Advances in Computer Vision and Spectroscopy Techniques for Non-Destructive Quality Assessment of Citrus Fruits: A Comprehensive Review
Abstract
1. Introduction
2. Computer Vision Techniques
2.1. Traditional Computer Vision Techniques
2.2. Hyperspectral Imaging Technology
2.3. Multispectral Imaging Technology
2.4. Summary of Computer Vision and Related Imaging Technologies
3. Spectroscopy Techniques
3.1. Infrared Spectroscopy
3.2. Raman Spectroscopy
3.3. Fluorescence Spectroscopy
3.4. Terahertz Spectroscopy
3.5. Nuclear Magnetic Resonance Spectroscopy
3.6. Summary of Spectral Technologies
4. Computer Vision Analysis and Chemometrics
4.1. Computer Vision Analysis
4.2. Chemometrics
5. Quality Detection Applications for Citrus Fruits
5.1. Citrus Quality Detection and Grading
5.1.1. Citrus External Quality Detection
5.1.2. Citrus Internal Quality Detection
5.1.3. Citrus Physicochemical Quality Detection
5.1.4. Citrus Quality-Based Ripening and Harvesting Detection
5.2. Citrus Damage Detection
5.2.1. Citrus Defect Detection
5.2.2. Citrus Disease Detection
5.3. Citrus Adulteration and Traceability Detection
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef] [PubMed]
- Richa, R.; Kohli, D.; Vishwakarma, D.; Mishra, A.; Kabdal, B.; Kothakota, A.; Richa, S.; Sirohi, R.; Kumar, R.; Naik, B. Citrus fruit: Classification, value addition, nutritional and medicinal values, and relation with pandemic and hidden hunger. J. Agric. Food Res. 2023, 14, 100718. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Dong, W.; Luo, W.; Huang, Y.; Zhan, B.; Liu, X. Detection of common defects on mandarins by using visible and near infrared hyperspectral imaging. Infrared Phys. Technol. 2020, 108, 103341. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Citrus Fruits—Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas: Nutritional and Health Benefits; Hussain, S.Z., Naseer, B., Qadri, T., Fatima, T., Bhat, T.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 229–244. [Google Scholar]
- Shi, Y.-S.; Zhang, Y.; Li, H.-T.; Wu, C.-H.; El-Seedi, H.R.; Ye, W.-K.; Wang, Z.-W.; Li, C.-B.; Zhang, X.-F.; Kai, G.-Y. Limonoids from Citrus: Chemistry, anti-tumor potential, and other bioactivities. J. Funct. Foods 2020, 75, 104213. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef]
- Ana, M.C.; Dário, P.; Rosa, M.P.; Maria, D.A.; Rui, G. Nondestructive Assessment of Citrus Fruit Quality and Ripening by Visible–Near Infrared Reflectance Spectroscopy. In Citrus; Muhammad Sarwar, K., Iqrar Ahmad, K., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. 13. [Google Scholar]
- Chakraborty, S.K.; Subeesh, A.; Dubey, K.; Jat, D.; Chandel, N.S.; Potdar, R.; Rao, N.R.N.V.G.; Kumar, D. Development of an optimally designed real-time automatic citrus fruit grading–sorting machine leveraging computer vision-based adaptive deep learning model. Eng. Appl. Artif. Intell. 2023, 120, 105826. [Google Scholar] [CrossRef]
- Lu, J.; Chen, W.; Lan, Y.; Qiu, X.; Huang, J.; Luo, H. Design of citrus peel defect and fruit morphology detection method based on machine vision. Comput. Electron. Agric. 2024, 219, 108721. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Zapata, P.J.; Cubero, S.; Blasco, J.; Munera, S. Non-destructive assessment of ’Fino’ lemon quality through ripening using NIRS and chemometric analysis. Postharvest Biol. Technol. 2024, 212, 112870. [Google Scholar] [CrossRef]
- Torres, I.; Sánchez, M.-T.; de la Haba, M.-J.; Pérez-Marín, D. LOCAL regression applied to a citrus multispecies library to assess chemical quality parameters using near infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 206–214. [Google Scholar] [CrossRef]
- Peng, K.; Ma, W.; Lu, J.; Tian, Z.; Yang, Z. Application of Machine Vision Technology in Citrus Production. Appl. Sci. 2023, 13, 9334. [Google Scholar] [CrossRef]
- Palei, S.; Behera, S.K.; Sethy, P.K. A Systematic Review of Citrus Disease Perceptions and Fruit Grading Using Machine Vision. Procedia Comput. Sci. 2023, 218, 2504–2519. [Google Scholar] [CrossRef]
- Dhiman, P.; Kaur, A.; Balasaraswathi, V.R.; Gulzar, Y.; Alwan, A.A.; Hamid, Y. Image Acquisition, Preprocessing and Classification of Citrus Fruit Diseases: A Systematic Literature Review. Sustainability 2023, 15, 9643. [Google Scholar] [CrossRef]
- Wang, H.; Gu, J.; Wang, M. A review on the application of computer vision and machine learning in the tea industry. Front. Sustain. Food Syst. 2023, 7, 1172543. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Xu, B.; Sun, J.; Mujumdar, A.S. Artificial intelligence assisted technologies for controlling the drying of fruits and vegetables using physical fields: A review. Trends Food Sci. Technol. 2020, 105, 251–260. [Google Scholar] [CrossRef]
- Anjali; Jena, A.; Bamola, A.; Mishra, S.; Jain, I.; Pathak, N.; Sharma, N.; Joshi, N.; Pandey, R.; Kaparwal, S.; et al. State-of-the-art non-destructive approaches for maturity index determination in fruits and vegetables: Principles, applications, and future directions. Food Prod. Process. Nutr. 2024, 6, 56. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; He, H.-J.; Kamruzzaman, M. Advancements, limitations and challenges in hyperspectral imaging for comprehensive assessment of wheat quality: An up-to-date review. Food Chem. X 2024, 21, 101235. [Google Scholar] [CrossRef]
- Benalia, S.; Calogero, V.; Anello, M.; Zimbalatti, G.; Bernardi, B. Application of computer vision systems for assessing bergamot fruit external features. Adv. Hortic. Sci. 2023, 37, 111–116. [Google Scholar]
- Phate, V.R.; Malmathanraj, R.; Palanisamy, P. Classification and weighing of sweet lime (Citrus limetta) for packaging using computer vision system. J. Food Meas. Charact. 2019, 13, 1451–1468. [Google Scholar] [CrossRef]
- Jadhav, T.; Singh, K.; Abhyankar, A. Volumetric estimation using 3D reconstruction method for grading of fruits. Multimed. Tools Appl. 2019, 78, 1613–1634. [Google Scholar] [CrossRef]
- Barkah, M.F. Klasifikasi Rasa Buah Jeruk Pontianak Berdasarkan Warna Kulit Buah Jeruk Menggunakan Metode K-Nearest Neighbor. Coding J. Komput. Dan Apl. 2020, 8, 55–66. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Wang, Z.; Qiang, H.; Cai, G.; Tan, C.; Zhao, C. Detecting ripe fruits under natural occlusion and illumination conditions. Comput. Electron. Agric. 2021, 190, 106450. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Momeny, M.; Mahmoudi, M.; Zhang, Y.-D. Classification of sour lemons based on apparent defects using stochastic pooling mechanism in deep convolutional neural networks. Sci. Hortic. 2020, 263, 109133. [Google Scholar] [CrossRef]
- Gómez-Flores, W.; Garza-Saldaña, J.J.; Varela-Fuentes, S.E. Detection of Huanglongbing disease based on intensity-invariant texture analysis of images in the visible spectrum. Comput. Electron. Agric. 2019, 162, 825–835. [Google Scholar] [CrossRef]
- Tan, A.; Zhou, G.; He, M. Surface defect identification of Citrus based on KF-2D-Renyi and ABC-SVM. Multimed. Tools Appl. 2021, 80, 9109–9136. [Google Scholar] [CrossRef]
- Thao, L.Q.; Kien, D.T.; Thien, N.D.; Bach, N.C.; Van Hiep, V.; Khanh, D.G. Utilizing AI and silver nanoparticles for the detection and treatment monitoring of canker in pomelo trees. Sens. Actuators A Phys. 2024, 368, 115127. [Google Scholar] [CrossRef]
- Momeny, M.; Jahanbakhshi, A.; Neshat, A.A.; Hadipour-Rokni, R.; Zhang, Y.-D.; Ampatzidis, Y. Detection of citrus black spot disease and ripeness level in orange fruit using learning-to-augment incorporated deep networks. Ecol. Inform. 2022, 71, 101829. [Google Scholar] [CrossRef]
- Chakraborty, S.; Shamrat, F.M.J.M.; Billah, M.M.; Jubair, M.A.; Alauddin, M.; Ranjan, R. Implementation of Deep Learning Methods to Identify Rotten Fruits. In Proceedings of the 2021 5th International Conference on Trends in Electronics and Informatics (ICOEI) 2021, Tirunelveli, India, 3–5 June 2021; pp. 1207–1212. [Google Scholar] [CrossRef]
- Matenda, R.T.; Rip, D.; Marais, J.; Williams, P.J. Exploring the potential of hyperspectral imaging for microbial assessment of meat: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 315, 124261. [Google Scholar] [CrossRef]
- Wieme, J.; Mollazade, K.; Malounas, I.; Zude-Sasse, M.; Zhao, M.; Gowen, A.; Argyropoulos, D.; Fountas, S.; Van Beek, J. Application of hyperspectral imaging systems and artificial intelligence for quality assessment of fruit, vegetables and mushrooms: A review. Biosyst. Eng. 2022, 222, 156–176. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, H.; Feng, Q.; Xu, L.; Lin, Q.; Cai, K. Rapid Detection of Pomelo Fruit Quality Using Near-Infrared Hyperspectral Imaging Combined with Chemometric Methods. Front. Bioeng. Biotechnol. 2021, 8, 616943. [Google Scholar] [CrossRef]
- Antony, M.M.; Suchand Sandeep, C.S.; Vadakke Matham, M. Hyperspectral vision beyond 3D: A review. Opt. Lasers Eng. 2024, 178, 108238. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Liu, S.; Huang, H.; Zhan, B.; Fan, G.; Zhang, H. Prediction of soluble solid content in Nanfeng mandarin by combining hyperspectral imaging and effective wavelength selection. J. Food Compos. Anal. 2024, 126, 105939. [Google Scholar] [CrossRef]
- Patel, D.; Bhise, S.; Kapdi, S.S.; Bhatt, T. Non-destructive hyperspectral imaging technology to assess the quality and safety of food: A review. Food Prod. Process. Nutr. 2024, 6, 69. [Google Scholar] [CrossRef]
- Basile, T.; Mallardi, D.; Cardone, M.F. Spectroscopy, a Tool for the Non-Destructive Sensory Analysis of Plant-Based Foods and Beverages: A Comprehensive Review. Chemosensors 2023, 11, 579. [Google Scholar] [CrossRef]
- Jie, D.; Wu, S.; Wang, P.; Li, Y.; Ye, D.; Wei, X. Research on Citrus grandis Granulation Determination Based on Hyperspectral Imaging through Deep Learning. Food Anal. Methods 2021, 14, 280–289. [Google Scholar] [CrossRef]
- Tang, N.; Sun, J.; Yao, K.; Zhou, X.; Tian, Y.; Cao, Y.; Nirere, A. Identification of varieties based on hyperspectral imaging technique and competitive adaptive reweighted sampling-whale optimization algorithm-support vector machine. J. Food Process Eng. 2021, 44, e13603. [Google Scholar] [CrossRef]
- Sabzi, S.; Javadikia, H.; Arribas, J.I. A three-variety automatic and non-intrusive computer vision system for the estimation of orange fruit pH value. Measurement 2020, 152, 107298. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, H.; Lin, B.; Xu, G.; Tang, G.; Cai, K. Study of modeling optimization for hyperspectral imaging quantitative determination of naringin content in pomelo peel. Comput. Electron. Agric. 2019, 157, 410–416. [Google Scholar] [CrossRef]
- Teerachaichayut, S.; Ho, H.T. Non-destructive prediction of total soluble solids, titratable acidity and maturity index of limes by near infrared hyperspectral imaging. Postharvest Biol. Technol. 2017, 133, 20–25. [Google Scholar] [CrossRef]
- Shivers, S.W.; Roberts, D.A.; McFadden, J.P. Using paired thermal and hyperspectral aerial imagery to quantify land surface temperature variability and assess crop stress within California orchards. Remote Sens. Environ. 2019, 222, 215–231. [Google Scholar] [CrossRef]
- Abdulridha, J.; Batuman, O.; Ampatzidis, Y. UAV-Based Remote Sensing Technique to Detect Citrus Canker Disease Utilizing Hyperspectral Imaging and Machine Learning. Remote Sens. 2019, 11, 1373. [Google Scholar] [CrossRef]
- Peng, Z.; Guan, L.; Liao, Y.; Lian, S. Estimating Total Leaf Chlorophyll Content of Gannan Navel Orange Leaves Using Hyperspectral Data Based on Partial Least Squares Regression. IEEE Access 2019, 7, 155540–155551. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Fernández-Novales, J.; Garde-Cerdán, T.; Marín-San Román, S.; Tardaguila, J.; Diago, M.P. Multi-sensor spectral fusion to model grape composition using deep learning. Inf. Fusion 2023, 99, 101865. [Google Scholar] [CrossRef]
- Toosi, A.; Javan, F.D.; Samadzadegan, F.; Mehravar, S.; Kurban, A.; Azadi, H. Citrus orchard mapping in Juybar, Iran: Analysis of NDVI time series and feature fusion of multi-source satellite imageries. Ecol. Inform. 2022, 70, 101733. [Google Scholar] [CrossRef]
- Ma, S.; Li, Y.; Peng, Y. Spectroscopy and computer vision techniques for noninvasive analysis of legumes: A review. Comput. Electron. Agric. 2023, 206, 107695. [Google Scholar] [CrossRef]
- Goyal, R.; Singha, P.; Singh, S.K. Spectroscopic food adulteration detection using machine learning: Current challenges and future prospects. Trends Food Sci. Technol. 2024, 146, 104377. [Google Scholar] [CrossRef]
- Donmez, C.; Villi, O.; Berberoglu, S.; Cilek, A. Computer vision-based citrus tree detection in a cultivated environment using UAV imagery. Comput. Electron. Agric. 2021, 187, 106273. [Google Scholar] [CrossRef]
- Longo-Minnolo, G.; Consoli, S.; Vanella, D.; Pappalardo, S.; Guarrera, S.; Manetto, G.; Cerruto, E. Delineating citrus management zones using spatial interpolation and UAV-based multispectral approaches. Comput. Electron. Agric. 2024, 222, 109098. [Google Scholar] [CrossRef]
- Ojo, I.A.; Costa, L.; Ampatzidis, Y.; Alferez, F.; Shukla, S. Citrus Fruit Maturity Prediction Utilizing UAV Multispectral Imaging and Machine Learning. In Proceedings of the 2021 ASABE Annual International Virtual Meeting, Online, 12–16 July 2021; p. 2100495. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, Z.; Deng, X.; Zhu, Z.; Huang, H.; Zheng, Z.; Lian, B.; Zeng, G.; Tong, Z. Comparison of machine learning methods for citrus greening detection on UAV multispectral images. Comput. Electron. Agric. 2020, 171, 105234. [Google Scholar] [CrossRef]
- Tian, S.; Lu, L.; Labavitch, J.M.; Webb, S.M.; Yang, X.; Brown, P.H.; He, Z. Spatial imaging of Zn and other elements in Huanglongbing-affected grapefruit by synchrotron-based micro X-ray fluorescence investigation. J. Exp. Bot. 2014, 65, 953–964. [Google Scholar] [CrossRef]
- Cai, J.; Zou, C.; Yin, L.; Jiang, S.; El-Seedi, H.R.; Guo, Z. Characterization and recognition of citrus fruit spoilage fungi using Raman scattering spectroscopic imaging. Vib. Spectrosc. 2023, 124, 103474. [Google Scholar] [CrossRef]
- Siregar, T.H.; Ahmad, U.; Sutrisno; Maddu, A. Mechanical Damage Detection of Indonesia Local Citrus Based on Fluorescence Imaging. IOP Conf. Ser. Earth Environ. Sci. 2018, 147, 012006. [Google Scholar] [CrossRef]
- Zur, N.; Shlizerman, L.; Ben-Ari, G.; Sadka, A. Use of Magnetic Resonance Imaging (MRI) to Study and Predict Fruit Splitting in Citrus. Hortic. J. 2017, 86, 151–158. [Google Scholar] [CrossRef]
- Hsiao, W.-T.; Kuo, W.-C.; Lin, H.-H.; Lai, L.-H. Assessment and Feasibility Study of Lemon Ripening Using X-ray Image of Information Visualization. Appl. Sci. 2021, 11, 3261. [Google Scholar] [CrossRef]
- Gan, H.; Lee, W.S.; Alchanatis, V.; Abd-Elrahman, A. Active thermal imaging for immature citrus fruit detection. Biosyst. Eng. 2020, 198, 291–303. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.M.; Muncan, J. A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]
- Fakhlaei, R.; Babadi, A.A.; Sun, C.; Ariffin, N.M.; Khatib, A.; Selamat, J.; Xiaobo, Z. Application, challenges and future prospects of recent nondestructive techniques based on the electromagnetic spectrum in food quality and safety. Food Chem. 2024, 441, 138402. [Google Scholar] [CrossRef]
- Kutsanedzie, F.Y.H.; Guo, Z.; Chen, Q. Advances in Nondestructive Methods for Meat Quality and Safety Monitoring. Food Rev. Int. 2019, 35, 536–562. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; He, Z.; Liang, X. Non-destructive determination of internal soluble solid content in pomelo using visible/near infrared full-transmission spectroscopy. Postharvest Biol. Technol. 2024, 214, 112990. [Google Scholar] [CrossRef]
- Santos, C.S.P.; Cruz, R.; Gonçalves, D.B.; Queirós, R.; Bloore, M.; Kovács, Z.; Hoffmann, I.; Casal, S. Non-Destructive Measurement of the Internal Quality of Citrus Fruits Using a Portable NIR Device. J. AOAC Int. 2021, 104, 61–67. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Clarke, A.D.; Lin, M.Y.; Yılmaz, B. Antioxidant properties of citrus fibre and the prediction of oxidation in ground beef meatballs made with citrus fibre by ATR-FTIR spectroscopy with principal component analysis. Int. Food Res. J. 2021, 28, 129. [Google Scholar] [CrossRef]
- Tian, S.; Wang, S.; Xu, H. Early detection of freezing damage in oranges by online Vis/NIR transmission coupled with diameter correction method and deep 1D-CNN. Comput. Electron. Agric. 2022, 193, 106638. [Google Scholar] [CrossRef]
- Li, P.; Su, G.; Du, G.; Jiang, L.; Dong, Y.; Shan, Y. Portable LWNIR and SWNIR spectroscopy with pattern recognition technology for accurate and nondestructive detection of hidden mold infection in citrus. Microchem. J. 2023, 193, 109203. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Ghanei Ghooshkhaneh, N.; Golzarian, M.R.; Mollazade, K. VIS-NIR spectroscopy for detection of citrus core rot caused by Alternaria alternata. Food Control 2023, 144, 109320. [Google Scholar] [CrossRef]
- Wu, L.; Tang, X.; Wu, T.; Zeng, W.; Zhu, X.; Hu, B.; Zhang, S. A review on current progress of Raman-based techniques in food safety: From normal Raman spectroscopy to SESORS. Food Res. Int. 2023, 169, 112944. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jianbo, X.; Mahunu, G.K.; Jiyong, S.; Xu, J.-L.; Sun, D.-W. Recent Progress in Rapid Analyses of Vitamins, Phenolic, and Volatile Compounds in Foods Using Vibrational Spectroscopy Combined with Chemometrics: A Review. Food Anal. Methods 2019, 12, 2361–2382. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Q.; Belwal, T.; Lin, X.; Luo, Z. Insights into chemometric algorithms for quality attributes and hazards detection in foodstuffs using Raman/surface enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2476–2507. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhao, C.; Tian, G.; Zhang, H.; Xiao, H.; He, L.; Zheng, J. Chemical Mapping of Essential Oils, Flavonoids and Carotenoids in Citrus Peels by Raman Microscopy. J. Food Sci. 2017, 82, 2840–2846. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Lu, C.; Zhou, S.; Tian, G.; He, L.; Bao, Y.; Fauconnier, M.-L.; Xiao, H.; Zheng, J. Simultaneous determination of 14 bioactive citrus flavonoids using thin-layer chromatography combined with surface enhanced Raman spectroscopy. Food Chem. 2021, 338, 128115. [Google Scholar] [CrossRef]
- Nekvapil, F.; Brezestean, I.; Barchewitz, D.; Glamuzina, B.; Chiş, V.; Cintă Pinzaru, S. Citrus fruits freshness assessment using Raman spectroscopy. Food Chem. 2018, 242, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Q.; Zhu, Z. Rapid Classification of Citrus Fruits Based on Raman Spectroscopy and Pattern Recognition Techniques. Food Sci. Technol. Res. 2013, 19, 1077–1084. [Google Scholar] [CrossRef]
- Pan, H.; Ahmad, W.; Jiao, T.; Zhu, A.; Ouyang, Q.; Chen, Q. Label-free Au NRs-based SERS coupled with chemometrics for rapid quantitative detection of thiabendazole residues in citrus. Food Chem. 2022, 375, 131681. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, L.; Chen, W.; Zhang, F.; Du, Y.; Wu, T. Rapid In Situ SERS Analysis of Pesticide Residues on Plant Surfaces Based on Micelle Extraction of Targets and Stabilization of Ag Nanoparticle Aggregates. Food Anal. Methods 2018, 11, 3161–3169. [Google Scholar] [CrossRef]
- Sanchez, L.; Pant, S.; Xing, Z.; Mandadi, K.; Kurouski, D. Rapid and noninvasive diagnostics of Huanglongbing and nutrient deficits on citrus trees with a handheld Raman spectrometer. Anal. Bioanal. Chem. 2019, 411, 3125–3133. [Google Scholar] [CrossRef]
- Gu, H.; Hu, L.; Dong, Y.; Chen, Q.; Wei, Z.; Lv, R.; Zhou, Q. Evolving trends in fluorescence spectroscopy techniques for food quality and safety: A review. J. Food Compos. Anal. 2024, 131, 106212. [Google Scholar] [CrossRef]
- Khaliduzzaman, A.; Omwange, K.A.; Al Riza, D.F.; Konagaya, K.; Kamruzzaman, M.; Alom, M.S.; Gao, T.; Saito, Y.; Kondo, N. Antioxidant assessment of agricultural produce using fluorescence techniques: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3704–3715. [Google Scholar] [CrossRef]
- Kakkar, S.; Gupta, P.; Kumar, N.; Kant, K. Progress in Fluorescence Biosensing and Food Safety towards Point-of-Detection (PoD) System. Biosensors 2023, 13, 249. [Google Scholar] [CrossRef]
- Itakura, K.; Saito, Y.; Suzuki, T.; Kondo, N.; Hosoi, F. Estimation of Citrus Maturity with Fluorescence Spectroscopy Using Deep Learning. Horticulturae 2019, 5, 2. [Google Scholar] [CrossRef]
- Al Riza, D.F.; Yolanda, J.; Tulsi, A.A.; Ikarini, I.a.; Hanif, Z.; Nasution, A.; Widodo, S. Mandarin orange (Citrus reticulata Blanco cv. Batu 55) ripeness level prediction using combination reflectance-fluorescence spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123061. [Google Scholar] [CrossRef]
- Muharfiza, M.; Al Riza, D.F.; Saito, Y.; Itakura, K.; Kohno, Y.; Suzuki, T.; Kuramoto, M.; Kondo, N. Monitoring of Fluorescence Characteristics of Satsuma Mandarin (Citrus unshiu Marc.) during the Maturation Period. Horticulturae 2017, 3, 51. [Google Scholar] [CrossRef]
- Wu, X.; Liang, X.; Wang, Y.; Wu, B.; Sun, J. Non-Destructive Techniques for the Analysis and Evaluation of Meat Quality and Safety: A Review. Foods 2022, 11, 3713. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Ding, L.; Han, K.; Zou, X.; Wang, S.; Wen, Z.; Ye, Y.; Ren, X. Detection of carbendazim in oranges with metal grating integrated microfluidic sensor in terahertz. Food Addit. Contam. Part A 2022, 39, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-H.; Otani, C.; Ogawa, Y. Innovatively identifying naringin and hesperidin by using terahertz spectroscopy and evaluating flavonoids extracts from waste orange peels by coupling with multivariate analysis. Food Control. 2022, 137, 108897. [Google Scholar] [CrossRef]
- Zang, Z.; Li, Z.; Wang, J.; Lu, X.; Lyu, Q.; Tang, M.; Cui, H.-L.; Yan, S. Terahertz spectroscopic monitoring and analysis of citrus leaf water status under low temperature stress. Plant Physiol. Biochem. 2023, 194, 52–59. [Google Scholar] [CrossRef]
- Salvino, R.A.; Colella, M.F.; De Luca, G. NMR-based metabolomics analysis of Calabrian citrus fruit juices and its application to industrial process quality control. Food Control 2021, 121, 107619. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Almeida, F.D.L.; Cavalcante, R.S.; de Brito, E.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. 1H NMR spectroscopy and chemometrics evaluation of non-thermal processing of orange juice. Food Chem. 2016, 204, 102–107. [Google Scholar] [CrossRef]
- Lin, H.; He, C.; Liu, H.; Shen, G.; Xia, F.; Feng, J. NMR-based quantitative component analysis and geographical origin identification of China’s sweet orange. Food Control 2021, 130, 108292. [Google Scholar] [CrossRef]
- Salazar, M.O.; Pisano, P.L.; González Sierra, M.; Furlan, R.L.E. NMR and multivariate data analysis to assess traceability of argentine citrus. Microchem. J. 2018, 141, 264–270. [Google Scholar] [CrossRef]
- Marchetti, L.; Pellati, F.; Benvenuti, S.; Bertelli, D. Use of 1H NMR to Detect the Percentage of Pure Fruit Juices in Blends. Molecules 2019, 24, 2592. [Google Scholar] [CrossRef]
- Migues, I.; Hodos, N.; Moltini, A.I.; Gámbaro, A.; Rivas, F.; Moyna, G.; Heinzen, H. 1H NMR metabolic profiles as selection tools of new mandarin cultivars based on fruit acceptability. Sci. Hortic. 2021, 287, 110262. [Google Scholar] [CrossRef]
- Do Prado Apparecido, R.; Carlos, E.F.; Lião, L.M.; Vieira, L.G.E.; Alcantara, G.B. NMR-based metabolomics of transgenic and non-transgenic sweet orange reveals different responses in primary metabolism during citrus canker development. Metabolomics 2017, 13, 20. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Salomé-Abarca, L.F.; Gonçalves Pereira, R.; Brandão Seibert, J.; Silva-Junior, G.J.; Das Graças Fernandes da Silva, M.F.; Choi, Y.H. Metabolomic Investigation of Citrus latifolia and the Putative Role of Coumarins in Resistance to Black Spot Disease. Front. Mol. Biosci. 2022, 9, 934401. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Tsuchida, T.; Tsuchikawa, S. Time-of-flight Near-infrared Spectroscopy for Nondestructive Measurement of Internal Quality in Grapefruit. J. Am. Soc. Hort. Sci. 2013, 138, 225–228. [Google Scholar] [CrossRef]
- Yao, M.; Fu, G.; Xu, J.; Li, T.; Zhang, L.; Liu, M.; Yang, P.; Xu, Y.; Rao, H. In situ diagnosis of mature HLB-asymptomatic citrus fruits by laser-induced breakdown spectroscopy. Appl. Opt. 2021, 60, 5846–5853. [Google Scholar] [CrossRef]
- Ropelewska, E.; Rady, A.M.; Watson, N.J. Apricot Stone Classification Using Image Analysis and Machine Learning. Sustainability 2023, 15, 9259. [Google Scholar] [CrossRef]
- Olorunfemi, B.O.; Nwulu, N.I.; Adebo, O.A.; Kavadias, K.A. Advancements in machine visions for fruit sorting and grading: A bibliometric analysis, systematic review, and future research directions. J. Agric. Food Res. 2024, 16, 101154. [Google Scholar] [CrossRef]
- Iqbal, Z.; Khan, M.A.; Sharif, M.; Shah, J.H.; ur Rehman, M.H.; Javed, K. An automated detection and classification of citrus plant diseases using image processing techniques: A review. Comput. Electron. Agric. 2018, 153, 12–32. [Google Scholar] [CrossRef]
- Kukreja, V.; Dhiman, P. A Deep Neural Network based disease detection scheme for Citrus fruits. In Proceedings of the 2020 International Conference on Smart Electronics and Communication (ICOSEC) 2020, Trichy, India, 10–12 September 2020; pp. 97–101. [Google Scholar] [CrossRef]
- Pathmanaban, P.; Gnanavel, B.K.; Anandan, S.S. Recent application of imaging techniques for fruit quality assessment. Trends Food Sci. Technol. 2019, 94, 32–42. [Google Scholar] [CrossRef]
- Al-Sammarraie, M.A.; Gierz, Ł.; Przybył, K.; Koszela, K.; Szychta, M.; Brzykcy, J.; Baranowska, H.M. Predicting Fruit’s Sweetness Using Artificial Intelligence—Case Study: Orange. Appl. Sci. 2022, 12, 8233. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Wang, Y.; Li, Y.; Xiong, Z. A smart fruit size measuring method and system in natural environment. J. Food Eng. 2024, 373, 112020. [Google Scholar] [CrossRef]
- Phate, V.R.; Malmathanraj, R.; Palanisamy, P. Classification and Indirect Weighing of Sweet Lime Fruit through Machine Learning and Meta-heuristic Approach. Int. J. Fruit Sci. 2021, 21, 528–545. [Google Scholar] [CrossRef]
- Bhargava, A.; Bansal, A. Fruits and vegetables quality evaluation using computer vision: A review. J. King Saud Univ.—Comput. Inf. Sci. 2021, 33, 243–257. [Google Scholar] [CrossRef]
- Nuño-Maganda, M.A.; Dávila-Rodríguez, I.A.; Hernández-Mier, Y.; Barrón-Zambrano, J.H.; Elizondo-Leal, J.C.; Díaz-Manriquez, A.; Polanco-Martagón, S. Real-Time Embedded Vision System for Online Monitoring and Sorting of Citrus Fruits. Electronics 2023, 12, 3891. [Google Scholar] [CrossRef]
- Bhargava, A.; Bansal, A. Automatic Detection and Grading of Multiple Fruits by Machine Learning. Food Anal. Methods 2020, 13, 751–761. [Google Scholar] [CrossRef]
- Qadri, S.; Furqan Qadri, S.; Husnain, M.; Saad Missen, M.M.; Khan, D.M.; Muzammil Ul, R.; Razzaq, A.; Ullah, S. Machine vision approach for classification of citrus leaves using fused features. Int. J. Food Prop. 2019, 22, 2072–2089. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; Ference, C.; Zhang, Q. An Accuracy Improvement Method Based on Multi-Source Information Fusion and Deep Learning for TSSC and Water Content Nondestructive Detection in “Luogang” Orange. Electronics 2021, 10, 80. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Yi, S.; Jin, G.; Qiu, X.; Li, Y. Nondestructive determining the soluble solids content of citrus using near infrared transmittance technology combined with the variable selection algorithm. Artif. Intell. Agric. 2020, 4, 48–57. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Hong, S.-J.; Kim, E.; Lee, C.-H.; Kim, G. Application of ensemble neural-network method to integrated sugar content prediction model for citrus fruit using Vis/NIR spectroscopy. J. Food Eng. 2023, 338, 111254. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; Ference, C.; Qiu, G.; Liang, X. Rapid Nondestructive Detection of Water Content and Granulation in Postharvest “Shatian” Pomelo Using Visible/Near-Infrared Spectroscopy. Biosensors 2020, 10, 41. [Google Scholar] [CrossRef]
- Zeb, A.; Qureshi, W.S.; Ghafoor, A.; Malik, A.; Imran, M.; Mirza, A.; Tiwana, M.I.; Alanazi, E. Towards sweetness classification of orange cultivars using short-wave NIR spectroscopy. Sci. Rep. 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Theanjumpol, P.; Wongzeewasakun, K.; Muenmanee, N.; Wongsaipun, S.; Krongchai, C.; Changrue, V.; Boonyakiat, D.; Kittiwachana, S. Non-destructive identification and estimation of granulation in ‘Sai Num Pung’ tangerine fruit using near infrared spectroscopy and chemometrics. Postharvest Biol. Technol. 2019, 153, 13–20. [Google Scholar] [CrossRef]
- Borba, K.R.; Spricigo, P.C.; Aykas, D.P.; Mitsuyuki, M.C.; Colnago, L.A.; Ferreira, M.D. Non-invasive quantification of vitamin C, citric acid, and sugar in ’Valência’ oranges using infrared spectroscopies. J. Food Sci. Technol. 2021, 58, 731–738. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pérez-Hernández, N.; Zepeda-Vallejo, L.G.; Quiroz-Acosta, T.; Mendieta-Moctezuma, A.; Montoya-García, C.; García-Nava, M.L.; Becerra-Martínez, E. 1H-NMR Based Metabolomics Profiling of Citrus Juices Produced in Veracruz, México. Chem. Biodivers. 2019, 16, e1800479. [Google Scholar] [CrossRef]
- Srivastava, S.; Vani, B.; Sadistap, S. Machine-vision based handheld embedded system to extract quality parameters of citrus cultivars. J. Food Meas. Charact. 2020, 14, 2746–2759. [Google Scholar] [CrossRef]
- Srivastava, S.; Vani, B.; Sadistap, S. Handheld, smartphone based spectrometer for rapid and nondestructive testing of citrus cultivars. J. Food Meas. Charact. 2021, 15, 892–904. [Google Scholar] [CrossRef]
- Srivastava, S.; Sadistap, S. Data fusion for fruit quality authentication: Combining non-destructive sensing techniques to predict quality parameters of citrus cultivars. J. Food Meas. Charact. 2022, 16, 344–365. [Google Scholar] [CrossRef]
- Zakiyyah, A.; Hanif, Z.; Indriani, D.; Iqbal, Z.; Damayanti, R.; Al Riza, D. Characterization and Classification of Citrus reticulata var. Keprok Batu 55 Using Image Processing and Artificial Intelligence. Univers. J. Agric. Res. 2022, 10, 397–404. [Google Scholar] [CrossRef]
- Sannidhan, M.S.; Martis, J.E.; Suhas, M.V.; Aithal, S.K. Predicting Citrus Limon Maturity with Precision Using Transfer Learning. In Proceedings of the 2023 International Conference on Recent Advances in Information Technology for Sustainable Development (ICRAIS) 2023, Manipal, India, 6–7 November 2023; pp. 182–187. [Google Scholar] [CrossRef]
- Liu, T.-H.; Ehsani, R.; Toudeshki, A.; Zou, X.-J.; Wang, H.-J. Identifying immature and mature pomelo fruits in trees by elliptical model fitting in the Cr–Cb color space. Precis. Agric. 2019, 20, 138–156. [Google Scholar] [CrossRef]
- Chen, S.; Xiong, J.; Jiao, J.; Xie, Z.; Huo, Z.; Hu, W. Citrus fruits maturity detection in natural environments based on convolutional neural networks and visual saliency map. Precis. Agric. 2022, 23, 1515–1531. [Google Scholar] [CrossRef]
- Apolo-Apolo, O.E.; Martínez-Guanter, J.; Egea, G.; Raja, P.; Pérez-Ruiz, M. Deep learning techniques for estimation of the yield and size of citrus fruits using a UAV. Eur. J. Agron. 2020, 115, 126030. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, H.; Chen, J.; Cheng, Y.; Xu, R.; Li, S.; Chen, Y. Improving human intuition for vision-based freshness prediction of Citrus reticulata Blanco using machine learning. Sci. Hortic. 2023, 321, 112300. [Google Scholar] [CrossRef]
- Pires, R.; Guerra, R.; Cruz, S.P.; Antunes, M.D.; Brázio, A.; Afonso, A.M.; Daniel, M.; Panagopoulos, T.; Gonçalves, I.; Cavaco, A.M. Ripening assessment of ‘Ortanique’ (Citrus reticulata Blanco x Citrus sinensis (L) Osbeck) on tree by SW-NIR reflectance spectroscopy-based calibration models. Postharvest Biol. Technol. 2022, 183, 111750. [Google Scholar] [CrossRef]
- Al Riza, D.F.; Ikrom, A.M.; Tulsi, A.A.; Darmanto; Hendrawan, Y. Mandarin orange (Citrus reticulata Blanco cv. Batu 55) ripeness parameters prediction using combined reflectance-fluorescence images and deep convolutional neural network (DCNN) regression model. Sci. Hortic. 2024, 331, 113089. [Google Scholar] [CrossRef]
- Sandra; Said, A.; Tulsi, A.A.; Indriani, D.W.; Yulianingsih, R.; Hawa, L.C.; Kondo, N.; Al Riza, D.F. Developing a prediction method for physicochemical characteristics of Pontianak Siam orange (Citrus suhuiensis cv. Pontianak) based on combined reflectance-Fluorescence spectroscopy and artificial neural network. Talanta Open 2024, 9, 100303. [Google Scholar] [CrossRef]
- Zhang, H.; Zhan, B.; Pan, F.; Luo, W. Determination of soluble solids content in oranges using visible and near infrared full transmittance hyperspectral imaging with comparative analysis of models. Postharvest Biol. Technol. 2020, 163, 111148. [Google Scholar] [CrossRef]
- Hu, W.; Xiong, J.; Liang, J.; Xie, Z.; Liu, Z.; Huang, Q.; Yang, Z. A method of citrus epidermis defects detection based on an improved YOLOv5. Biosyst. Eng. 2023, 227, 19–35. [Google Scholar] [CrossRef]
- Sun, X.; Xu, S.; Lu, H. Non-Destructive Identification and Estimation of Granulation in Honey Pomelo Using Visible and Near-Infrared Transmittance Spectroscopy Combined with Machine Vision Technology. Appl. Sci. 2020, 10, 5399. [Google Scholar] [CrossRef]
- Xu, Q.; Cai, J.-R.; Zhang, W.; Bai, J.-W.; Li, Z.-Q.; Tan, B.; Sun, L. Detection of citrus Huanglongbing (HLB) based on the HLB-induced leaf starch accumulation using a home-made computer vision system. Biosyst. Eng. 2022, 218, 163–174. [Google Scholar] [CrossRef]
- Arthi, A.; Sharmili, N.; Althubiti, S.A.; Laxmi Lydia, E.; Alharbi, M.; Alkhayyat, A.; Gupta, D. Duck optimization with enhanced capsule network based citrus disease detection for sustainable crop management. Sustain. Energy Technol. Assess. 2023, 58, 103355. [Google Scholar] [CrossRef]
- Cai, Z.; Sun, C.; Zhang, H.; Zhang, Y.; Li, J. Developing universal classification models for the detection of early decayed citrus by structured-illumination reflectance imaging coupling with deep learning methods. Postharvest Biol. Technol. 2024, 210, 112788. [Google Scholar] [CrossRef]
- Xie, C.; Lee, W.S. Detection of citrus black spot symptoms using spectral reflectance. Postharvest Biol. Technol. 2021, 180, 111627. [Google Scholar] [CrossRef]
- Li, J.; Luo, W.; Han, L.; Cai, Z.; Guo, Z. Two-wavelength image detection of early decayed oranges by coupling spectral classification with image processing. J. Food Compos. Anal. 2022, 111, 104642. [Google Scholar] [CrossRef]
- Li, J.; Zhang, R.; Li, J.; Wang, Z.; Zhang, H.; Zhan, B.; Jiang, Y. Detection of early decayed oranges based on multispectral principal component image combining both bi-dimensional empirical mode decomposition and watershed segmentation method. Postharvest Biol. Technol. 2019, 158, 110986. [Google Scholar] [CrossRef]
- Luo, W.; Fan, G.; Tian, P.; Dong, W.; Zhang, H.; Zhan, B. Spectrum classification of citrus tissues infected by fungi and multispectral image identification of early rotten oranges. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121412. [Google Scholar] [CrossRef]
- He, C.; Li, X.; Liu, Y.; Yang, B.; Wu, Z.; Tan, S.; Ye, D.; Weng, H. Combining multicolor fluorescence imaging with multispectral reflectance imaging for rapid citrus Huanglongbing detection based on lightweight convolutional neural network using a handheld device. Comput. Electron. Agric. 2022, 194, 106808. [Google Scholar] [CrossRef]
- Dhiman, P.; Manoharan, P.; Lilhore, U.K.; Alroobaea, R.; Kaur, A.; Iwendi, C.; Alsafyani, M.; Baqasah, A.M.; Raahemifar, K. PFDI: A precise fruit disease identification model based on context data fusion with faster-CNN in edge computing environment. EURASIP J. Adv. Signal Process. 2023, 2023, 72. [Google Scholar] [CrossRef]
- Ruggiero, L.; Amalfitano, C.; Di Vaio, C.; Adamo, P. Use of near-infrared spectroscopy combined with chemometrics for authentication and traceability of intact lemon fruits. Food Chem. 2022, 375, 131822. [Google Scholar] [CrossRef]
- Mohammadian, A.; Barzegar, M.; Mani-Varnosfaderani, A. Detection of fraud in lime juice using pattern recognition techniques and FT-IR spectroscopy. Food Sci. Nutr. 2021, 9, 3026–3038. [Google Scholar] [CrossRef]
- Liu, N.; Townsend, P.A.; Naber, M.R.; Bethke, P.C.; Hills, W.B.; Wang, Y. Hyperspectral imagery to monitor crop nutrient status within and across growing seasons. Remote Sens. Environ. 2021, 255, 112303. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Riccioli, C.; Pérez-Marín, D.; Garrido-Varo, A. Optimizing spatial data reduction in hyperspectral imaging for the prediction of quality parameters in intact oranges. Postharvest Biol. Technol. 2021, 176, 111504. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, B.; Guo, Z. Fresh-cut orange preservation based on nano-zinc oxide combined with pressurized argon treatment. LWT 2021, 135, 110036. [Google Scholar] [CrossRef]
- Sun, C.; Aernouts, B.; Saeys, W. Characterisation and optical detection of puffy Satsuma mandarin. Biosyst. Eng. 2023, 229, 18–31. [Google Scholar] [CrossRef]
- Yang, Q.; Qian, X.; Routledge, M.N.; Wu, X.; Shi, Y.; Zhu, Q.; Zhang, H. Metabonomics analysis of postharvest citrus response to Penicillium digitatum infection. LWT 2021, 152, 112371. [Google Scholar] [CrossRef]
- Srivastava, S.; Sadistap, S. Data processing approaches and strategies for non-destructive fruits quality inspection and authentication: A review. J. Food Meas. Charact. 2018, 12, 2758–2794. [Google Scholar] [CrossRef]
- Sun, J.; Wu, M.; Hang, Y.; Lu, B.; Wu, X.; Chen, Q. Estimating cadmium content in lettuce leaves based on deep brief network and hyperspectral imaging technology. J. Food Process Eng. 2019, 42, e13293. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Luo, S.M.; Hou, C.J.; Tang, Y.; He, Y.; Xue, X.Y. Detection of orchard citrus fruits using a monocular machine vision-based method for automatic fruit picking applications. Comput. Electron. Agric. 2018, 152, 64–73. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, J.; Yao, K.; Xu, M.; Tian, Y.; Dai, C. A decision fusion method based on hyperspectral imaging and electronic nose techniques for moisture content prediction in frozen-thawed pork. LWT 2022, 165, 113778. [Google Scholar] [CrossRef]
- Li, L.; Xie, S.; Ning, J.; Chen, Q.; Zhang, Z. Evaluating green tea quality based on multisensor data fusion combining hyperspectral imaging and olfactory visualization systems. J. Sci. Food Agric. 2019, 99, 1787–1794. [Google Scholar] [CrossRef]
- Azcarate, S.M.; Ríos-Reina, R.; Amigo, J.M.; Goicoechea, H.C. Data handling in data fusion: Methodologies and applications. TrAC Trends Anal. Chem. 2021, 143, 116355. [Google Scholar] [CrossRef]
- Lu, P.; Dai, F. An Overview of Multi-sensor Information Fusion. In Proceedings of the 2021 6th International Conference on Intelligent Informatics and Biomedical Sciences (ICIIBMS) 2021, Oita, Japan, 25–27 November 2021; pp. 5–9. [Google Scholar] [CrossRef]
- Xu, F.; Hao, Z.; Huang, L.; Liu, M.; Chen, T.; Chen, J.; Zhang, L.; Zhou, H.; Yao, M. Comparative identification of citrus huanglongbing by analyzing leaves using laser-induced breakdown spectroscopy and near-infrared spectroscopy. Appl. Phys. B 2020, 126, 43. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Ali, S.; Li, H.; Ouyang, Q.; Wu, X.; Hashim, M.M.; Chen, Q. Recent advances in assessing qualitative and quantitative aspects of cereals using nondestructive techniques: A review. Trends Food Sci. Technol. 2021, 116, 815–828. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment—A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- Zheng, Z.; Xiong, J.; Lin, H.; Han, Y.; Sun, B.; Xie, Z.; Yang, Z.; Wang, C. A Method of Green Citrus Detection in Natural Environments Using a Deep Convolutional Neural Network. Front. Plant Sci. 2021, 12, 705737. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Fu, L.; Xu, M.; Tang, N.; Cao, Y.; Yao, K.; Jing, J. Identification of red jujube varieties based on hyperspectral imaging technology combined with CARS-IRIV and SSA-SVM. J. Food Process Eng. 2022, 45, e14137. [Google Scholar] [CrossRef]
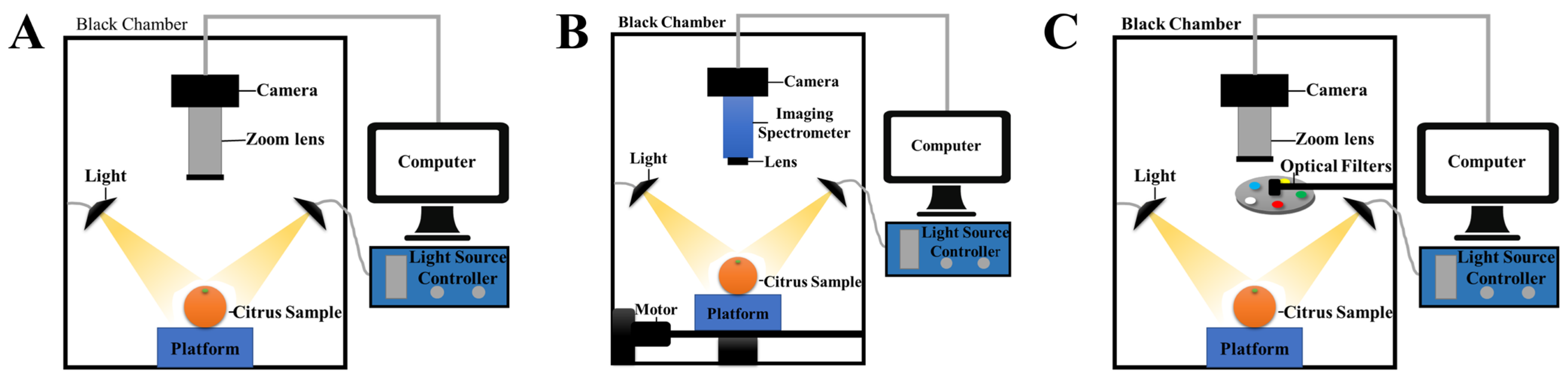

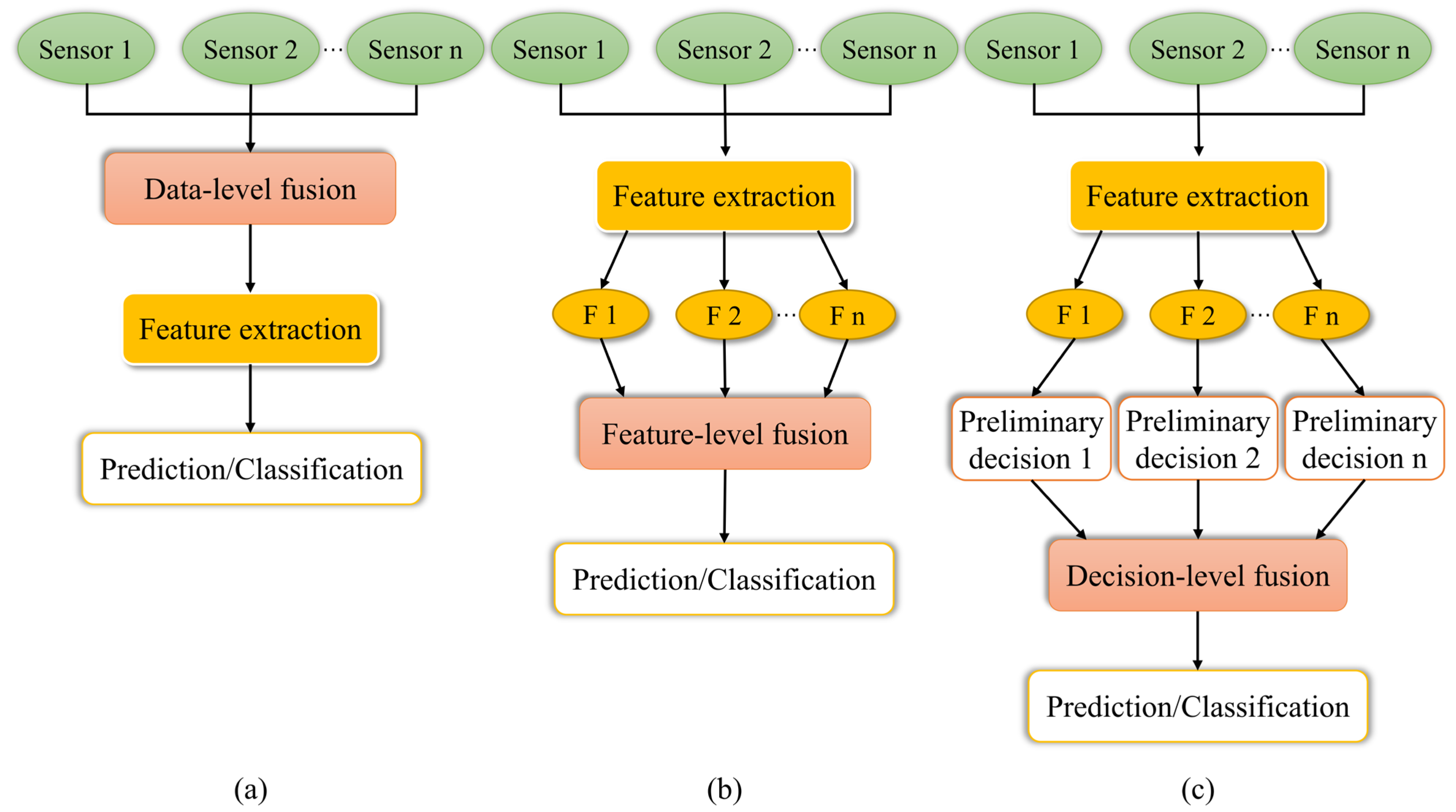
| Detection Technology | Sample | Measurement Properties | Preprocessing | Feature Selection and Extraction | Modeling Technology | Best Performance | Reference |
|---|---|---|---|---|---|---|---|
| Computer vision | Three kinds of bergamots | Peel color, dimensional features, and hardness | White balance, color correction, standardization | RGB to Hunter L, a, b, shape, PCA | LDA | Accuracy = 80.49% | [20] |
| Computer vision | Pontianak Siam oranges | Fruity flavor | Digitization | RGB | KNN | Accuracy = 80% | [23] |
| Computer vision | Oranges | Color and sweetness | Contrast, sharpening, smoothing, edge detection, filtering | RGB | KNN, DT, SVM, Neural Network, LR | LR: accuracy = 97% | [105] |
| Computer vision | Oranges | Size measuring | Data augmentation, find contour, crop, resize, median filter | Binary image, CNN, Cycle GAN, RGB, HSV, YCrCb, contours | YOLOv5, PLS | Accuracy = 95.6%, the overall error = 10.12% | [106] |
| Computer vision | Oranges and other fruits | Size and maturity | OTSU, voxel mapping, projection matrix estimation | RGB to HSV, contours, 3D reconstruction, volume conversion | FRBC | Classification accuracy = 98.5% | [22] |
| Computer vision | Sweet lime fruit | Weight | Median filter, grayscale conversion, OTSU, binarization | Canny, 1D, 2D | DA, NR, FFANN | R2 = 0.9931, MAPE = 2.306% | [21] |
| Computer vision | Sweet lime fruit | Weight | Channel separation, median filter, grayscale, OTSU | 1D, 2D | SVM, GA-ANFIS, PSO-ANFIS | R2p = 0.9536, RMSEP = 4.3113 | [107] |
| Computer vision | Citrus fruits | Surface feature and weight | Image resizing | - | SortNet | Classification accuracy = 97%, grader accuracy = 91.3% | [9] |
| Computer vision | Citrus fruits | Fruit segmentation, color, and size classification | Histogram equalization, rotation, zoom | - | DT | Segmentation accuracy = 97%, color accuracy = 94%, size accuracy = 90% | [109] |
| Computer vision | Oranges, avocados, bananas and apples | Grading and classification | Background separation, image scaling, Gaussian filtering, fuzzy segmentation | Color, statistical, texture, geometric features | KNN, SVM, SRC, ANN | Accuracy = 98.48% | [110] |
| Computer vision | Grapefruit, Moussami, Malta, lemon, Kinnow, Local lemon, Fuetrells, and Malta Shakri | Classification | ROI, Binary, histogram, texture, spectral, data augmentation | CFS | MLP, RF, J48 and Naive Bayes | MLP: accuracy = 98.14% | [111] |
| Computer vision | Bam, Blood, and Thomson orange | pH | Threshold segmentation | Color, texture, histogram, moments, shape | ANN-PSO, MLP | Bam: R2 = 0.950, Blood: R2 = 0.935, Thomson: R2 = 0.957 | [40] |
| HSI | Nanfeng mandarin | SSC | SGS-MSC | BOSS, CARS, IRIV | PLSR, LSSVM | R2p = 0.9376, RMSEP = 0.3986 | [35] |
| HSI | Pomelo | Sugar, vitamin C, organic acid | ROI | - | RBF-PLS | Sugar: R2T = 0.872, RMSET = 1.404%; Vitamin C: R2T = 0.872, RMSET = 61.540 mg/kg; organic acid: R2T = 0.866, RMSET = 1.573 g/kg | [33] |
| HSI | Pomelo fruits | Naringin content | SG | - | PLS | R2CV = 0.933, RMSECV = 0.345 | [41] |
| VIS/NIR, computer vision, electronic nose | “Luogang” Orange | TSSC, and water content | SG | GA | CNN-PLSR | TSSC: R2 = 0.8580, RMSE = 0.4276; water content: R2 = 0.7013, RMSE = 0.0063 | [112] |
| Vis/NIR | “Gannan” navel orange | SSC | Smoothing, MSC, SNV, 1D | SPA, CARS, GA | PLS | R2p = 0.9165, RMSEP = 0.5684 | [113] |
| Vis/NIR | Unshiu, Cheonhyehyang, Hallabong | Sugar content | MSC, SNV, SG, MM | - | PLSR, VIP-PLSR, Full-ANN, PCA-ANN, PLS-ANN, 1D-CNN, Ensemble Type-1, 2, 3, 4 | R2T = 0.839, RMSET = 0.516 | [114] |
| Vis/NIR | “Shatian” pomelo | Water content and granulation degree | 1D, SR, LM, IM, SG, MSC | RCA, MI-SPA, GA, PCA, LDA | PLSR | R2v = 0.712, RMSEV = 0.0488; accuracy = 100% | [115] |
| Vis/NIR | Pomelo | SSC | SNV, MSC, 2D | CARS, SPA, PCA | PLSR, SVR | R2v = 0.85, RMSE = 0.98 | [63] |
| NIR | “Fino” lemons | TSS, and TA | MSC | Spectral conversion | PLS-R, PLS-DA | TSS: R2 = 0.84, RMSEP = 0.42; TA: R2 = 0.72, RMSEP = 0.45 | [11] |
| NIR | Red Blood, Mosambi, and Succari oranges | Brix, TA, Brix: TA, BrimA, and sweetness classification | SG | PCA | PLSR, Tree, Ensemble, KNN, LDA, SVM | Brix: R2 = 0.57, TA: R2 = 0.73, Brix: TA: R2 = 0.66, BrimA: R2 = 0.55, classification accuracy = 80.03% | [116] |
| NIR | Citrus fiber | Total polyphenol, total flavonoid, oxygen radical absorbance capacity values, and the pH | Fixed block mean, polynomial subtract (1st order), smoothing | PCA | GLM | R2 = 0.96 | [65] |
| NIR | Oranges, lemons, clementines, tangerines, and Tahiti limes | Ascorbic acid, dehydroascorbic acid, total vitamin C, soluble solids, total acidity, and juiciness | SNV, SG, 1D, 2D, MSC, normalization | PCA | LDA, PLSR | Vitamin C: R2 = 0.77–0.86 | [64] |
| NIR | “Sai Num Pung” tangerine fruit | MC, SSC, TA, and granulation rate | SNV, MSC, normalization, derivatives | PCA | PLS, LDA, QDA, PLS-DA, KNN, SSOM | Predictive ability = 93.7%, model stability = 95.3%, correctly classified = 94.0% | [117] |
| NIR, MIR | “Valencia” oranges | Vitamin C, citric acid, total and reducing sugar content | Mean center, SNV, SG, normalization | - | PLS | MIR models had lower prediction errors than NIR models | [118] |
| THz | Valencia sweet orange | Naringin, and hesperidin | MSC, SNV, 1D, 2D | - | PLSR | Naringin: R2 = 0.99, RMSEP = 2.97%; hesperidin: R2 = 0.97, RMSEP = 4.48% | [88] |
| NMR | Lemons, tangerines, oranges, and grapefruits | Specific amino acids, sugars, and organic acids | - | PCA | OPLS-DA | Valencia oranges had the highest concentration of ascorbic acid (>2 mM) | [119] |
| NMR | 8 Citrus varieties grown in Uruguay | Sugar, citric acid | Zero padding, Fourier transform, phase correction, baseline correction, normalization | PCA | PLS-DA, OPLS-DA | Sweetening power/citric acid: R2 = 0.79 | [95] |
| Computer vision | Citrus | Chlorophyll, sugar, TSS, pH, weight, volume | Grayscale, OTSU, morphological operations, watershed | Dominant color method, Color and texture characteristics | PCR, PLSR, MLR, ANN | Ch a: accuracy = 70.38%; Ch b: accuracy = 79.72%; TSS: accuracy = 78.94%; sugar: accuracy = 73.97%; weight: accuracy = 68.68%; volume: accuracy = 48.98%; pH: accuracy = 63.11% | [120] |
| UV-Vis-NIR | Citrus | Chlorophyll, sugar, TSS, pH, weight and volume | SNV, spectral average | PCA | ANN, MLP, PLSR, PCR | Ch a: accuracy = 76.71%; Ch b: accuracy = 82.86%; TSS: accuracy = 87.88%; sugar: accuracy = 77.33%; weight: accuracy = 62.47%; volume: accuracy = 18.98%; pH: accuracy = 80.64% | [121] |
| Computer vision, UV-Vis-NIR spectroscopy, ultrasound, and electronic nose | Citrus fruits | Chlorophyll, sugar, TSS, pH, weight and volume | Baseline correction, segmentation, noise elimination, amplitude and time of flight extraction, scaling and normalization, color and texture extraction, multiple to single spectrum conversion, attenuation and propagation delay conversion | PCA | Statistical modeling methods (MLR, PCR, PLSR) and Five Different ANN modeling methods | TSS accuracy = 95.64%; chlorophyll (Ch a accuracy = 96.78%, Ch b accuracy = 97.76%); sugar accuracy = 97.36%; pH accuracy = 78.31%; weight accuracy = 91.45%; Volume accuracy = 36.64% | [122] |
| Computer vision | Orange | Maturity | OTSU, histogram pattern, thresholding, binarization | RGB, L*, a*, b, HSV | LR, DT, RF, SVM | SVM: accuracy = 88.71% | [123] |
| Computer vision | Lemon | Maturity | Image resizing, filter, color space conversion, grayscale, OTSU | ROI | VGG, ResNet, DenseNet, NASNet Large, MobileNet, Inception V3 | VGG: accuracy = 96.134% | [124] |
| Computer vision | Grapefruit | Maturity | RGB to Y’CbCr, elliptical boundary model segmentation, morphological operations | Color area selection, ellipse fitting, Douglas–Peucker algorithm | Polynomial Fitting | Total correct recognition rate = 93.5% | [125] |
| Computer vision | Tangerine | Maturity | Data augmentation | MSSS | YOLOv5, ResNet34 | Accuracy = 95.07% | [126] |
| Computer vision | Citrus | Maturity | RGB, HIS, graying, OTSU, binarization, morphological operations | Area evaluation, Canny, corner detection, edge labeling algorithm, extract contour fragments, Hough transform | Morphological characteristics statistics | Accuracy = 97.44% | [24] |
| Computer vision | Citrus orchard | Fruit production, and fruit size | ROI, data augmentation | CNN | Faster R-CNN, LSTM | Estimate error = 7.22% | [127] |
| Computer vision | Ponkan mandarins | Freshness | Image masking, data augmentation | ResNet-18 | CNN | Prediction accuracy = 95.6% | [128] |
| NIR | “Ortanique” Citrus | pH, SSC, TA, and MI | SNV, PSNV, MSC, Norris derivative, SPLINE, SG, CR | - | PLS | pH: R2 = 0.80; SSC: R2 = 0.79; TA: R2 = 0.73; MI: R2 = 0.69 | [129] |
| Fluorescence spectroscopy | Satsuma mandarin | Brix–acid ratio, and maturity | - | - | CNN, PCR | Absolute error = 2.48 | [83] |
| Vis-NIR, fluorescence spectroscopy | Mandarin Batu 55 oranges | SSC, TA, and maturity | MA, SG, SNV, MSC | PCA | PLSR | R2 = 0.91, RMSE = 2.4555 | [84] |
| Computer vision, fluorescence imaging | Mandarin Batu 55 oranges | Maturity, SSC, acidity, firmness, and Brix–acid ratio | MA, SG, SNV, MSC | PCA | DCNN | Acidity: R2 = 0.83; Brix–acid ratio: R2 = 0.94; SSC: R2 = 0.86; firmness: R2 = 0.91 | [130] |
| Vis-NIR, fluorescence spectroscopy | Pontianak Siam oranges | TSS, acidity, firmness, and maturity | MA, SG | PCA | ANN | TSS: R2 = 0.89; acidity: R2 = 0.96; firmness: R2 = 0.97; maturity: R2 = 0.99 | [131] |
| Computer vision | Sour lemons | Defect | ROI, normalization, data augmentation | - | CNN, KNN, ANN, Fuzzy, SVM, DT | Accuracy = 100% | [25] |
| Computer vision | Citrus fruits | Peel defects, and fruit morphological characteristics | Image stitching, data augmentation | Image-processing technology | Yolo-FD, PSO-ELM | Yolo-FD: average accuracy = 98.7%; PSO-ELM: accuracy 91.42%, R2 = 0.9044, MSE = 0.8497 | [10] |
| HSI | “GuanXiMiYou” Citrus | Granulation | Image correction, data augmentation | - | LS-SVM, BP-NN, CNN, CNN with batch normalization | Training set accuracy = 100% | [38] |
| HSI | Citrus | SSC, and TA | OTSU, MSC | CARS, SPA, CARS-SPA | PLS, MLR, LS-SVM | R2p = 0.911, RMSEP = 0.4032 | [132] |
| MSI | “Nanfeng” mandarins | Defects | Image calibration | PCA | Defect detection algorithm based on PC-2 image and ratio image combined with simple threshold method | Classification accuracy = 96.63% | [4] |
| Fluorescence imaging | Citrus | Epidermal defects | Mark, scale, crop and add noise | CBAM, FPN, PAN | YOLOv5 | Map = 95.5%, precision = 94.0%, recall = 95.1% | [133] |
| Vis/NIR | “Orah” oranges | Freezing damage | DCM | CARS, SPA, | PLSDA, SVM, CNN | Overall accuracy = 91.96% | [66] |
| Vis/NIR, computer vision | Honey pomelos | SSC, TA, and moisture content | Normalization, SG, MSC | PCA | LDA, SVM, GRNN | Moving average = 0.9950, classification sensitivity = 0.9750, classification specificity = 0.9934 | [134] |
| Computer vision | Citrus leaves | HLB | Threshold segmentation, connectivity analysis, morphology, fitted ellipse, affine transformed | GLCM, grayscale histogram | MLP, RF, LR | Reflection modes accuracy = 96.67%, transmission modes accuracy = 88.33% | [135] |
| Computer vision | Pomelo trees | Canker | - | - | CitrusNet, SVM | Accuracy = 92.33% | [28] |
| Computer vision | Citrus | Ripeness level, and Black Spot | Data augmentation, CAE | - | GoogleNet, ResNet18, ResNet50, ShuffleNet, MobileNetv2, DenseNet201 | Ripeness level accuracy = 99.5% and Black Spot disease F-measure = 100% | [29] |
| Computer vision | Oranges, bananas, and apples | Rottenness | Normalization, data augmentation | - | CNN, MobileNetV2 | Validation set accuracy = 99.61% | [30] |
| Computer vision | Citrus | Citrus disease defects | DCP, KF-2D-Renyi | Extract texture, edge, and shape features | ABC-SVM | Average recognition rate = 98.45% | [27] |
| Computer vision | Citrus | Disease | Normalization, image brightness adjustment, contrast enhancement | CNN | Accuracy = 89.1% | [103] | |
| Computer vision | Citrus | Common Citrus diseases | Noise filtering, data augmentation, image segmentation | ECN, DOA | DOA-ECN-DSSAE | Accuracy = 98.4% | [136] |
| SIRI | Four types of Citrus | Rot | Image demodulation | - | CNN | Overall classification accuracy = 90.6% | [137] |
| HSI | Sugar Belle leaves and immature fruit | Citrus canker in various disease stages | - | - | RBF, KNN | RBF: asymptomatic accuracy = 94%; early accuracy = 96%; late accuracy = 100% | [44] |
| HSI | Citrus | Citrus Black Spot | SG, spectral calibration | PLS analysis | KNN | Healthy samples: accuracy = 100%; early disease samples: accuracy = 93.8%; late disease samples: accuracy = 80.2% | [138] |
| HSI | Oranges | Rot | Correction, ROI, threshold segmentation | PCA | PLS-DA, BP-ANN | Overall classification accuracy = 96.6% | [139] |
| MSI | Citrus fruit trees | Healthy and HLB-infected trees | Image stitching, liner stretch | PCA, autoencoder | SVM, KNN, LR, Naive Bayes, AdaBoost, Neural Network | AdaBoost: accuracy = 100% | [53] |
| MSI | Navel orange | Rot | BEMD | PCA | Improved watershed segmentation | Rotten fruits: accuracy = 97.3%; healthy fruits: accuracy = 100% | [140] |
| MSI | Newhall navel orange | Rot | Image correction | BOSS, BOSS-SPA, PCA | PLS-DA | BOSS-PLS-DA: accuracy = 97.1%; BOSS-SPA-PLS-DA: accuracy = 100% | [141] |
| Vis/NIR | Thompson and Jaffa oranges | Black rot, pH, TA, and SSC | SG, MN, SNV, CFS | PCA | SVM, BPNN | Thompson accuracy = 93%, Jaffa accuracy = 97% | [69] |
| NIR | Citrus | Hidden mold infection | De-bias, detrend, 1D, 2D, CWT, MM, MSC, SNV | PCA | PCA-FLD, SIMCA, SVM, PLS-DA | Detection accuracy = 100% | [67] |
| Raman | Orange and grapefruit leaves | Health, nutritional deficiencies, early and late HLB infection | Baseline correction, data normalization | - | OPLS-DA | Grapefruit: detection rate = 98%; orange tree: detection rate = 87% | [79] |
| Raman | Mandarin | Carotenoids and corruption | Polynomial smoothing and filtering, poly baseline correction | PCA | LDA, KNN, SVM | A. alternate: Rp2 = 1.000; A. niger: Rp2 = 0.900; P. italicum: Rp2 = 0.800 | [55] |
| Fluorescence imaging, MSI | Navel orange | HLB | Correction, ROI | - | MobileNetV3 | Total accuracy = 96.5% | [142] |
| NIR, computer vision | Citrus | Citrus diseases | Data augmentation, normalization | - | Faster-CNN | Canker accuracy = 97%, Scab accuracy = 95%, Melanosis accuracy = 99%, HLB accuracy = 97%, Black Spot accuracy = 97%, healthy accuracy = 97% | [143] |
| NIR | Limone Costa d’Amalfi and Limone di Sorrento | Lemon equatorial diameter, peel thickness, juice yield, color; SSC, TA, pH, mineral content, and cation molar concentration | MSC, SNV, 1D, 2D | PCA, MLR, LDA | PCA, MLR, LDA | Distinguish between breeds and geographical origins | [144] |
| NIR | Different types of lemon juice | Adulteration | Mean centering, self-scaling processing | PCA | VIP-PLS-DA, CPANN | Accuracy = 96% | [145] |
| NMR | Sweet orange | 62 ingredients in sweet orange | FT, phase adjustment, baseline correction | PCA | PLS-DA, OPLS-DA | Accurate classification of sweet oranges of different geographical origins | [92] |
| NMR | Citrus juice from San Pedro and Entre Ríos, Argentina | TA, carbohydrate, and signal from the ethanol region | - | PCA | PCA, PLS-DA | Accuracy = 100% | [93] |
| NMR | Orange and other four kinds of pure juice | Relative percentage of pure juice | Noise reduction, baseline correction, and normalization | Non-targeted approach | PLS | Orange: R2P = 0.950, RMSEP = 4.435 | [94] |
| Detection Technology | Spectral Range | Detection Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Computer vision | 400–700 nm | External quality inspection. Citrus grading and classification. Citrus disease detection. Picking identification and positioning. | Simple operation, low-cost, fast, and wide application. | Data redundancy. Sensitive to external light. Image information depends on camera characteristics. Inability to detect internal quality. |
| HSI | 200–2500 nm | Sugar content, acidity, hardness, maturity, flavonoids, and other natural active substances. Detection of agricultural product quality and defects. Disease detection. | Capable of simultaneously collecting images and spectral features to detect internal chemical composition information. | Data redundancy. High equipment cost. |
| MSI | 400–1100 nm | Monitoring Citrus vegetation, water stress, and maturity. | Faster detection and lower equipment cost compared with HIS technology. | Low detection accuracy. Insufficient information for some specific tasks. |
| IR | 780–1,000,000 nm | The most commonly used spectral technology for detecting the internal components of Citrus, such as SSC and TA, maturity, grading, and damage. | Simple operation, low-cost, fast, and can detect multiple chemical components in fruits with wide usage. | Large spectral range and requires chemometric knowledge to analyze. |
| Raman | 0–4000 cm−1 | Flavonoids. Disease detection. | Fast detection and high sensitivity. | Susceptible to interference from factors such as fluorescence, sample moisture content, and temperature. Limited detection range and high equipment cost. |
| Fluorescence spectroscopy | 200–1000 nm | Fluorescent compounds. such as chlorophyll, flavonoids, carotenoids, acidity, and vitamin C. | Fast detection and high sensitivity. | Spectral analysis is complex. and the applicability of fluorescent groups is limited. |
| THz | 0.1–10 THz | Flavonoid detection. | Low energy, strong penetration. | High equipment cost. Spectral features are difficult to distinguish. |
| NMR | 1–900 MHz | Various ingredients of Citrus. Product traceability. | Fast, reproducible, and stable. High sensitivity. | Complicated operation and high sample processing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Zhong, M.; Zhu, W.; Rashid, A.; Han, R.; Virk, M.S.; Duan, K.; Zhao, Y.; Ren, X. Advances in Computer Vision and Spectroscopy Techniques for Non-Destructive Quality Assessment of Citrus Fruits: A Comprehensive Review. Foods 2025, 14, 386. https://doi.org/10.3390/foods14030386
Yu K, Zhong M, Zhu W, Rashid A, Han R, Virk MS, Duan K, Zhao Y, Ren X. Advances in Computer Vision and Spectroscopy Techniques for Non-Destructive Quality Assessment of Citrus Fruits: A Comprehensive Review. Foods. 2025; 14(3):386. https://doi.org/10.3390/foods14030386
Chicago/Turabian StyleYu, Kai, Mingming Zhong, Wenjing Zhu, Arif Rashid, Rongwei Han, Muhammad Safiullah Virk, Kaiwen Duan, Yongjun Zhao, and Xiaofeng Ren. 2025. "Advances in Computer Vision and Spectroscopy Techniques for Non-Destructive Quality Assessment of Citrus Fruits: A Comprehensive Review" Foods 14, no. 3: 386. https://doi.org/10.3390/foods14030386
APA StyleYu, K., Zhong, M., Zhu, W., Rashid, A., Han, R., Virk, M. S., Duan, K., Zhao, Y., & Ren, X. (2025). Advances in Computer Vision and Spectroscopy Techniques for Non-Destructive Quality Assessment of Citrus Fruits: A Comprehensive Review. Foods, 14(3), 386. https://doi.org/10.3390/foods14030386








