, S.-M.; Park, I.-K. Antibacterial Mode of Action of Trans-Cinnamaldehyde Derived from Cinnamon Bark (Cinnamomum verum) Essential Oil against Agrobacterium Tumefaciens. Pestic. Biochem. Physiol. 2020, 165, 104546. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hua, H.; Selvaraj, J.N.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Growth Inhibition and Morphological Alterations of Fusarium Verticillioides by Cinnamon Oil and Cinnamaldehyde. Food Control 2014, 46, 343–350. [Google Scholar] [CrossRef]
- da Silva, A.S.R.; Fernandes, C.C.; dos Santos, D.A.; Mazza, M.C.M.; Silva, J.B.A.; Magalhães, L.G.; Pires, R.H.; Miranda, M.L.D.; Crotti, A.E.M. Antileishmanial and Antifungal Activities of Volatile Oils from Cinnamomum cassia Bark and Schinus Molle Leaves. Chem. Biodivers. 2024, 21, e202401076. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, H.; Feng, Y.; Yu, M.; Xu, Y.; Zhao, Y.; Zheng, B.; Lin, J.; Miao, W.; Zhou, R.; et al. Emulsions Containing Composite (Clove, Oregano, and Cinnamon) Essential Oils: Phase Inversion Preparation, Physicochemical Properties and Antibacterial Mechanism. Food Chem. 2023, 421, 136201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, M.; Wei, Y.; Xu, F.; Jiang, S.; Chen, Y.; Ding, P.; Shao, X. Cinnamon Essential Oil Causes Cell Membrane Rupture and Oxidative Damage of Rhizopus Stolonifer to Control Soft Rot of Peaches. Food Control 2025, 170, 111039. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, X.; Jing, G.; OuYang, Q.; Tao, N. Cinnamaldehyde Inhibits the Mycelial Growth of Geotrichum Citri-Aurantii and Induces Defense Responses against Sour Rot in Citrus Fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Dias, A.L.B.; Santos, J.G.d.; da Silva, I.J.M.M.; Miranda, M.L.D. Antifungal and Allelopathic Effects of Essential Oil from Calyptranthes concinna DC. Dried Leaves and of Its Major Constituent Elemicin. Agronomy 2024, 14, 1527. [Google Scholar] [CrossRef]
- de Groot, A.C.; Frosch, P.J. Adverse Reactions to Fragrances. Contact Dermat. 2006, 36, 57–86. [Google Scholar] [CrossRef] [PubMed]
- Ballin, N.Z.; Sørensen, A.T. Coumarin Content in Cinnamon Containing Food Products on the Danish Market. Food Control 2014, 38, 198–203. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (Afc) Related to Coumarin. EFSA J. 2004, 2, 104. [Google Scholar] [CrossRef]




| Months | June | July | August | September | October |
|---|---|---|---|---|---|
| Average high temperature (°C) | 31 | 32 | 31 | 26 | 21 |
| Average low temperature (°C) | 14 | 19 | 18 | 13 | 4 |
| Total monthly precipitation (cm) | 14.1 | 16.5 | 76.3 | 4.8 | 17.8 |
| Sample | A. tubingensis | A. terreus | A. flavus | A. fumigatus | A. niger |
|---|---|---|---|---|---|
| detection limit | 40 | 47 | 100 | 93 | 78 |
| Frequency | 40% | 47% | 100% | 93% | 78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Luo, K.; Wen, S.; Zhou, Q.; Li, B.; Liang, W.; Di, J. Isolation and Identification of Aspergillus spp. from Rotted Walnuts and Inhibition Mechanism of Aspergillus flavus via Cinnamon Essential Oil. Foods 2025, 14, 357. https://doi.org/10.3390/foods14030357
Zhang D, Luo K, Wen S, Zhou Q, Li B, Liang W, Di J. Isolation and Identification of Aspergillus spp. from Rotted Walnuts and Inhibition Mechanism of Aspergillus flavus via Cinnamon Essential Oil. Foods. 2025; 14(3):357. https://doi.org/10.3390/foods14030357
Chicago/Turabian StyleZhang, Doudou, Kangjing Luo, Shaocong Wen, Qing Zhou, Bochao Li, Wenhui Liang, and Jianbing Di. 2025. "Isolation and Identification of Aspergillus spp. from Rotted Walnuts and Inhibition Mechanism of Aspergillus flavus via Cinnamon Essential Oil" Foods 14, no. 3: 357. https://doi.org/10.3390/foods14030357
APA StyleZhang, D., Luo, K., Wen, S., Zhou, Q., Li, B., Liang, W., & Di, J. (2025). Isolation and Identification of Aspergillus spp. from Rotted Walnuts and Inhibition Mechanism of Aspergillus flavus via Cinnamon Essential Oil. Foods, 14(3), 357. https://doi.org/10.3390/foods14030357





