Impact of Five Soy Proteins on Lean Chicken Breast Systems with Varying Moisture Contents: Cooking Loss, Texture, Microstructure, and T2 NMR
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
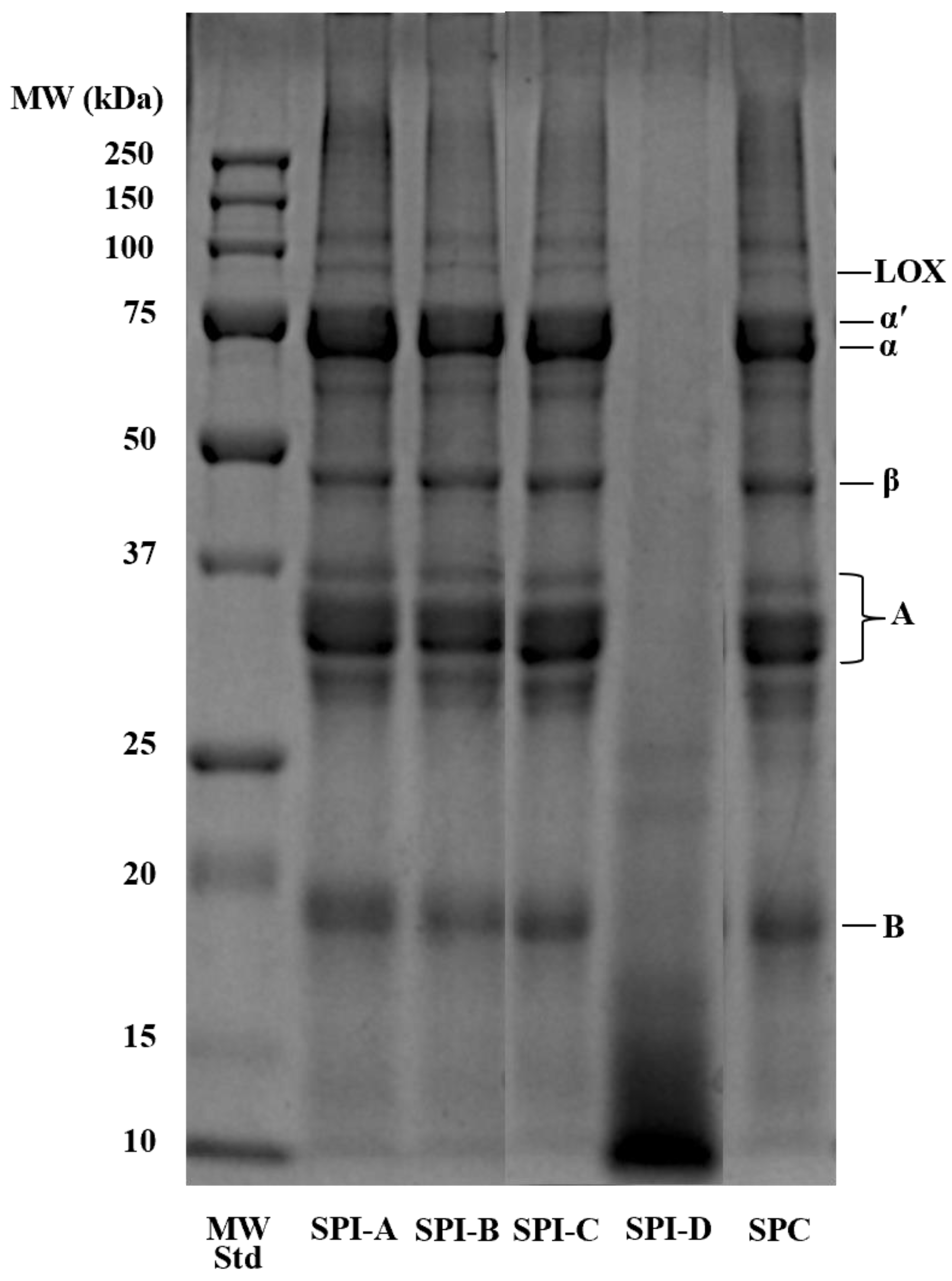
2.2. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.3. Soy Protein Hydration and Hybrid Meat Batter Production
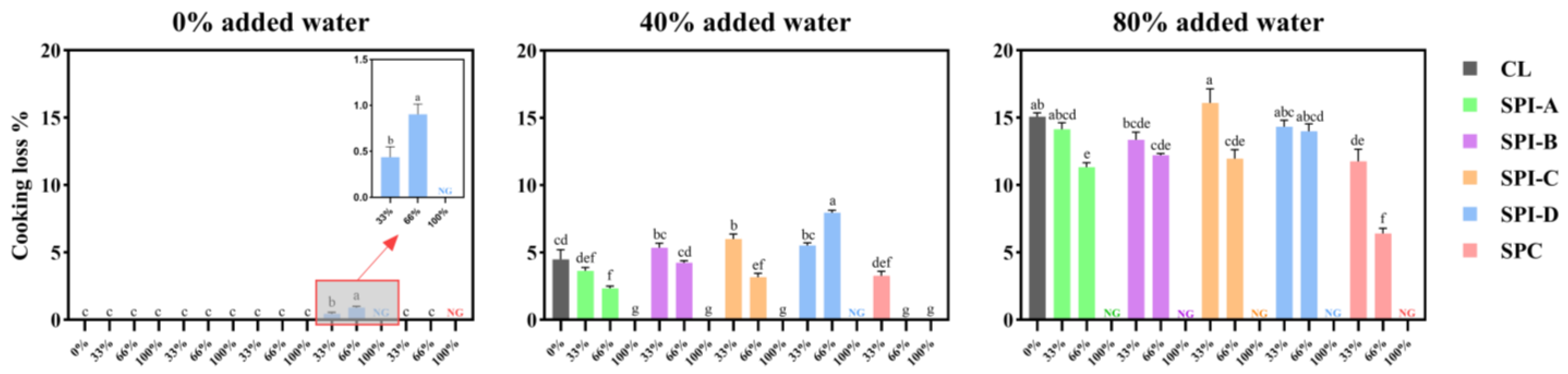
2.4. Cooking and Cooking Loss
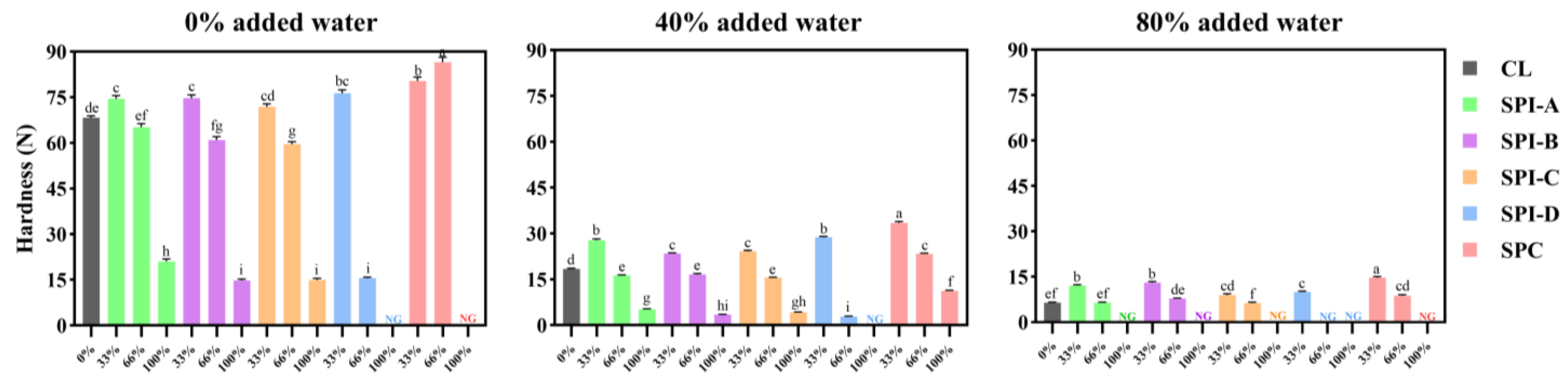
2.5. Texture Profile Analysis
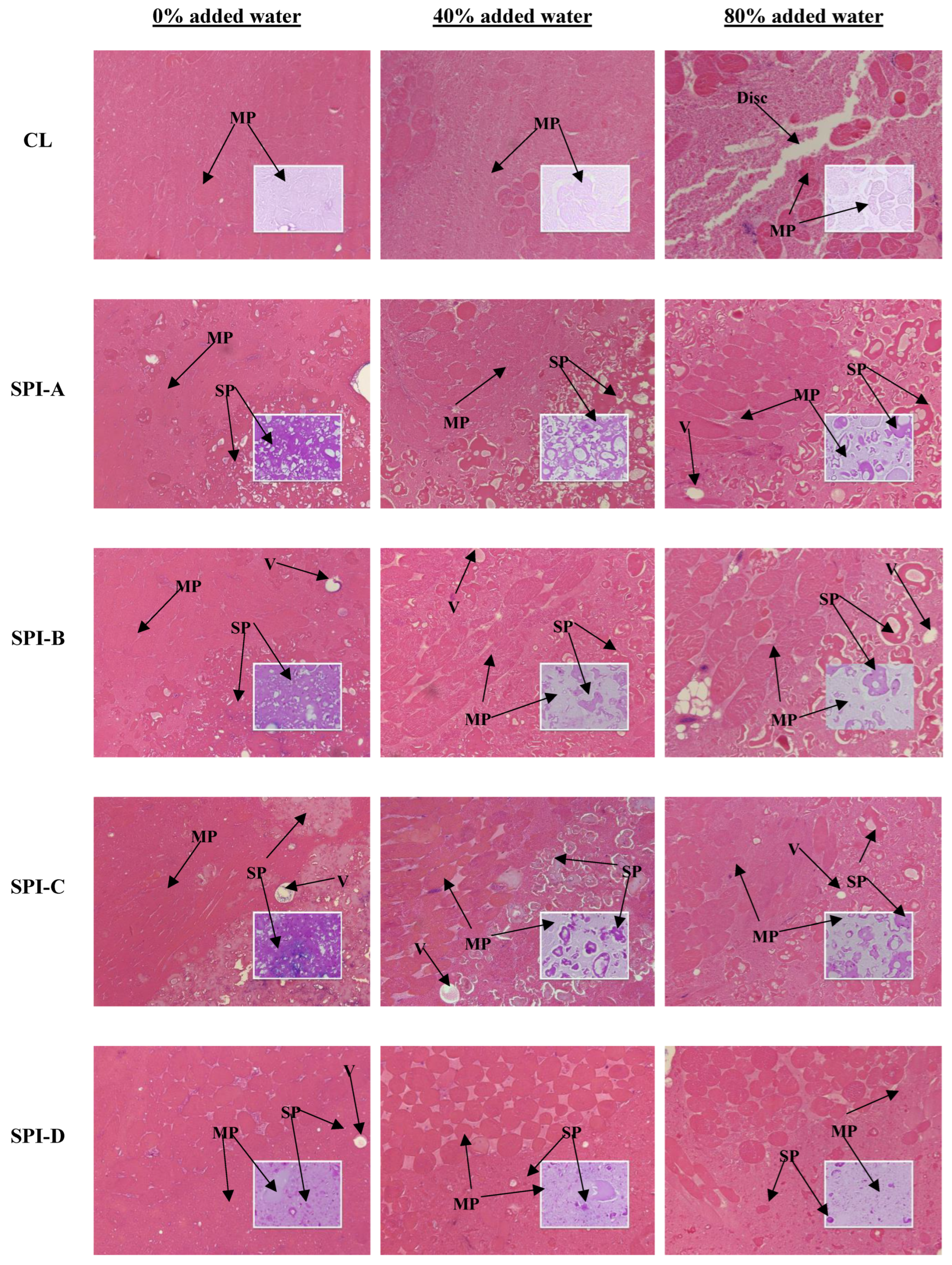
2.6. Light Microscopy
2.7. Pulsed NMR T2 Relaxometry
2.8. Color Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. SDS PAGE
3.2. Cooking Loss
3.3. Texture Profile Analysis
3.3.1. Hardness
3.3.2. Other Texture Parameters
3.4. Light Microscopy
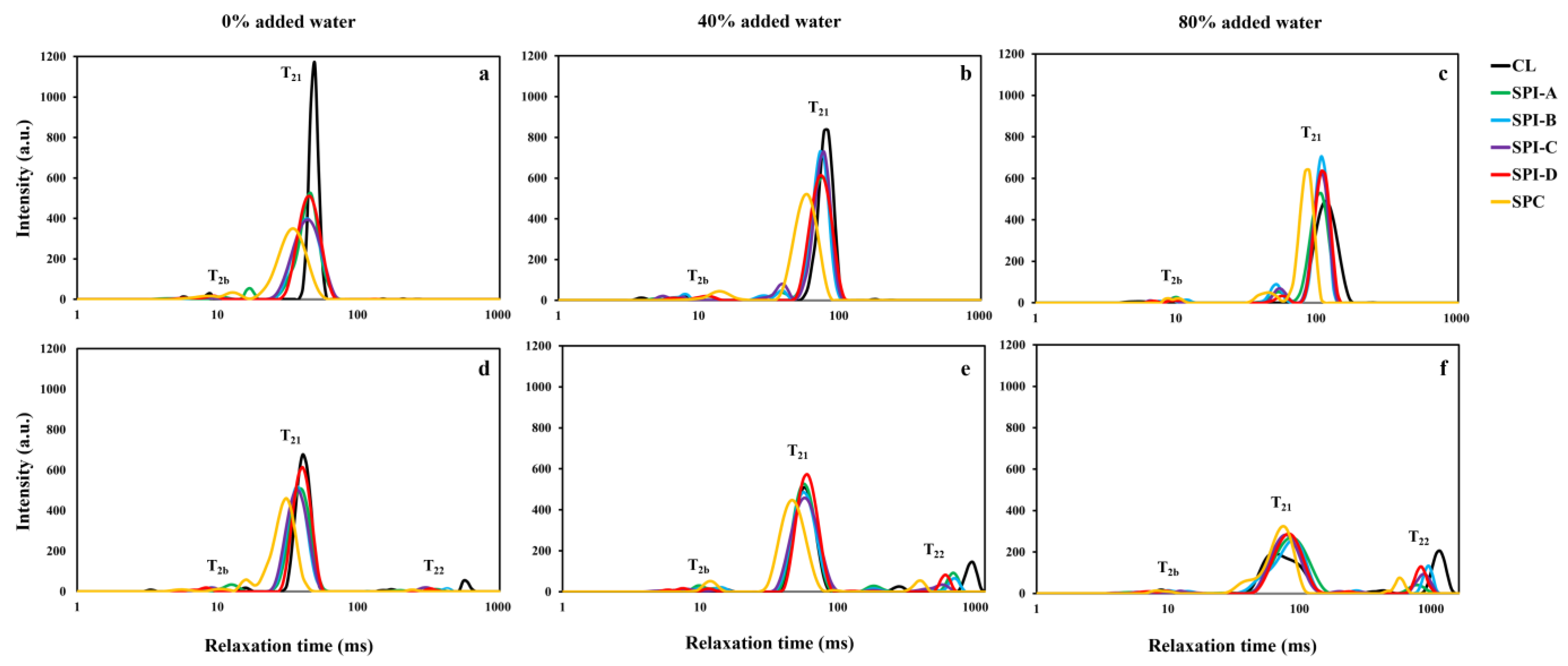
3.5. NMR T2 Relaxometry
3.6. Color Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs Population Division. World Population Prospects 2024, Online Edition; United Nations: New York, NY, USA, 2024. [Google Scholar]
- Bonnet, C.; Coinon, M. Environmental co-benefits of health policies to reduce meat consumption: A narrative review. Health Policy 2024, 143, 105017. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2024–2033; OECD Publishing: Paris, France, 2024. [Google Scholar]
- Samad, A.; Alam, A.N.; Kumari, S.; Hossain, M.J.; Lee, E.Y.; Hwang, Y.H.; Joo, S.T. Modern concepts of restructured meat production and market opportunities. Food Sci. Anim. Resour. 2024, 44, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. Principles of Meat Processing. In The Science of Poultry and Meat Processing; University of Guelph: Guelph, ON, Canada, 2015. [Google Scholar]
- Judge, M.D.; Haugh, C.G.; Zachariah, G.L.; Parmelee, C.E.; Pyle, R.L. Soya additives in beef patties. J. Food Sci. 1974, 39, 137–139. [Google Scholar] [CrossRef]
- Odiase, O.M.; Igene, J.O.; Evivie, S.E.; Ebabhamiegbebho, P.A. Determination and sensory evaluation of soy flour-meat combinations in the production of meatballs. J. Appl. Nat. Sci. 2013, 5, 482–487. [Google Scholar] [CrossRef]
- Berry, B.W.; Leddy, K.F.; Bodwell, C.E. Sensory characteristics, shear values and cooking properties of ground beef patties extended with iron- and zinc-fortified soy isolate, concentrate or flour. J. Food Sci. 1985, 50, 1556–1559. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Cofrades, S.; Jiménez-Colmenero, F. Raman spectroscopic determination of structural changes in meat batters upon soy protein addition and heat treatment. Food Res. Int. 2008, 41, 765–772. [Google Scholar] [CrossRef]
- Ensor, S.A.; Mandigo, R.W.; Calkins, C.R.; Quint, L.N. Comparative evaluation of whey protein concentrate, soy protein isolate and calcium-reduced nonfat dry milk as binders in an emulsion-type sausage. J. Food Sci. 1987, 52, 1155–1158. [Google Scholar] [CrossRef]
- Akesowan, A. Quality characteristics of light pork burgers fortified with soy protein isolate. Food Sci. Biotechnol. 2010, 19, 1143–1149. [Google Scholar] [CrossRef]
- Danowska-Oziewicz, M. Effect of soy protein isolate on physicochemical properties, lipid oxidation and sensory quality of low-fat pork patties stored in vacuum, MAP and frozen state. J. Food Process. Preserv. 2014, 38, 641–654. [Google Scholar] [CrossRef]
- Youssef, M.K.; Barbut, S. Effects of two types of soy protein isolates, native and preheated whey protein isolates on emulsified meat batters prepared at different protein levels. Meat Sci. 2011, 87, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Suwal, S.; Britten, M.; Pouliot, Y.; Doyen, A. The use of high hydrostatic pressure to modulate milk protein interactions for the production of an alpha-lactalbumin enriched-fraction. Green Chem. 2018, 20, 515–524. [Google Scholar] [CrossRef]
- Lin, W.; Barbut, S. Hybrid meat batter system: Effects of plant proteins (pea, brown rice, faba bean) and concentrations (3–12%) on texture, microstructure, rheology, water binding, and color. Poult. Sci. 2024, 103, 103822. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 32, 62–68. [Google Scholar]
- Youssef, M.K.; Barbut, S. Effects of protein level and fat/oil on emulsion stability, texture, microstructure and color of meat batters. Meat Sci. 2009, 82, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, J.; Sun, L.; Weng, Z.; Fang, Y.; Tang, X.; Zhao, T.; Shen, X. Study on the tofu quality evaluation method and the establishment of a model for suitable soybean varieties for Chinese traditional tofu processing. LWT 2020, 117, 108441. [Google Scholar] [CrossRef]
- Zheng, L.; Regenstein, J.M.; Zhou, L.; Wang, Z. Soy protein isolates: A review of their composition, aggregation, and gelation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1940–1957. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. Research note: Producing lean poultry meat composite gels with different carbohydrate-based breadcrumbs. Poult. Sci. 2023, 102, 102914. [Google Scholar] [CrossRef] [PubMed]
- Bertram, H.C.; Purslow, P.P.; Andersen, H.J. Relationship between meat structure, water mobility, and distribution: A low-field nuclear magnetic resonance study. J. Agric. Food Chem. 2002, 50, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.d.; Rocha, D.A.V.F.d.; Bernardinelli, O.D.; Oliveira Júnior, F.D.; de Sousa, D.G.; Sabadini, E.; da Cunha, R.L.; Trindade, M.A.; Pollonio, M.A.R. Understanding the performance of plant protein concentrates as partial meat substitutes in hybrid meat emulsions. Foods 2022, 11, 3311. [Google Scholar] [CrossRef] [PubMed]

| 0% Added Water | 40% Added Water | 80% Added Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Replacement (%) | L* | a* | b* | L* | a* | b* | L* | a* | b* |
| CL | 0 | 77.9 ± 0.38 a | 2.1 ± 0.08 bc | 12.8 ± 0.10 c | 78.2 ± 0.11 a | 1.0 ± 0.03 ab | 11.4 ± 0.15 cd | 78.3 ± 0.26 a | 0.5 ± 0.03 a | 10.3 ± 0.13 bc |
| SPI-A | 33 | 69.1 ± 0.25 g | 1.7 ± 0.11 d | 10.5 ± 0.21 ef | 72.5 ± 0.13 e | 0.2 ± 0.08 de | 10.4 ± 0.09 ef | 73.2 ± 0.19 d | −0.2 ± 0.10 bc | 9.9 ± 0.17 cd |
| 66 | 62.8 ± 0.15 i | −0.2 ± 0.07 h | 10.3 ± 0.19 fg | 65.9 ± 0.21 g | −0.9 ± 0.04 f | 10.2 ± 0.09 fg | 65.6 ± 0.17 f | −1.3 ± 0.04 d | 9.7 ± 0.11 cde | |
| 100 | 55.1 ± 0.24 k | 0.5 ± 0.05 g | 10.9 ± 0.23 e | 56.9 ± 0.21 i | −0.7 ± 0.02 f | 8.5 ± 0.06 h | NG | NG | NG | |
| SPI-B | 33 | 73.3 ± 0.40 bc | 1.8 ± 0.03 cd | 10.9 ± 0.11 e | 75.5 ± 0.14 b | 0.8 ± 0.09 bc | 10.0 ± 0.13 fg | 75.7 ± 0.19 b | −0.1 ± 0.09 b | 9.2 ± 0.15 ef |
| 66 | 71.3 ± 0.29 ef | 0.0 ± 0.05 h | 10.6 ± 0.08 ef | 72.6 ± 0.21 e | −0.6 ± 0.04 f | 9.7 ± 0.14 g | 72.4 ± 0.15 d | −0.9 ± 0.06 d | 8.8 ± 0.12 f | |
| 100 | 67.0 ± 0.12 h | −0.3 ± 0.05 h | 9.8 ± 0.09 g | 69.4 ± 0.15 f | −0.6 ± 0.02 f | 8.8 ± 0.13 h | NG | NG | NG | |
| SPI-C | 33 | 72.0 ± 0.27 de | 0.9 ± 0.09 f | 10.8 ± 0.06 ef | 74.2 ± 0.22 cd | 0.0 ± 0.13 e | 9.9 ± 0.11 fg | 74.6 ± 0.13 c | −0.6 ± 0.14 c | 9.4 ± 0.17 def |
| 66 | 66.8 ± 0.28 h | −1.2 ± 0.11 i | 9.6 ± 0.09 gh | 69.2 ± 0.23 f | −1.8 ± 0.12 g | 8.8 ± 0.10 h | 69.3 ± 0.35 e | −2.1 ± 0.06 e | 8.0 ± 0.14 g | |
| 100 | 60.6 ± 0.19 j | −1.5 ± 0.02 i | 9.1 ± 0.20 h | 62.5 ± 0.11 h | −2.5 ± 0.06 h | 6.8 ± 0.11 i | NG | NG | NG | |
| SPI-D | 33 | 74.2 ± 0.11 b | 2.7 ± 0.09 a | 11.8 ± 0.09 d | 75.7 ± 0.27 b | 1.3 ± 0.10 a | 10.9 ± 0.11 de | 76.5 ± 0.22 b | 0.5 ± 0.13 a | 10.7 ± 0.10 b |
| 66 | 73.4 ± 0.18 bc | 1.3 ± 0.06 e | 14.4 ± 0.10 b | 74.8 ± 0.18 bc | 0.6 ± 0.06 cd | 12.8 ± 0.13 b | NG | NG | NG | |
| 100 | NG | NG | NG | NG | NG | NG | NG | NG | NG | |
| SPC | 33 | 73.0 ± 0.19 cd | 2.3 ± 0.04 b | 12.4 ± 0.09 cd | 75.6 ± 0.20 b | 1.0 ± 0.06 ab | 11.8 ± 0.11 c | 75.8 ± 0.17 b | 0.4 ± 0.05 a | 10.8 ± 0.19 b |
| 66 | 70.7 ± 0.18 f | 1.6 ± 0.06 de | 15.4 ± 0.10 a | 73.4 ± 0.20 de | 0.4 ± 0.05 d | 13.3 ± 0.23 b | 72.6 ± 0.13 d | −0.1 ± 0.04 b | 12.1 ± 0.11 a | |
| 100 | NG | NG | NG | 70.0 ± 0.10 f | 0.9 ± 0.04 bc | 14.7 ± 0.08 a | NG | NG | NG | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Barbut, S. Impact of Five Soy Proteins on Lean Chicken Breast Systems with Varying Moisture Contents: Cooking Loss, Texture, Microstructure, and T2 NMR. Foods 2025, 14, 427. https://doi.org/10.3390/foods14030427
Lin W, Barbut S. Impact of Five Soy Proteins on Lean Chicken Breast Systems with Varying Moisture Contents: Cooking Loss, Texture, Microstructure, and T2 NMR. Foods. 2025; 14(3):427. https://doi.org/10.3390/foods14030427
Chicago/Turabian StyleLin, Weilun, and Shai Barbut. 2025. "Impact of Five Soy Proteins on Lean Chicken Breast Systems with Varying Moisture Contents: Cooking Loss, Texture, Microstructure, and T2 NMR" Foods 14, no. 3: 427. https://doi.org/10.3390/foods14030427
APA StyleLin, W., & Barbut, S. (2025). Impact of Five Soy Proteins on Lean Chicken Breast Systems with Varying Moisture Contents: Cooking Loss, Texture, Microstructure, and T2 NMR. Foods, 14(3), 427. https://doi.org/10.3390/foods14030427





