Research Progress Regarding Psychrotrophic Pseudomonas in Aquatic Products: Psychrophilic Characteristics, Spoilage Mechanisms, Detection Methods, and Control Strategies
Abstract
1. Introduction
2. Characteristics of Pseudomonas spp.
2.1. Species of Pseudomonas spp. in Different Aquatic Products
2.2. Cold Adaptation Mechanism of Pseudomonas spp.
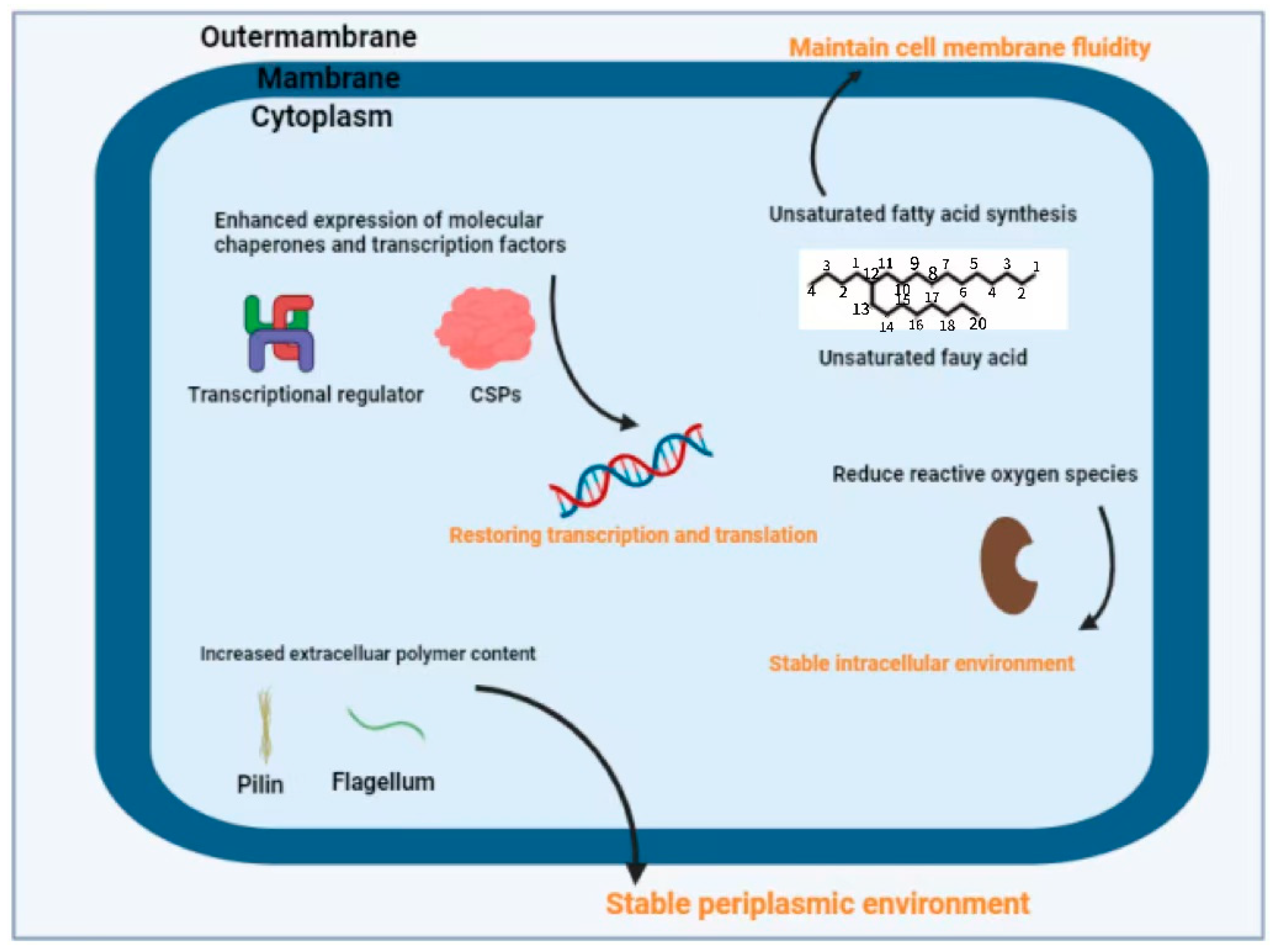
2.2.1. Regulation of Cell Membranes
- (i)
- Branched-chain fatty acids
- (ii)
- Polyunsaturated fatty acids
- (iii)
- Fatty acid desaturases
2.2.2. Low Temperature Protease
2.2.3. Cold-Adapted Proteins
- (i)
- CSPs
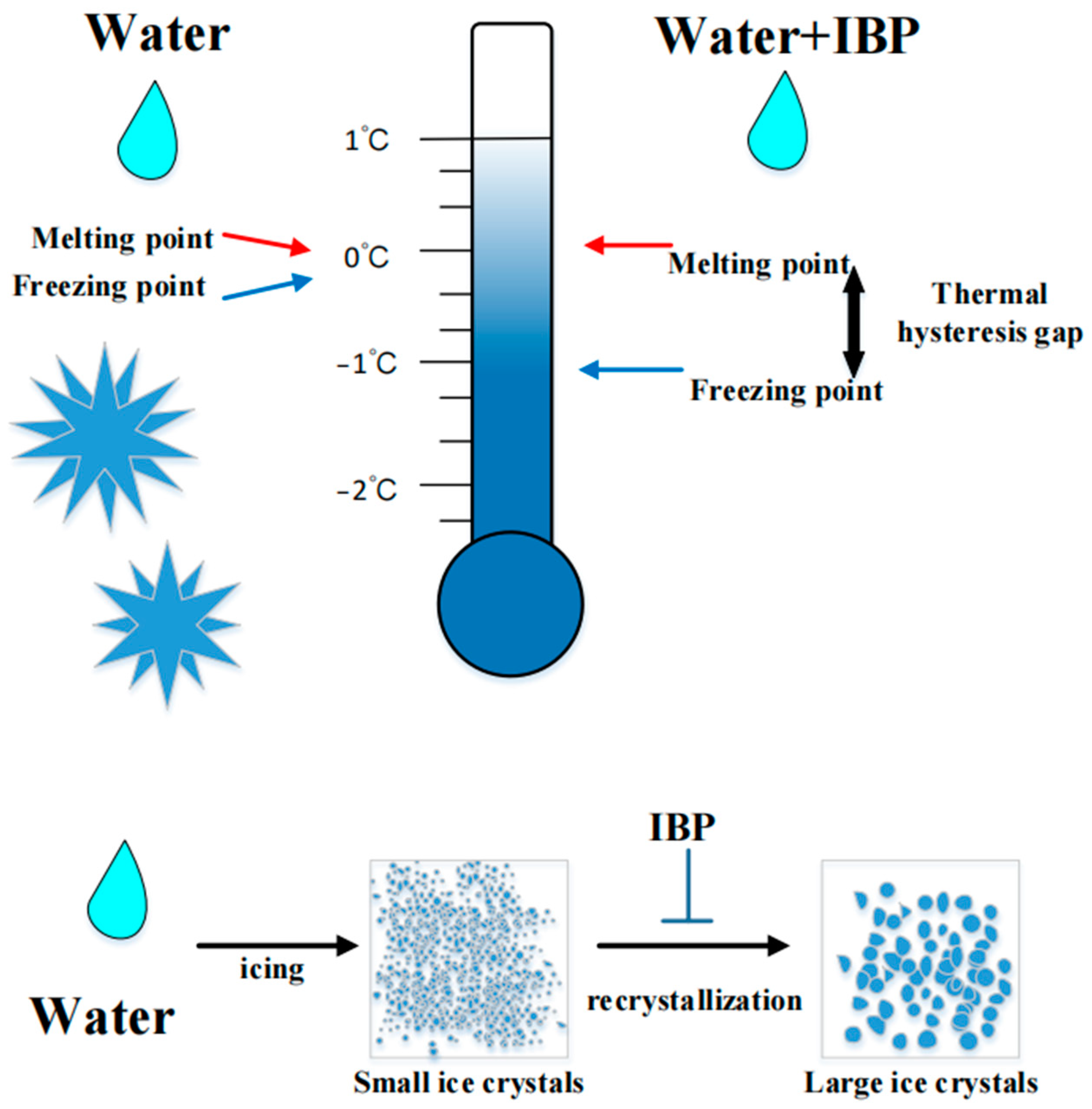
- (ii)
- IBPs
2.3. Regulatory Mechanisms of Biofilm Formation
2.3.1. Cyclic Di-Guanosine Monophosphate (C-di-GMP) Signaling Factor
2.3.2. Quorum Sensing (QS) Systems
2.3.3. Two-Component Control System (TCS)
3. The Spoilage Indicators of Pseudomonas spp.
3.1. Volatile Organic Compounds (VOCs)
3.1.1. BAs
3.1.2. TMA
3.1.3. Total Volatile Base Nitrogen (TVB-N)
| Categories | Representatives | Source | Hazard | References |
|---|---|---|---|---|
| Alcohols | 1-penten-3-ol, ethanol, methyl mercaptan, 0-methyl-4-butanol, isopropanol, 37-ethyl-0-hexanol, 4-penten-8-ol, etc. | Oxidation of polyunsaturated fatty acids | Fishy, fatty, mushroomy, or grassy flavor | [93,109] |
| Aldehydes | Nonanal, hexanal, decanal, 3-methylbutyraldehyde, trans-2-octenal, 8-methylbutyraldehyde and lauric aldehyde, etc. | Alkoxy radicals and derivatives of unsaturated fatty acids | Causes nausea, vomiting, abdominal pain and other digestive symptoms | [110] |
| Ketones | 2-octanone, 4-methyl-2-pentanone, 2-pentanone, 2-heptanone, n-nonanone, 2-undecanone, etc. | Oxidation or degradation of the unsaturated fatty acids and degradation of amino acids | Toxic, with a pungent odor Affects appetite, causes headaches | [111] |
| Esters | Butyl butyrate, isobutyl isobutyrate, ethyl 2-methylbutyrate, etc. | Composed of the reaction products of alcohols and acids resulting from acid–alcohol condensation | Has a strong smell of corruption, may cause allergic reactions or gastrointestinal discomfort | [112] |
3.2. Thiobarbituric Acid (TBA)
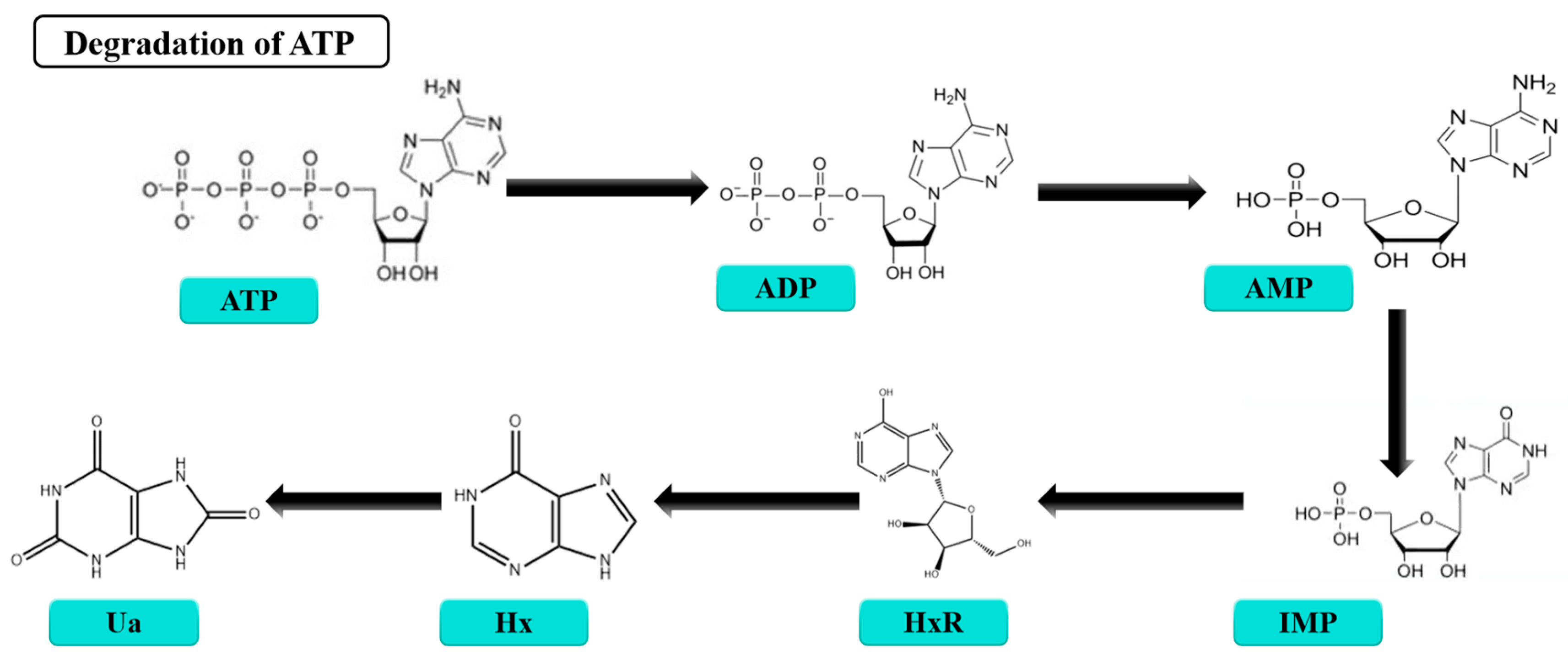
3.3. K-Value
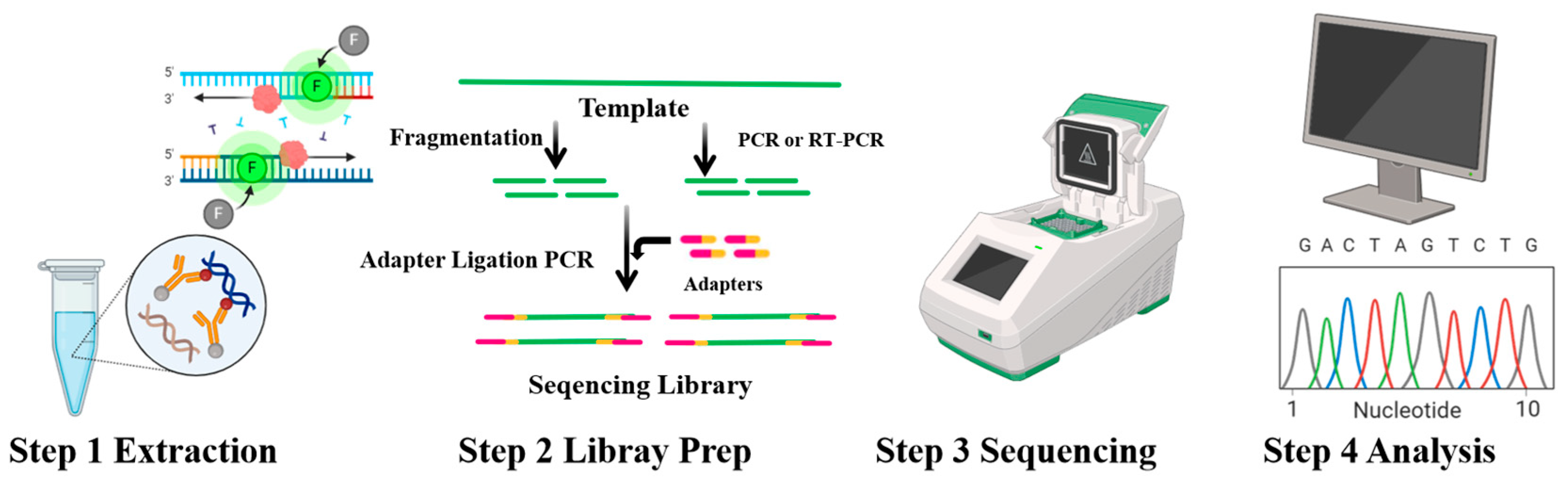
4. Technologies for Detection of Pseudomonas spp.
4.1. RPA
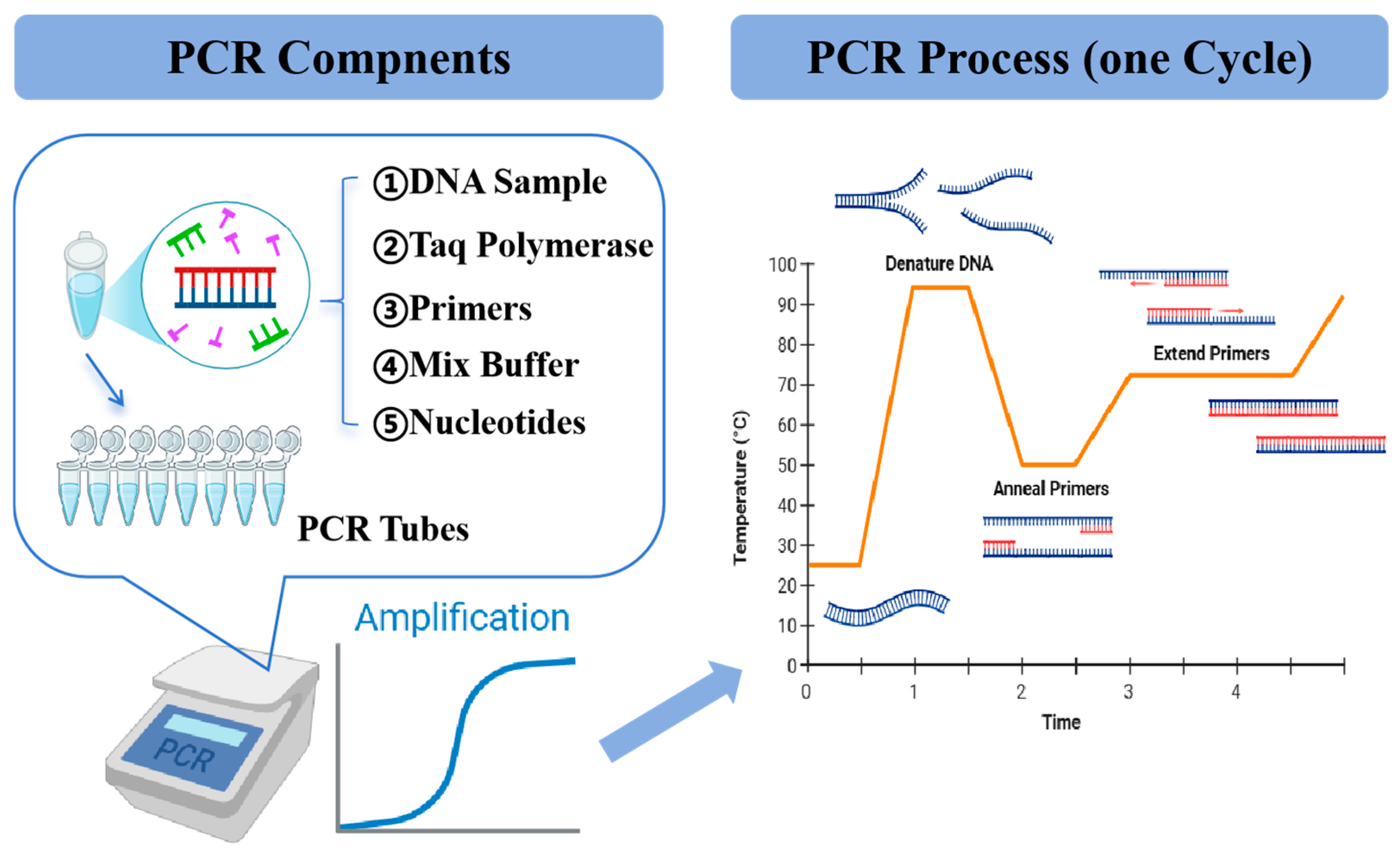
4.2. PCR
4.3. LAMP
4.4. NGS
4.5. FISH
| Methods | Principle | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| RPA | The rapid amplification of target DNA is achieved by the synergistic effect of DNA recombinase and polymerase | High sensitivity, high specificity, rapid turnaround time, easy to use | Limited multiplexing, low throughput, poor stability, high cost. | [135] |
| PCR | Amplifies specific genetic sequences using a polymerase enzyme and targeted primers | Quantitation possible, sensitivity, specificity, speed, versatility | PCR system affects the effectiveness, complexity, false positives, high cost | [136] |
| LAMP | Four primers are designed for the six regions of the target gene, and the amplification reaction is carried out using the strand displacement DNA polymerase under constant temperature conditions | High sensitivity, specificity, rapid turnaround time, simplicity | Limited multiplexing, poor performance with complex DNA templates, inability to detect DNA deletions or insertions, limited commercial availability | [127] |
| NGS | Improves sequencing speed and reduce costs by sequencing a large number of DNA fragments in parallel | High throughput, high accuracy, multiplexing, large scale, high resolution, versatility | Technical expertise, sample quality, data analysis, limited access | [132] |
| FISH | A labeled single-stranded nucleic acid probe binds to the unknown single-stranded nucleic acid in the sample based on base complementarity, forming a detectable hybrid double-stranded nucleic acid | High sensitivity, high specificity, rapid, Easy to visualize | Photobleaching, autofluorescence, limited to specific sequences | [133] |
5. Control Strategies
5.1. The Control of Bacterial Biofilms in Aquatic Products
5.1.1. Physical Methods
5.1.2. Chemical Methods
5.1.3. Biological Methods
| Method | Categories | Mechanism of Inhibition | Reference |
|---|---|---|---|
| Non-thermal plasma | Physical method | Ionization of gases by heat or a strong magnetic field generates a variety of active ingredients (superoxide, photons, etc.) that work in synergy to remove the bacterial biofilm | [137] |
| Electron beam radiation | Elevation of ROS levels by electron beam irradiation leading to damage to the bacterial cytoplasmic membrane and damage to bacterial nucleic acids | [138] | |
| Phage method | Biological method | Production of depolymerases to disrupt extracellular polysaccharides in biofilms | [145] |
| Methyl phthalate | Reduces P. aeruginosa biofilm formation, motility, protease activity, and production of AHLs (high serine lactones) | [152] | |
| Cinnamaldehyde | Disrupts the QS system by destroying the bacteriophage’s LuxR-type proteins | [153] | |
| Resveratrol | Inhibition of biofilms by disruption of diketopiperazine analogues (DKPs) in population sensing | [154] | |
| EOs | Chemical method | Flavonoids contained in EOs can disrupt biofilms and cause bacterial cell death | [140] |
| 2,6-Di-tert-butyl-4-methylphenol (DTBMP) | Prevents initial bacterial adhesion and biofilm formation and interferes with bacterial adhesion to aquatic products | [142] |
5.2. Inhibiting Bacterial Growth
5.2.1. Control Oxygen Content
5.2.2. Use of Antimicrobials
5.2.3. Preservation Coating Technology
5.3. Anti-Bacterial Packaging Materials
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| CSPs | Cold shock proteins |
| Pro | Proline |
| Gly | Glycine |
| 3-D | Three-dimensional |
| IBPs | Ice binding proteins |
| AFPs | Antifreeze proteins |
| INPs | Ice nucleation proteins |
| TH | Thermal hysteresis |
| EPS | Extracellular polymers |
| C-di-GMP | Cyclic di-guanosine monophosphate |
| DGC | Diguanylate cyclase |
| PDE | Phosphodiesterases |
| GGDEF | Gly-Gly-Asp-Glu-Phe |
| QS | Quorum sensing |
| AHLs | N-acyl-homoserine lactones |
| AIPs | Autoinducing peptides |
| AI-2 | Autoinducer-2 |
| DKPs | Diketopiperazines |
| TCS | Two-component control system |
| HK | Histidine kinase |
| RP | Regulatory protein |
| TVC | Total viable counts |
| VOCs | Volatile organic compounds |
| BAs | Biogenic amines |
| TMA | Trimethylamines |
| HS-SPME | Headspace solid-phase microextraction |
| GC-MS | Chromatography-mass spectrometry |
| ADC | Arginine decarboxylases |
| HPLC | High-performance liquid chromatography |
| GC | Gas chromatography |
| CZE | Capillary zone electrophoresis |
| IEC | Ion-exchange chromatography |
| DMA | Dimethylamine |
| FA | Formaldehyde |
| IMS | Ion mobility spectrometry |
| TVB-N | Total Volatile Base Nitrogen |
| SSOs | Specific spoilage organisms |
| TBA | Thiobarbituric Acid |
| ATP | Adenosine triphosphate |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| IMP | Inosine monophosphate |
| HxR | Inosine |
| Hx | Hypoxanthine |
| RPA | Polymerase amplification |
| PCR | Polymerase chain reaction |
| LAMP | Loop-mediated isothermal amplification |
| NGS | Next-generation sequencing |
| FISH | Fluorescence in situ hybridization |
| MLST | Multilocus sequence typing |
| ROS | Reactive oxygen species |
| EO | Essential oils |
| AEW | Acidic electrolytic water |
| DTBMP | 2,6-di-tert-butyl-4-methylphenol |
| QSIs | Quorum-sensing inhibitors |
| Quorum quenching | |
| GAC8 | Octyl gallate |
| NPs | Nanoparticles |
| PCs | Proanthocyanidins |
| CH | Chitosan |
| CS | Chondroitin sulfate |
References
- Tigchelaar, M.; Leape, J.; Micheli, F.; Allison, E.H.; Basurto, X.; Bennett, A.; Bush, S.R.; Cao, L.; Cheung, W.W.; Crona, B.; et al. The vital roles of blue foods in the global food system. Glob. Food Secur. 2022, 33, 100637. [Google Scholar] [CrossRef]
- Bi, J. Aquatic Food Products: Processing Technology and Quality Control. Foods 2024, 13, 2806. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.-S.; Fang, K.; Yang, X.-T.; Han, J.-W. Ensuring the quality of meat in cold chain logistics: A comprehensive review. Trends Food Sci. Technol. 2022, 119, 133–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Yuan, Y.; Yue, T. Diversity and characterization of spoilage-associated psychrotrophs in food in cold chain. Int. J. Food Microbiol. 2019, 290, 86–95. [Google Scholar] [CrossRef]
- Errampalli, D.; Brubacher, N.R. Biological and integrated control of postharvest blue mold (Penicillium expansum) of apples by Pseudomonas syringae and cyprodinil. Biol. Control 2006, 36, 49–56. [Google Scholar] [CrossRef]
- Craig, K.; Johnson, B.R.; Grunden, A. Leveraging Pseudomonas Stress Response Mechanisms for Industrial Applications. Front. Microbiol. 2021, 12, 660134. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Yao, W.; Wu, Z. Research progress in relationships between freshwater bivalves and algae. Ecotoxicol. Environ. Saf. 2022, 239, 113665. [Google Scholar] [CrossRef] [PubMed]
- Tahiluddin, A.B.; Mindanao State University Tawi-Tawi College of Technology and Oceanography; Maribao, I.P.; Amlani, M.Q.; Sarri, J.H. A Review on Spoilage Microorganisms in Fresh and Processed Aquatic Food Products. Food Bull. 2022, 1, 21–36. [Google Scholar] [CrossRef]
- Nakazawa, N.; Okazaki, E. Recent research on factors influencing the quality of frozen seafood. Fish. Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Residential Bacteria on Surfaces in the Food Industry and Their Implications for Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef]
- Abee, T.; Kovács, Á.T.; Kuipers, O.P.; van der Veen, S. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 2011, 22, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xie, J. Research Progress on Biofilm Formation and Regulatory Mechanisms of Spoilage Bacteria in Aquatic Products. J. Chin. Inst. Food Sci. Technol. 2022, 22, 342–352. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Growth Kinetics and Spoilage Potential of Co-culturing Acinetobacter johnsonii and Pseudomonas fluorescens from Bigeye Tuna (Thunnus obesus) During Refrigerated Storage. Curr. Microbiol. 2020, 77, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Jia, Z.; An, J.; Ding, Y.; Chang, J.; Wang, Y.; Zhou, X. Insights into the fish protein degradation induced by the fish-borne spoiler Pseudomonas psychrophila and Shewanella putrefaciens: From whole genome sequencing to quality changes. Int. J. Food Microbiol. 2024, 416, 110675. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Gomila, M.; Mulet, M.; Zaruma, A.; García-Valdés, E. Past, present and future of the boundaries of the Pseudomonas genus: Proposal of Stutzerimonas gen. Nov. Syst. Appl. Microbiol. 2022, 45, 126289. [Google Scholar] [CrossRef] [PubMed]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonasgenomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, F.; Ren, L.; Tan, X.; Lv, X.; Li, Q.; Li, J.; Li, T. Complete Genome Analysis Reveals the Quorum Sensing-Related Spoilage Potential of Pseudomonas fluorescens PF08, a Specific Spoilage Organism of Turbot (Scophthalmus maximus). Front. Microbiol. 2022, 13, 856802. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.; El-Tahlawy, A.S.; Abdelmoneim, H.M.; Abdallah, K.M.; El Bayomi, R.M. Pseudomonas aeruginosa in Fish and Fish Products: A review on the Incidence, Public Health Significance, Virulence Factors, Antimicrobial Resistance, and Biofilm Formation. J. Adv. Vet. Res. 2023, 13, 1464–1468. [Google Scholar]
- Boziaris, I.S.; Parlapani, F.F. Chapter 3—Specific Spoilage Organisms (SSOs) in Fish. In The Microbiological Quality of Food; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2017; pp. 61–98. [Google Scholar] [CrossRef]
- Zhuang, S.; Tian, L.; Liu, Y.; Wang, L.; Hong, H.; Luo, Y. Amino acid degradation and related quality changes caused by common spoilage bacteria in chill-stored grass carp (Ctenopharyngodon idella). Food Chem. 2023, 399, 133989. [Google Scholar] [CrossRef]
- Huang, W.; Xie, J. Characterization of the Volatiles and Quality of Hybrid Grouper and Their Relationship to Changes of Microbial Community During Storage at 4 °C. Molecules 2020, 25, 818. [Google Scholar] [CrossRef] [PubMed]
- Jeyasekaran, G.; Ganesan, P.; Anandaraj, R.; Shakila, R.J.; Sukumar, D. Quantitative and qualitative studies on the bacteriological quality of Indian white shrimp (Penaeus indicus) stored in dry ice. Food Microbiol. 2006, 23, 526–533. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, R.; Gui, M.; Jiang, Z.; Li, P. Identification of the Specific Spoilage Organism in Farmed Sturgeon (Acipenser baerii) Fillets and Its Associated Quality and Flavour Change During Ice Storage. Foods 2021, 10, 2021. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Cai, W.-Q.; Shi, Y.-G.; Dong, X.-P.; Bai, F.; Shen, S.-K.; Jiao, R.; Zhang, X.-Y.; Zhu, X. Effects of different salt con-centrations and vacuum packaging on the shelf-stability of Russian sturgeon (Acipenser gueldenstaedti) stored at 4 °C. Food Control 2020, 109, 106865. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Ren, L.; Mei, Y.; Ding, T.; Li, Q.; Chen, H.; Li, J. Involvement of Exogenous N-Acyl-Homoserine Lactones in Spoilage Potential of Pseudomonas fluorescens Isolated from Refrigerated Turbot. Front. Microbiol. 2019, 10, 2716. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, G.M.B.; Reiche, T.; Tennfjord, C.E.; Mehli, L. Antibiotic Resistance Properties among Pseudomonas spp. Associated with Salmon Processing Environments. Microorganisms 2022, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Zhou, D.; Lam, Y.L.N.; Li, X.; Chen, G.; Bi, J.; Lou, X.; Chen, L.; Yang, H. Nanoemulsified clove essential oils-based edible coating controls Pseudomonas spp.-causing spoilage of tilapia (Oreochromis niloticus) fillets: Working mechanism and bacteria metabolic responses. Food Res. Int. 2022, 159, 111594. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Liu, Y.; Mei, J.; Xie, J. Effects of different slaughtering methods on the energy metabolism, apoptosis process and quality of grouper (Epinephelus fuscoguttatus) during cold storage at 4 °C. J. Sci. Food Agric. 2025, 105, 661–670. [Google Scholar] [CrossRef]
- Ma, X.; Mei, J.; Xie, J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea). Ultrason. Sonochemistry 2021, 76, 105657. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Suyal, D.C.; Yadav, A.; Shouche, Y.; Goel, R. Psychrophilic Pseudomonas helmanticensis proteome under simulated cold stress. Cell Stress Chaperon. 2020, 25, 1025–1032. [Google Scholar] [CrossRef]
- Cao-Hoang, L.; Dumont, F.; Marechal, P.A.; Le-Thanh, M.; Gervais, P. Rates of chilling to 0 °C: Implications for the survival of microorganisms and relationship with membrane fluidity modifications. Appl. Microbiol. Biotechnol. 2008, 77, 1379–1387. [Google Scholar] [CrossRef]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Tribelli, P.M.; López, N.I. Reporting Key Features in Cold-Adapted Bacteria. Life 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.; Shkryl, Y.; Slepchenko, L.; Cheraneva, D.; Podvolotskaya, A.; Bakunina, I.; Nedashkovskaya, O.; Son, O.; Tekutyeva, L. Genomic Features of a Food-Derived Pseudomonas aeruginosa Strain PAEM and Biofilm-Associated Gene Expression under a Marine Bacterial α-Galactosidase. Int. J. Mol. Sci. 2020, 21, 7666. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.P.; Raghunandanan, S.; Gopinath, V.; Suryaletha, K.; Thomas, S. Deciphering the Cold Adaptive Mechanisms in Pseudomonas psychrophila MTCC12324 Isolated from the Arctic at 79° N. Curr. Microbiol. 2020, 77, 2345–2355. [Google Scholar] [CrossRef]
- Renne, M.F.; Ernst, R. Membrane homeostasis beyond fluidity: Control of membrane compressibility. Trends Biochem. Sci. 2023, 48, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Schulze, P.S.C.; Kiron, V.; Wijffels, R.H. Temperature-Dependent Lipid Accumulation in the Polar Marine Microalga Chlamydomonas malina RCC2488. Front. Plant Sci. 2020, 11, 619064. [Google Scholar] [CrossRef]
- Hingston, P.A.; Hansen, L.T.; Pombert, J.-F.; Wang, S. Characterization of Listeria monocytogenes enhanced cold-tolerance variants isolated during prolonged cold storage. Int. J. Food Microbiol. 2019, 306, 108262. [Google Scholar] [CrossRef] [PubMed]
- Saita, E.; Albanesi, D.; de Mendoza, D. Sensing membrane thickness: Lessons learned from cold stress. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 837–846. [Google Scholar] [CrossRef]
- Flegler, A.; Iswara, J.; Mänz, A.T.; Schocke, F.S.; Faßbender, W.A.; Hölzl, G.; Lipski, A. Exogenous fatty acids affect membrane properties and cold adaptation of Listeria monocytogenes. Sci. Rep. 2022, 12, 1499. [Google Scholar] [CrossRef]
- Králová, S. Role of fatty acids in cold adaptation of Antarctic psychrophilic Flavobacterium spp. Syst. Appl. Microbiol. 2017, 40, 329–333. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial lipids: Metabolism and membrane homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef]
- Mansilla, M.C.; de Mendoza, D. The Bacillus subtilis desaturase: A model to understand phospholipid modification and temperature sensing. Arch. Microbiol. 2005, 183, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Andreeva, I.S.; Zhirakovskiy, V.Y.; Pechurkina, N.I.; Puchkova, L.I.; Saranina, I.V.; Emelyanova, E.K.; Kamynina, T.P. Antibiotic resistance and cold-adaptive enzymes of antarctic culturable bacteria from King George Island. Polar Sci. 2021, 31, 100756. [Google Scholar] [CrossRef]
- Hassan, N.; Anesio, A.M.; Rafiq, M.; Holtvoeth, J.; Bull, I.; Haleem, A.; Shah, A.A.; Hasan, F. Temperature Driven Membrane Lipid Adaptation in Glacial Psychrophilic Bacteria. Front. Microbiol. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Li, M.; Zhao, X.; Shi, J.; Liu, Y.; Zhang, N.; Zhou, Y.; Ma, J.; Chen, G.; Zhang, S.; et al. Mining of key genes for cold adaptation from Pseudomonas fragi D12 and analysis of its cold-adaptation mechanism. Front. Microbiol. 2023, 14, 1215837. [Google Scholar] [CrossRef]
- Fan, S.; Ren, H.; Fu, X.; Kong, X.; Wu, H.; Lu, Z. Genome streamlining of Pseudomonas putida B6-2 for bioremediation. mSystems 2024, 9, e0084524. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Jordá, T.; Rozès, N.; Martínez-Pastor, M.T.; Puig, S. Regulation of yeast fatty acid desaturase in response to iron deficiency. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2018, 1863, 657–668. [Google Scholar] [CrossRef]
- Choi, T.-R.; Park, Y.-L.; Song, H.-S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Kim, H.-J.; Bhatia, S.K.; Gurav, R.; Lee, Y.K.; et al. Effects of a Δ-9-fatty acid desaturase and a cyclopropane-fatty acid synthase from the novel psychrophile Pseudomonas sp. B14-6 on bacterial membrane properties. J. Ind. Microbiol. Biotechnol. 2020, 47, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, Z.; Ji, X.; Luo, Y.; Wei, Y.; Zhang, Q. Novel Histidine Kinase Gene HisK2301 from Rhodosporidium kratochvilovae Contributes to Cold Adaption by Promoting Biosynthesis of Polyunsaturated Fatty Acids and Glycerol. J. Agric. Food Chem. 2019, 67, 653–660. [Google Scholar] [CrossRef] [PubMed]
- de Mendoza, D.; Pilon, M. Control of membrane lipid homeostasis by lipid-bilayer associated sensors: A mechanism conserved from bacteria to humans. Prog. Lipid Res. 2019, 76, 100996. [Google Scholar] [CrossRef]
- Garba, L.; Yussoff, M.A.M.; Halim, K.B.A.; Ishak, S.N.H.; Ali, M.S.M.; Oslan, S.N.; Rahman, R.N.Z.R.A. Homology modeling and docking studies of a Δ9-fatty acid desaturase from a Cold-tolerant Pseudomonas sp. AMS8. PeerJ 2018, 6, e4347. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Schmidt, F.; Klockgether, J.; Davenport, C.F.; Salazar, M.G.; Völker, U.; Tümmler, B. Functional genomics of the initial phase of cold adaptation of Pseudomonas putida KT2440. FEMS Microbiol. Lett. 2011, 318, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Furhan, J. Adaptation, production, and biotechnological potential of cold-adapted proteases from psychrophiles and psychrotrophs: Recent overview. J. Genet. Eng. Biotechnol. 2020, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, K.; Chen, H.; Wang, Z.; Zhao, W.; Zhu, L. Cold-adapted enzymes: Mechanisms, engineering and biotechnological application. Bioprocess Biosyst. Eng. 2023, 46, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Rapuano, R.; Graziano, G. Some Clues about Enzymes from Psychrophilic Microorganisms. Microorganisms 2022, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Öten, A.M.; Atak, E.; Karaca, B.T.; Fırtına, S.; Kutlu, A. Discussing the roles of proline and glycine from the perspective of cold adaptation in lipases and cellulases. Biocatal. Biotransform. 2023, 41, 243–260. [Google Scholar] [CrossRef]
- Zafra, F.; Piniella, D. Proximity labeling methods for proteomic analysis of membrane proteins. J. Proteom. 2022, 264, 104620. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.; Wu, Q.; Tang, X.; Huang, Z. Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst. Int. J. Mol. Sci. 2023, 24, 8532. [Google Scholar] [CrossRef]
- Kim, J.; Ha, S.; Park, W. Expression and deletion analyses of cspE encoding cold-shock protein E in Acinetobacter oleivorans DR1. Res. Microbiol. 2018, 169, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Mangiagalli, M.; Brocca, S.; Orlando, M.; Lotti, M. The “cold revolution”. Present and future applications of cold-active enzymes and ice-binding proteins. New Biotechnol. 2020, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chai, H.; Zhong, Y.; Shen, Y.; Yang, W.; Chen, J.; Xin, Z.; Shi, H. The DEAD-box RNA helicase SHI2 functions in repression of salt-inducible genes and regulation of cold-inducible gene splicing. J. Exp. Bot. 2020, 71, 1598–1613. [Google Scholar] [CrossRef]
- Amir, M.; Kumar, V.; Dohare, R.; Rehman, T.; Hussain, A.; Alajmi, M.F.; El-Seedi, H.R.; Hassan, H.M.A.; Islam, A.; Ahmad, F.; et al. Investigating architecture and structure-function relationships in cold shock DNA-binding domain family using structural genomics-based approach. Int. J. Biol. Macromol. 2019, 133, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Lindae, A.; Eberle, R.J.; Caruso, I.P.; Coronado, M.A.; de Moraes, F.R.; Azevedo, V.; Arni, R.K. Expression, purification and characterization of cold shock protein A of Corynebacterium pseudotuberculosis. Protein Expr. Purif. 2015, 112, 15–20. [Google Scholar] [CrossRef]
- Trevors, J.T.; Bej, A.K.; Mojib, N.; van Elsas, J.D.; Van Overbeek, L. Bacterial gene expression at low temperatures. Extremophiles 2012, 16, 167–176. [Google Scholar] [CrossRef]
- Khan, M.; Bajpai, V.K.; Anasari, S.A.; Kumar, A.; Goel, R. Characterization and Localization of Fluorescent Pseudomonas Cold Shock Protein(s) by Monospecific Polyclonal Antibodies. Microbiol. Immunol. 2003, 47, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pakhomova, S.; Newcomer, M.E.; Christner, B.C.; Luo, B.-H. Structural basis of antifreeze activity of a bacterial multi-domain antifreeze protein. PLoS ONE 2017, 12, e0187169. [Google Scholar] [CrossRef] [PubMed]
- Venketesh, S.; Dayananda, C. Properties, Potentials, and Prospects of Antifreeze Proteins. Crit. Rev. Biotechnol. 2008, 28, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Firdaus-Raih, M.; Hashim, N.H.F.; Bharudin, I.; Abu Bakar, M.F.; Huang, K.K.; Alias, H.; Lee, B.K.B.; Isa, M.N.M.; Mat-Sharani, S.; Sulaiman, S.; et al. The Glaciozyma antarctica genome reveals an array of systems that provide sustained responses towards temperature variations in a persistently cold habitat. PLoS ONE 2018, 13, e0189947. [Google Scholar] [CrossRef]
- Cid, F.P.; Rilling, J.I.; Graether, S.P.; Bravo, L.A.; de La Luz Mora, M.; Jorquera, M.A. Properties and biotechnological applications of ice-binding proteins in bacteria. FEMS Microbiol. Lett. 2016, 363, fnw099. [Google Scholar] [CrossRef]
- Singh, P.; Hanada, Y.; Singh, S.M.; Tsuda, S. Antifreeze protein activity in Arctic cryoconite bacteria. FEMS Microbiol. Lett. 2014, 351, 14–22. [Google Scholar] [CrossRef]
- Vanderveer, T.L.; Choi, J.; Miao, D.; Walker, V.K. Expression and localization of an ice nucleating protein from a soil bacterium, Pseudomonas borealis. Cryobiology 2014, 69, 110–118. [Google Scholar] [CrossRef]
- Lorv, J.S.H.; Rose, D.R.; Glick, B.R. Bacterial Ice Crystal Controlling Proteins. Scientifica 2014, 2014, 976895. [Google Scholar] [CrossRef] [PubMed]
- Hudait, A.; Odendahl, N.; Qiu, Y.; Paesani, F.; Molinero, V. Ice-Nucleating and Antifreeze Proteins Recognize Ice through a Diversity of Anchored Clathrate and Ice-like Motifs. J. Am. Chem. Soc. 2018, 140, 4905–4912. [Google Scholar] [CrossRef] [PubMed]
- Sterniša, M.; Klančnik, A.; Možina, S.S. Spoilage Pseudomonas biofilm with Escherichia coli protection in fish meat at 5 °C. J. Sci. Food Agric. 2019, 99, 4635–4641. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, J.; Wu, Y.; Han, J.; Liu, D.; Lv, R. Transcriptomic analysis of biofilm formation by Bacillus cereus under different carbon source conditions. Food Qual. Saf. 2024, 8, fyad038. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, J.; Wang, J.; Li, P.; Lin, J. Treatment of Pseudomonas aeruginosa infectious biofilms: Challenges and strategies. Front. Microbiol. 2022, 13, 955286. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xie, J. Comparative Proteome Analysis of Shewanella putrefaciens WS13 Mature Biofilm Under Cold Stress. Front. Microbiol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Tomioka, Y.; Kouzuma, A.; Watanabe, K. Overexpression of the adenylate cyclase gene cyaC facilitates current generation by Shewanella oneidensis in bioelectrochemical systems. Bioelectrochemistry 2019, 129, 100–105. [Google Scholar] [CrossRef]
- Pang, X.; Yuk, H.-G. Effects of the colonization sequence of Listeria monocytogenes and Pseudomonas fluorescens on survival of biofilm cells under food-related stresses and transfer to salmon. Food Microbiol. 2019, 82, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zou, Y.; Zheng, J.; Qiu, S.; Liu, L.; Wei, C. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 273, 127414. [Google Scholar] [CrossRef]
- Papenfort, K.; E Silpe, J.; Schramma, K.R.; Cong, J.-P.; Seyedsayamdost, M.R.; Bassler, B.L. Erratum: A Vibrio cholerae autoinducer–receptor pair that controls biofilm formation. Nat. Chem. Biol. 2017, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhu, J.; Ye, X.; Ge, Y.; Li, J. Inhibition of biofilm development and spoilage potential of Shewanella baltica by quorum sensing signal in cell-free supernatant from Pseudomonas fluorescens. Int. J. Food Microbiol. 2016, 230, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, L.; Wang, X.; Li, J.; Zhu, J.; Sun, A. Role of RpoS in stress resistance, quorum sensing and spoilage potential of Pseudomonas fluorescens. Int. J. Food Microbiol. 2018, 270, 31–38. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, Y.; Wang, Y.; Qu, D. Competitive interaction on dual-species biofilm formation by spoilage bacteria, Shewanella baltica and Pseudomonas fluorescens. J. Appl. Microbiol. 2019, 126, 1175–1186. [Google Scholar] [CrossRef]
- Cheng, X.; de Bruijn, I.; van der Voort, M.; Loper, J.E.; Raaijmakers, J.M. The Gac regulon of Pseudomonas fluorescens SBW25. Environ. Microbiol. Rep. 2013, 5, 608–619. [Google Scholar] [CrossRef]
- Barahona, E.; Navazo, A.; Yousef-Coronado, F.; de Cárcer, D.A.; Martínez-Granero, F.; Espinosa-Urgel, M.; Martín, M.; Rivilla, R. Efficient rhizosphere colonization by Pseudomonas fluorescens f113 mutants unable to form biofilms on abiotic surfaces. Environ. Microbiol. 2010, 12, 3185–3195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent advances in fishy odour in aquatic fish products, from formation to control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Anagnostopoulos, D.A.; Karamani, E.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Growth and Volatile Organic Compound Production of Pseudomonas Fish Spoiler Strains on Fish Juice Agar Model Substrate at Different Temperatures. Microorganisms 2023, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, Y.; Liu, Z.; Zhou, H. Formation, Analytical Methods, Change Tendency, and Control Strategies of Biogenic Amines in Canned Aquatic Products: A Systematic Review. J. Food Prot. 2021, 84, 2020–2036. [Google Scholar] [CrossRef]
- Rahmani, J.; Miri, A.; Mohseni-Bandpei, A.; Fakhri, Y.; Bjørklund, G.; Keramati, H.; Moradi, B.; Amanidaz, N.; Shariatifar, N.; Khaneghah, A.M. Contamination and Prevalence of Histamine in Canned Tuna from Iran: A Systematic Review, Meta-Analysis, and Health Risk Assessment. J. Food Prot. 2018, 81, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Tırıs, G.; Yanıkoğlu, R.S.; Ceylan, B.; Egeli, D.; Tekkeli, E.K.; Önal, A. A review of the currently developed analytical methods for the determination of biogenic amines in food products. Food Chem. 2023, 398, 133919. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qiu, K.; Zhu, Y.; Zhang, X.; Yang, T.; Yi, G.; Xu, M.; Rao, Z. Production of Putrescine in Metabolic Engineering Corynebacterium crenatum by Mixed Sugar Fermentation. ACS Sustain. Chem. Eng. 2022, 10, 14407–14416. [Google Scholar] [CrossRef]
- Dong, H.; Gai, Y.; Fu, S.; Zhang, D. Application of Biotechnology in Specific Spoilage Organisms of Aquatic Products. Front. Bioeng. Biotechnol. 2022, 10, 895283. [Google Scholar] [CrossRef] [PubMed]
- Vasconi, M.; Bellagamba, F.; Bernardi, C.; Martino, P.A.; Moretti, V.M. Histamine Formation in a Dry Salted Twaite Shad (Alosa fallax lacustris) Product. J. Food Prot. 2017, 80, 127–135. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, C.; Huang, D.; Yang, G.; Tang, Y.; Shi, Y.; Kong, C.; Cao, P.; Cai, Y. Determination of 8 biogenic amines in aquatic products and their derived products by high-performance liquid chromatography-tandem mass spectrometry without derivatization. Food Chem. 2021, 361, 130044. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.-H.; Ho, C.-T. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2022, 405, 134911. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Z.; Yang, S.-P.; Cheng, Y.; Qian, Y.-F. Study on the spoilage potential of Pseudomonas fluorescens on salmon stored at different temperatures. J. Food Sci. Technol. 2018, 55, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, J.; Xie, J. Foodomics in aquatic products quality assessment during storage: An advanced and reliable approach. Food Biosci. 2024, 58, 103734. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, J.; Xu, S.; Wei, J.; Liu, H.; Xie, S.; Pan, Y.; Zhao, Y.; Zhu, Y. Ultra-efficient trimethylamine gas sensor based on Au nanoparticles sensitized WO3 nanosheets for rapid assessment of seafood freshness. Food Chem. 2022, 392, 133318. [Google Scholar] [CrossRef]
- Chen, B.; Mei, J.; Xie, J. Effects of packaging methods and temperature variations on the quality and microbial diversity of grouper (Epinephelus Lanceolatus) during cold storage. Food Biosci. 2024, 60, 104315. [Google Scholar] [CrossRef]
- Lu, W.-H.; Chiu, H.-H.; Kuo, H.-C.; Chen, G.-Y.; Chepyala, D.; Kuo, C.-H. Using matrix-induced ion suppression combined with LC-MS/MS for quantification of trimethylamine-N-oxide, choline, carnitine and acetylcarnitine in dried blood spot samples. Anal. Chim. Acta 2021, 1149, 338214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, Y.; Yang, S.; Wang, S.; Wu, J.; Jiao, T.; Wei, J.; Li, D.; Chen, X.; Chen, Q.; et al. Non-destructive prediction of total volatile basic nitrogen (TVB-N) content of Litopenaeus vannamei using A bi-channel data acquisition of Colorimetric sensing array. J. Food Compos. Anal. 2024, 128, 106026. [Google Scholar] [CrossRef]
- Li, P.; Chen, Z.; Tan, M.; Mei, J.; Xie, J. Evaluation of weakly acidic electrolyzed water and modified atmosphere packaging on the shelf life and quality of farmed puffer fish (Takifugu obscurus) during cold storage. J. Food Saf. 2020, 40, e12773. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography–ion mobility spectrometry (GC–IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Xie, J. Progress on odor deterioration of aquatic products: Characteristic volatile compounds, analysis methods, and formation mechanisms. Food Biosci. 2023, 53, 102666. [Google Scholar] [CrossRef]
- Zheng, R.; Xu, X.; Xing, J.; Cheng, H.; Zhang, S.; Shen, J.; Li, H. Quality Evaluation and Characterization of Specific Spoilage Organisms of Spanish Mackerel by High-Throughput Sequencing during 0 °C Cold Chain Logistics. Foods 2020, 9, 312. [Google Scholar] [CrossRef]
- Li, D.; Qin, N.; Zhang, L.; Li, Q.; Prinyawiwatkul, W.; Luo, Y. Degradation of adenosine triphosphate, water loss and textural changes in frozen common carp (Cyprinus carpio) fillets during storage at different temperatures. Int. J. Refrig. 2019, 98, 294–301. [Google Scholar] [CrossRef]
- Zhao, D.; Luo, T.; Liu, Y.; Shu, R.; Hong, H.; Luo, Y.; Tan, Y. Effects of different culture modes on the storage quality of tilapia (Oreochromis mossambicus) fillets at 4 °C. JSFA Rep. 2024, 4, 11–18. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Ma, H.; Qi, H.; Jia, S.; Li, W.; Li, J.; Zhuang, S.; Luo, Y. Microbiota Composition and Quality Changes of Tiger Puffer (Takifugu rubripes) Fillets during 4 °C Refrigerated and Ice Storage. J. Aquat. Food Prod. Technol. 2021, 30, 1109–1123. [Google Scholar] [CrossRef]
- Cao, X.; Li, P.; Feng, X.; Liu, D.; Wang, X.; Wang, L. Detection of 13 foodborne pathogens in aquatic products using visual chromogenic chips based on asymmetric multiplex polymerase chain reaction and nucleic acid hybridization. Food Control 2024, 155, 110100. [Google Scholar] [CrossRef]
- Zhan, Z.; He, S.; Cui, Y.; Yang, J.; Shi, X. Development of a multiplex recombinase polymerase amplification coupled with lateral flow dipsticks for the simultaneous rapid detection of Salmonella spp., Salmonella Typhimurium and Salmonella Enteritidis. Food Qual. Saf. 2024, 8, fyad059. [Google Scholar] [CrossRef]
- Tran, D.H.; Tran, H.T.; Pham, T.N.M.; Phung, H.T.T. Direct multiplex recombinase polymerase amplification for rapid detection of Staphylococcus aureus and Pseudomonas aeruginosa in food. Mol. Biol. Res. Commun. 2022, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; El-Matbouli, M. Rapid detection and differentiation of carp oedema virus and cyprinid herpes virus-3 in koi and common carp. J. Fish Dis. 2018, 41, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cao, Y.; Yu, Y.; Yan, S.; Wang, Y.; Pan, Y.; Zhang, W. Real-Time Recombinase Polymerase Amplification Assay for the Detection of Vibrio cholerae in Seafood. Food Anal. Methods 2017, 10, 2657–2666. [Google Scholar] [CrossRef]
- Zhang, C.; Tao, Z.; Ye, H.; Wang, P.; Jiang, M.; Benard, K.; Li, W.; Yan, X. Development and validation of a CRISPR/Cas12a-based platform for rapid and sensitive detection of the large yellow croaker iridovirus. Aquaculture 2024, 584, 740658. [Google Scholar] [CrossRef]
- Nair, A.V.; A Pradeep, M.; Vijayan, K.K. Molecular approach for the rapid detection of Bacillus and Pseudomonas genera—Dominant antagonistic groups—From diverse ecological niches using colony multiplex PCR. J. Ind. Microbiol. Biotechnol. 2014, 41, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Rees-George, J.; Vanneste, J.L.; Cornish, D.A.; Pushparajah, I.P.S.; Yu, J.; Templeton, M.D.; Everett, K.R. Detection of Pseudomonas syringae pv. actinidiae using polymerase chain reaction (PCR) primers based on the 16S–23S rDNA intertranscribed spacer region and comparison with PCR primers based on other gene regions. Plant Pathol. 2010, 59, 453–464. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.; Chen, B.; Wang, X.; Liang, Z.; Wang, L. The Detection of Foodborne Pathogenic Bacteria in Seafood Using a Multiplex Polymerase Chain Reaction System. Foods 2022, 11, 3909. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, C.; Lin, H.; Zhang, T.; Liu, H.; Huang, X. Portable rotary PCR system for real-time detection of Pseudomonas aeruginosa in milk. Lab Chip 2023, 23, 4592–4599. [Google Scholar] [CrossRef] [PubMed]
- Mayboroda, O.; Katakis, I.; O’Sullivan, C.K. Multiplexed isothermal nucleic acid amplification. Anal. Biochem. 2018, 545, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Ahmad, F.J.; Kar, S. Recent advances in loop-mediated isothermal amplification (LAMP) for rapid and efficient detection of pathogens. Curr. Res. Microb. Sci. 2022, 3, 100120. [Google Scholar] [CrossRef] [PubMed]
- Bernal, D.F.; Mosqueda, J.; Pérez-Sánchez, G.; Chávez, J.A.C.; Martínez, M.N.; Rodríguez, A.; Carvajal-Gamez, B. Loop-Mediated Isothermal Amplification Coupled with Reverse Line Blot Hybridization for the Detection of Pseudomonas aeruginosa. Microorganisms 2024, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-J.; Wang, L.; Chen, J.; Wang, R.-N.; Shi, Y.-H.; Li, C.-H.; Zhang, D.-M.; Yan, X.-J.; Zhang, Y.-J. Development and evaluation of a real-time fluorogenic loop-mediated isothermal amplification assay integrated on a microfluidic disc chip (on-chip LAMP) for rapid and simultaneous detection of ten pathogenic bacteria in aquatic animals. J. Microbiol. Methods 2014, 104, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Han, S.; Lv, L.; Li, L. Next-Generation Sequencing Applications for the Study of Fungal Pathogens. Microorganisms 2022, 10, 1882. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, L.; Gao, Y.G.; Xia, Y. Detection, Diagnosis, and Preventive Management of the Bacterial Plant Pathogen Pseudomonas syringae. Plants 2023, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-Y.; Zeng, Y.-L.; Yang, X.-Y.; Li, W.-B.; Lan, X.-P. Utility of aptamer-fluorescence in situ hybridization for rapid detection of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 30, 273–278. [Google Scholar] [CrossRef]
- Del’Duca, A.; Cesar, D.E.; Diniz, C.G.; Abreu, P.C. Evaluation of the presence and efficiency of potential probiotic bacteria in the gut of tilapia (Oreochromis niloticus) using the fluorescent in situ hybridization technique. Aquaculture 2013, 388–391, 115–121. [Google Scholar] [CrossRef]
- Zhu, X.-X.; Wang, Y.-S.; Li, S.-J.; Peng, R.-Q.; Wen, X.; Peng, H.; Shi, Q.-S.; Zhou, G.; Xie, X.-B.; Wang, J. Rapid detection of mexX in Pseudomonas aeruginosa based on CRISPR-Cas13a coupled with recombinase polymerase amplification. Front. Microbiol. 2024, 15, 1341179. [Google Scholar] [CrossRef] [PubMed]
- Mousivand, Z.; Haddadi, F.; Kamaladini, H. Colorimetric bacteria sensing of Pseudomonas aeruginosa using gold nanoparticle probes. J. Genet. Eng. Biotechnol. 2023, 21, 72. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Boziaris, I.S. Non-Thermal Methods for Ensuring the Microbiological Quality and Safety of Seafood. Appl. Sci. 2021, 11, 833. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Luan, B.; Zhang, X.; Fang, Z.; Xian, Y.; Lu, X.; Ostrikov, K.; Bazaka, K. Microplasma Bubbles: Reactive Vehicles for Biofilm Dispersal. ACS Appl. Mater. Interfaces 2019, 11, 20660–20669. [Google Scholar] [CrossRef] [PubMed]
- Angarano, V.; Smet, C.; Akkermans, S.; Watt, C.; Chieffi, A.; Van Impe, J.F.M. Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms. Antibiotics 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, Y.; Mei, J.; Xie, J. Effect of Ocimum basiicum L. essential oil microemulsion on the quality of snakehead (Channa argus) under different dipping conditions during cold storage. JSFA Rep. 2025, 5, 14–26. [Google Scholar] [CrossRef]
- Wang, J.; Chi, Z.; Zhao, K.; Wang, H.; Zhang, X.; Xu, F.; Shao, X.; Wei, Y. A transcriptome analysis of the antibacterial mechanism of flavonoids from Sedum aizoon L. against Shewanella putrefaciens. World J. Microbiol. Biotechnol. 2020, 36, 94. [Google Scholar] [CrossRef] [PubMed]
- Santhakumari, S.; Jayakumar, R.; Logalakshmi, R.; Prabhu, N.M.; Nazar, A.K.A.; Pandian, S.K.; Ravi, A.V. In vitro and in vivo effect of 2,6-Di-tert-butyl-4-methylphenol as an antibiofilm agent against quorum sensing mediated biofilm formation of Vibrio spp. Int. J. Food Microbiol. 2018, 281, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Güven, N.; Onurdağ, F.K. Investigation of antimicrobial and antibiofilm effects of some preservatives used in drugs, cosmetics and food products. Mikrobiyoloji Bul. 2014, 48, 94–105. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Azlin-Hasim, S.; Cruz-Romero, M.; Cummins, E.; Kerry, J.P.; Morris, M.A. Antimicrobial effect of benzoic and sorbic acid salts and nano-solubilisates against Staphylococcus aureus, Pseudomonas fluorescens and chicken microbiota biofilms. Food Control 2020, 107, 106786. [Google Scholar] [CrossRef]
- Yin, Y.; Ni, P.; Liu, D.; Yang, S.; Almeida, A.; Guo, Q.; Zhang, Z.; Deng, L.; Wang, D. Bacteriophage potential against Vibrio parahaemolyticus biofilms. Food Control 2019, 98, 156–163. [Google Scholar] [CrossRef]
- Čanak, I.; Markov, K.; Melvan, E.; Starčević, A.; Živković, M.; Zadravec, M.; Pleadin, J.; Jakopović, Ž.; Kostelac, D.; Frece, J. Isolation and Characterisation of L. plantarum O1 Producer of Plantaricin as Potential Starter Culture for the Biopreservation of Aquatic Food Products. Food Technol. Biotechnol. 2018, 56, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Puga, C.; Rodríguez-López, P.; Cabo, M.; SanJose, C.; Orgaz, B. Enzymatic dispersal of dual-species biofilms carrying Listeria monocytogenes and other associated food industry bacteria. Food Control 2018, 94, 222–228. [Google Scholar] [CrossRef]
- Wang, D.; Chen, H.; Li, J.; Li, T.; Ren, L.; Liu, J.; Shen, Y. Screening and validation of quorum quenching enzyme PF2571 from Pseudomonas fluorescens strain PF08 to inhibit the spoilage of red sea bream filets. Int. J. Food Microbiol. 2022, 362, 109476. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Lin, Q.; Tan, Y. Quality improvement in Scophthalmus maximus fillets during cold storage by coating with polylactic acid/hesperidin electrospun fiber. LWT-Food Sci. Technol. 2022, 170, 114080. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, J.; Koseki, S.; Yang, Q.; Yu, H.; Fu, L. Screening and preservation application of quorum sensing inhibitors of Pseudomonas fluorescens and Shewanella baltica in seafood products. LWT 2021, 149, 111749. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef]
- Li, T.; Sun, X.; Chen, H.; He, B.; Mei, Y.; Wang, D.; Li, J. Methyl anthranilate: A novel quorum sensing inhibitor and anti-biofilm agent against Aeromonas sobria. Food Microbiol. 2020, 86, 103356. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, D.; Liu, N.; Ma, Y.; Ding, T.; Mei, Y.; Li, J. Inhibition of quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas fluorescens by cinnamaldehyde. Int. J. Food Microbiol. 2018, 269, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Y.; Deng, J.; Jiang, H.; Zhuang, L.; Ye, W.; Ma, J.; Jiang, J.; Feng, L. Diketopiperazines Synthesis Gene in Shewanella baltica and Roles of Diketopiperazines and Resveratrol in Quorum Sensing. J. Agric. Food Chem. 2019, 67, 12013–12025. [Google Scholar] [CrossRef] [PubMed]
- Couvert, O.; Koullen, L.; Lochardet, A.; Huchet, V.; Thevenot, J.; Le Marc, Y. Effects of carbon dioxide and oxygen on the growth rate of various food spoilage bacteria. Food Microbiol. 2023, 114, 104289. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.P.; Wagai, D.T.; Hon, G.R.; Suraj, P.; Chaudhari, S.R.; Matche, R.S. Enhancement of the Shelf Life of Malabar Parathas through the Evaluation of Oxygen Scavenging. ACS Food Sci. Technol. 2024, 4, 3047–3058. [Google Scholar] [CrossRef]
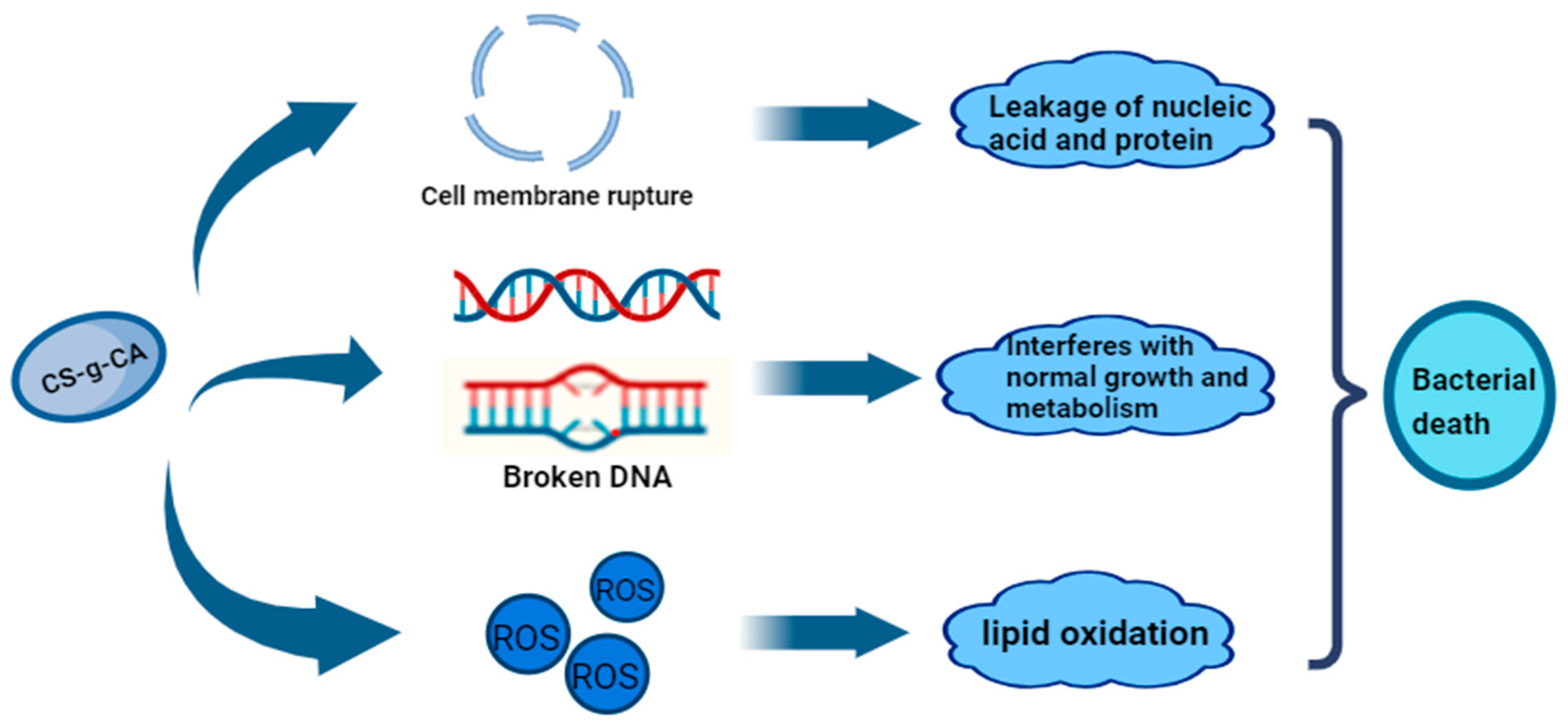
- Lan, W.; Du, J.; Sun, Y.; Xie, J. Insight into the antibacterial activity and mechanism of chitosan caffeic acid graft against Pseudomonas fluorescens. Int. J. Food Sci. Technol. 2023, 58, 1317–1325. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Shi, Y.-G.; Gu, Q.; Fang, M.; Chen, Y.-W.; Fang, S.; Dang, Y.-L.; Chen, J.-S. Antimicrobial effect and mechanism of non-antibiotic alkyl gallates against Pseudomonas fluorescens on the surface of Russian sturgeon (Acipenser gueldenstaedti). Int. J. Food Microbiol. 2021, 342, 109093. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, D.; Guo, S.; Zheng, X.; Yang, W.; Chen, H.; Zhang, Y.; Yu, Q. Dual-action defense: A photothermal and controlled nitric oxide-releasing coating for preventing biofilm formation. J. Colloid Interface Sci. 2025, 679, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Ni, C.; He, G.; Li, X.; Jiang, X.; Yu, L. Degradable polylactic acid-reinforced acrylate polymer coating for marine antifouling. Prog. Org. Coat. 2024, 197, 108824. [Google Scholar] [CrossRef]
- Yang, X.; Fang, S.; Xie, Y.; Mei, J.; Xie, J. Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage. Coatings 2024, 14, 338. [Google Scholar] [CrossRef]
- Chen, J.; Luo, L.; Cen, C.; Liu, Y.; Li, H.; Wang, Y. The nano antibacterial composite film carboxymethyl chitosan/gelatin/nano ZnO improves the mechanical strength of food packaging. Int. J. Biol. Macromol. 2022, 220, 462–471. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, Q.; Yu, D.; Dong, J.; Xu, Y.; Xia, W. Physical, antioxidant, and preservation properties of chitosan film doped with proanthocyanidins-loaded nanoparticles. Food Hydrocoll. 2022, 130, 107686. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Characterization of active films based on chitosan/polyvinyl alcohol integrated with ginger essential oil-loaded bacterial cellulose and application in sea bass (Lateolabrax japonicas) packaging. Food Chem. 2024, 441, 138343. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]


| Species | Compounds | Properties | Reference |
|---|---|---|---|
| Fish | Proteins, omega-3 polyunsaturated fatty acids, vitamin D and B vitamins, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) | Antioxidant, helps cardiovascular health, improves brain function, reduces inflammation, helps with bone health | [7] |
| Shrimp | Proteins, minerals, particularly selenium, astaxanthin | Antioxidant, antihypertensive, antibacterial | [8] |
| Crab | Bioactive peptides, minerals, chitin | Antiviral, enhances immunity, promotes blood circulation | [9] |
| Bivalves | Oyster peptide, alginate, vitamin B12, squalene, polysaccharide, sulfides, organic acids | Antioxidant, anti-fatigue, enhances immunity, maintains nervous system and red blood cell health | [10] |
| Fish Species | Species (And Strains) | Spoilage Characteristics | References |
|---|---|---|---|
| Sturgeon (Acipenser baerii) | P. fluorescens | Fat oxidation | [26,27] |
| Grass carp (Ctenopharyngodon idella) | P. malodorata | Degraded amino acid | [23] |
| Turbot (Scophthalmus maximus) | P. fluorescens PF08 | Protease production, biofilm formation, and sulfur and amine metabolism | [21,28] |
| Salmon (Salmo salar) | P. aeruginosa | Lipid oxidation, biofilm formation | [29] |
| Tilapia (Oreochromis niloticus) | P. fragi BBa3 | Protein hydrolysis and oxidation | [30] |
| Grouper (Epinephelus fuscoguttatus) | P. fluorescens, P. aeruginosa | Formation of undesirable odors and flavors | [31] |
| Large yellow croaker (Larimichthys crocea) | P. plecoglossicida, P. fluorescens | Discoloration and ulceration of fish | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xie, J.; Mei, J. Research Progress Regarding Psychrotrophic Pseudomonas in Aquatic Products: Psychrophilic Characteristics, Spoilage Mechanisms, Detection Methods, and Control Strategies. Foods 2025, 14, 363. https://doi.org/10.3390/foods14030363
Wang J, Xie J, Mei J. Research Progress Regarding Psychrotrophic Pseudomonas in Aquatic Products: Psychrophilic Characteristics, Spoilage Mechanisms, Detection Methods, and Control Strategies. Foods. 2025; 14(3):363. https://doi.org/10.3390/foods14030363
Chicago/Turabian StyleWang, Jingjing, Jing Xie, and Jun Mei. 2025. "Research Progress Regarding Psychrotrophic Pseudomonas in Aquatic Products: Psychrophilic Characteristics, Spoilage Mechanisms, Detection Methods, and Control Strategies" Foods 14, no. 3: 363. https://doi.org/10.3390/foods14030363
APA StyleWang, J., Xie, J., & Mei, J. (2025). Research Progress Regarding Psychrotrophic Pseudomonas in Aquatic Products: Psychrophilic Characteristics, Spoilage Mechanisms, Detection Methods, and Control Strategies. Foods, 14(3), 363. https://doi.org/10.3390/foods14030363







