From Traditional Amazon Use to Food Applications: Tapirira guianensis Seed Extracts as a Triad of Antiproliferative Effect, Oxidative Defense, and Antimalarial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Cell Lines
2.2. Plant Source and Ultrasound-Assisted Solvent Extraction
2.3. Chemical Profile and Antioxidant Capacity
2.3.1. Phenolic Compounds
2.3.2. Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QToF/MS) Analyses
2.3.3. Nuclear Magnetic Resonance (NMR) Analysis
2.3.4. Chemical Antioxidant Capacity
2.3.5. Lipid Peroxidation Assay
2.4. Cell Culture: Cytotoxicity and Antioxidant Activity
2.4.1. Cell Viability Assessment
2.4.2. Intracellular Reactive Oxygen Species (ROS) Generation
2.4.3. Protection Against Chromosomal Aberration
2.4.4. Erythrocyte Cellular Antioxidant Activity and Protection
2.5. In Vitro Antimalarial Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Profile
3.2. Chemical Antioxidant Capacity of T. guianensis Seed Extracts
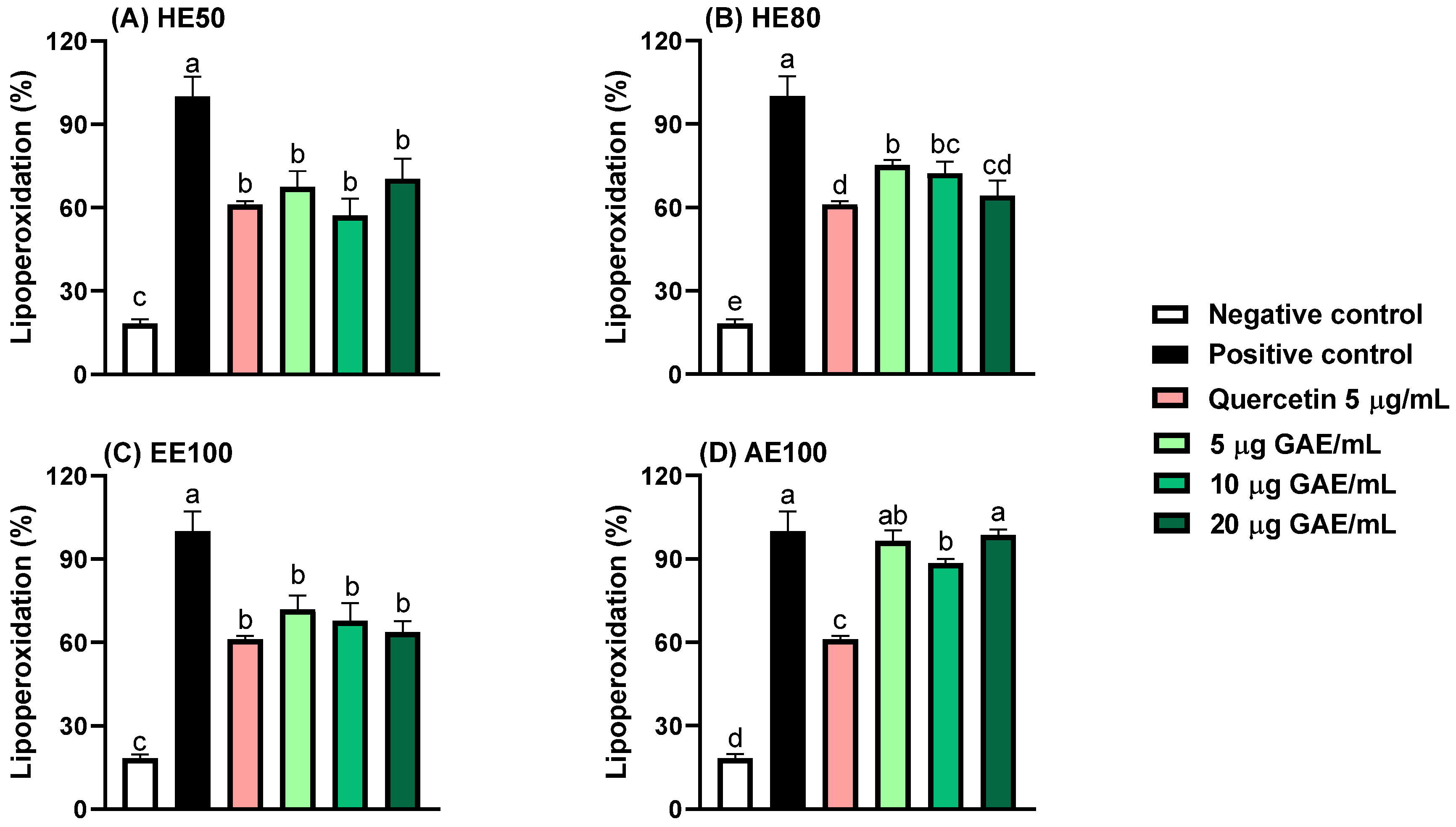
3.3. Protection Against Lipid Peroxidation
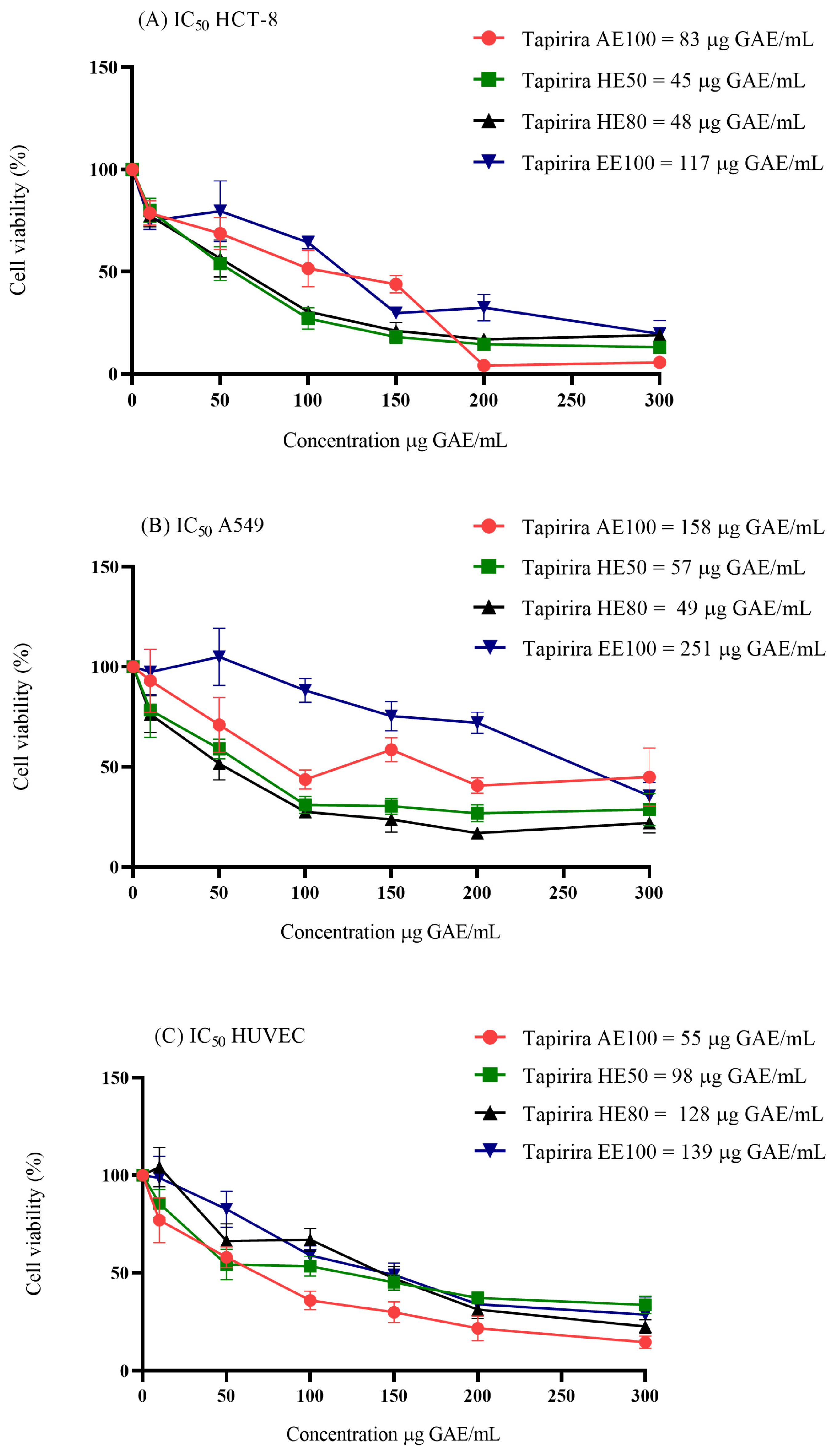
3.4. Cytotoxicity in Cell Culture
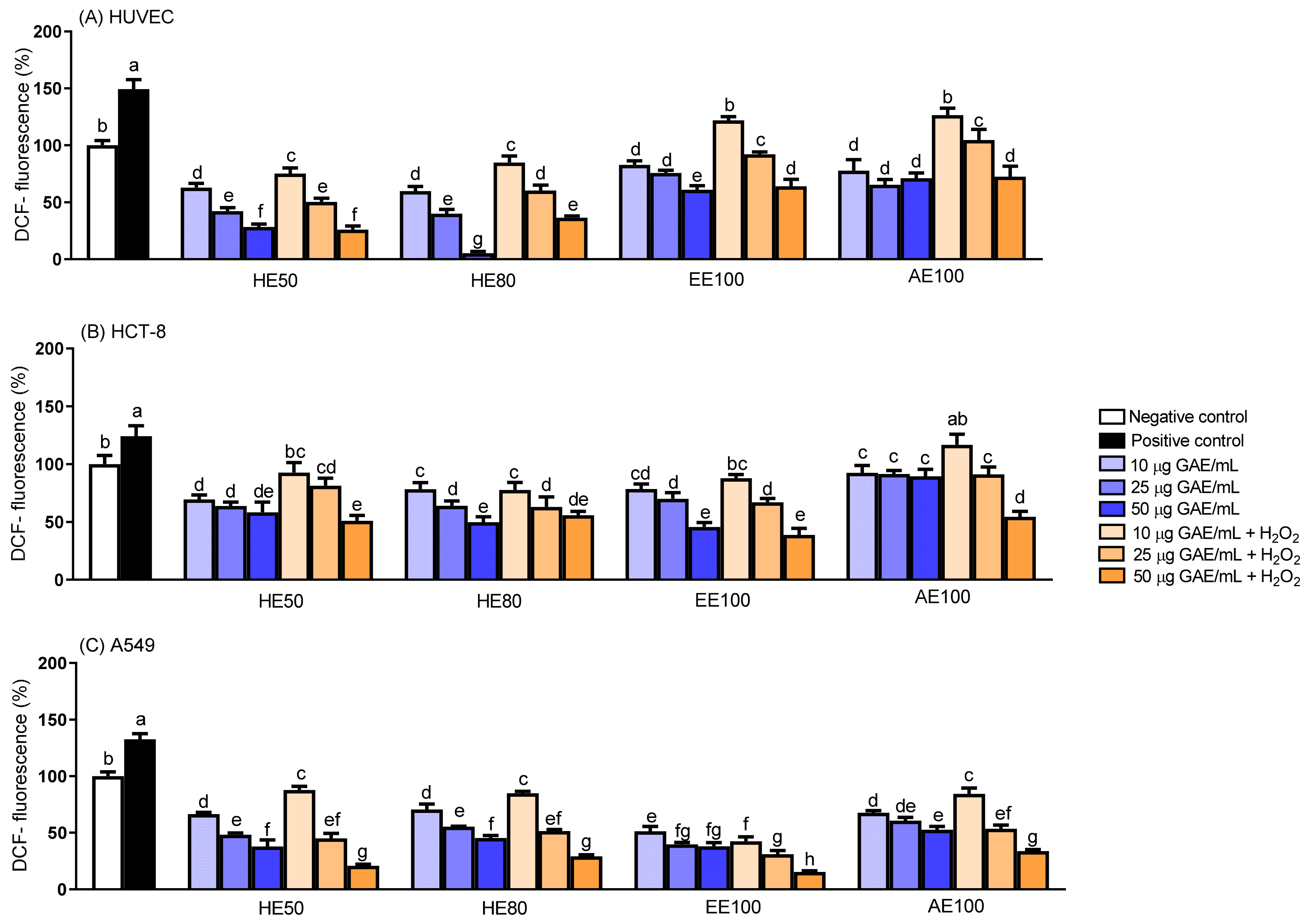
3.5. Intracellular Generation of Reactive Oxygen Species (ROS)
3.6. Chromosomal Aberrations
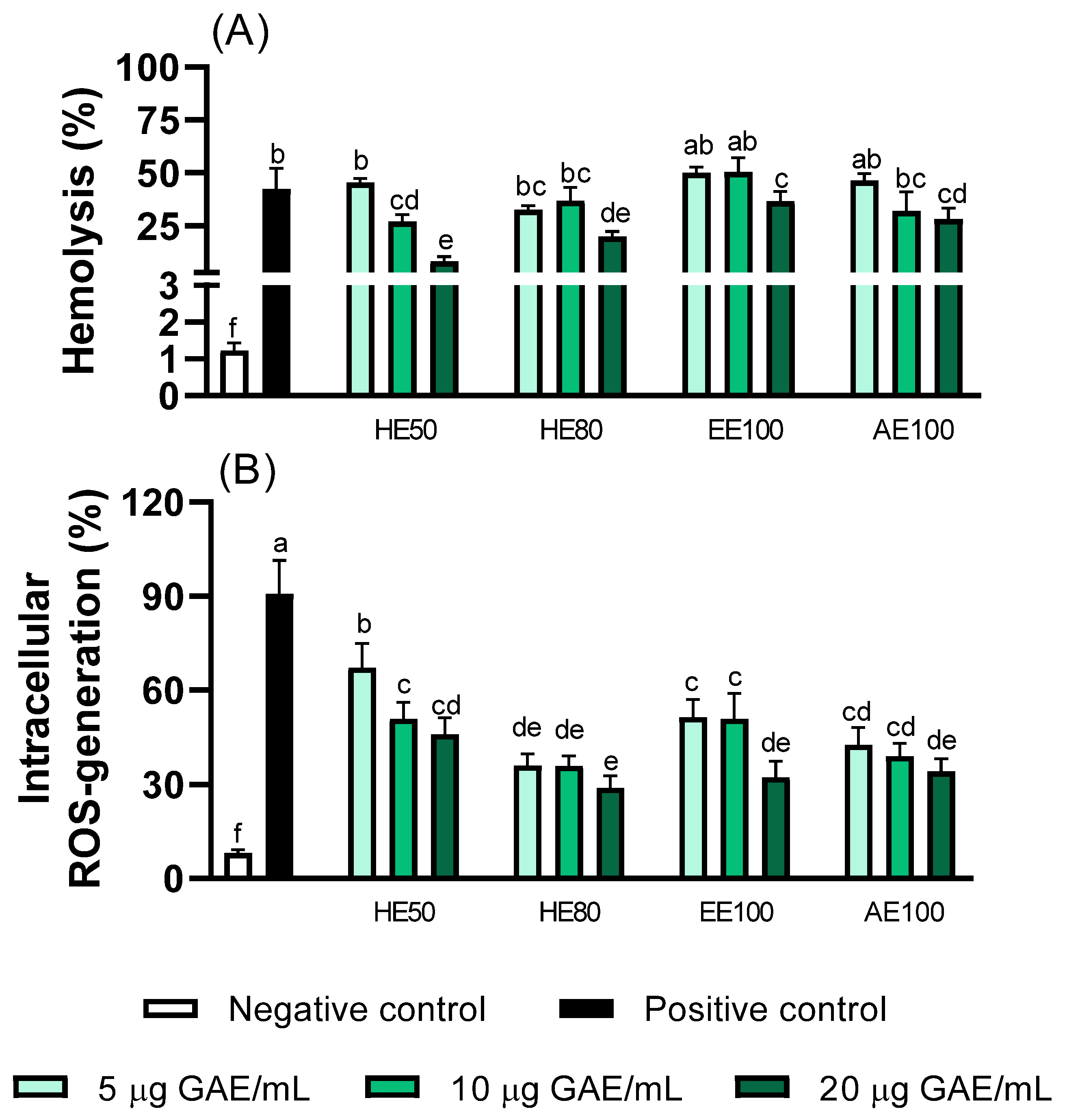
3.7. Erythrocyte Protection Effects of T. guianensis Extracts
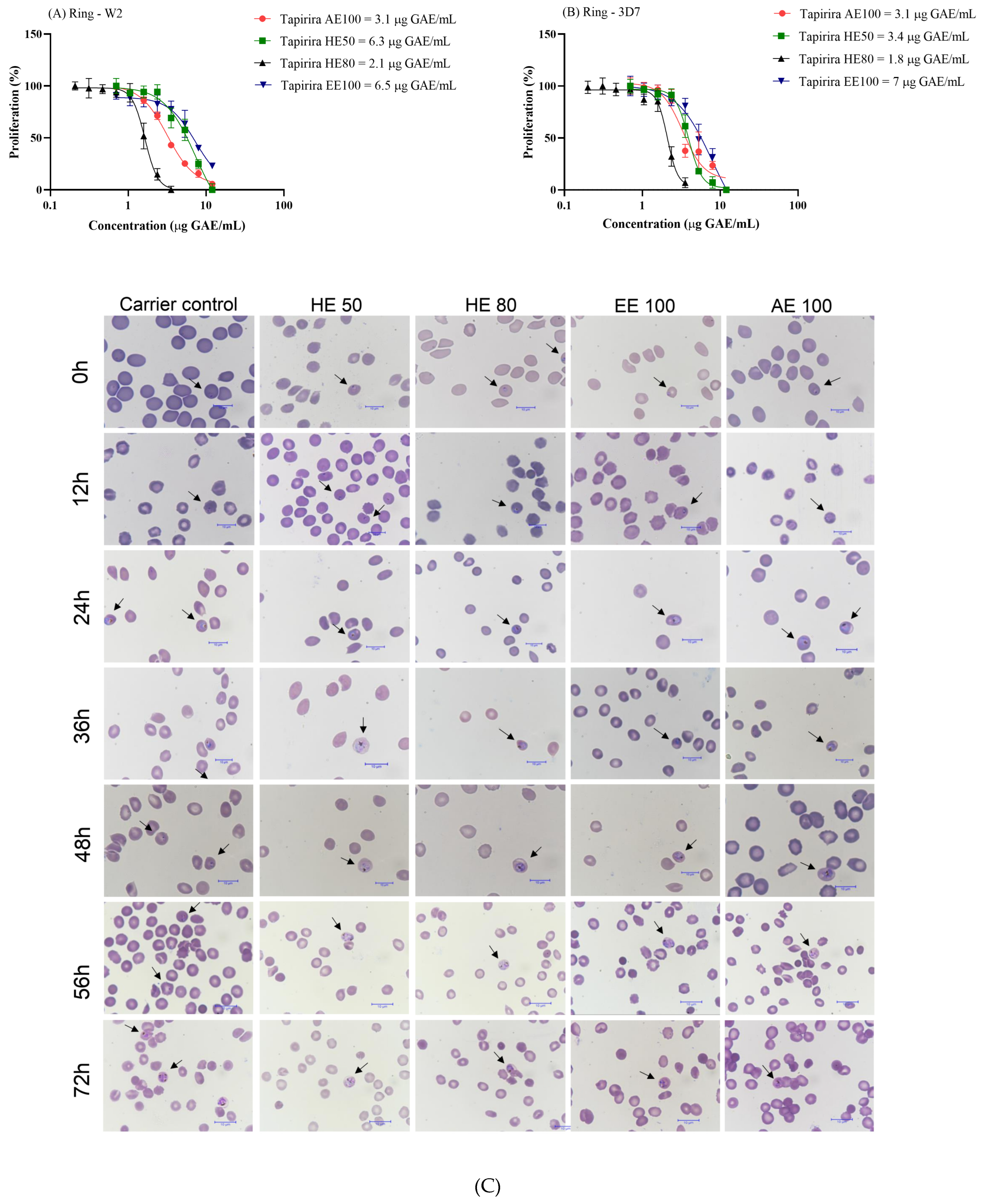
3.8. T. guianensis Extracts Present Antimalarial Activity Against P. falciparum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, E.; David, J.; David, J.P.; Garcia, G.; Silva, M. Chemical composition of biological active extracts of Tapirira guianensis (Anacardiaceae). Química Nova 2020, 43, 1216–1219. [Google Scholar] [CrossRef]
- Mar, J.M.; Corrêa, R.F.; Ramos, A.d.S.; Kinupp, V.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.d.A. Enhancing Bioactive Compound Bioaccessibility in Tapirira guianensis Juices through Ultrasound-Assisted Applications. Processes 2023, 11, 2718. [Google Scholar] [CrossRef]
- Patient, A.; Jean-Marie, E.; Robinson, J.C.; Martial, K.; Meudec, E.; Levalois-Grützmacher, J.; Closs, B.; Bereau, D. Polyphenol Composition and Antioxidant Activity of Tapirira guianensis Aubl. (Anacardiaceae) Leaves. Plants 2022, 11, 326. [Google Scholar] [CrossRef]
- Roumy, V.; Fabre, N.; Portet, B.; Bourdy, G.; Acebey, L.; Vigor, C.; Valentin, A.; Moulis, C. Four anti-protozoal and anti-bacterial compounds from Tapirira guianensis. Phytochemistry 2009, 70, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- World Health Organization. The Global Burden of Disease: 2020 Update; World Health Organization: Geneva, Switzerland, 2022.
- World Health Organization. World Malaria Report. 2024: Addressing Inequity in the Global Malaria Response; WHO: Geneva, Switzerland, 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 12 December 2024).
- Rosenthal, P.J. The interplay between drug resistance and fitness in malaria parasites. Mol. Microbiol. 2013, 89, 1025–1038. [Google Scholar] [CrossRef]
- Haldar, K.; Bhattacharjee, S.; Safeukui, I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 2018, 16, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, M.; Granato, D.; Azevedo, L. Antioxidant/pro-oxidant and antiproliferative activities of phenolic-rich foods and extracts: A cell-based point of view. In Advances in Food and Nutrition Research, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 1–28. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem 2018, 48, 538–549. [Google Scholar] [CrossRef]
- dos Santos Lima, A.; de Oliveira Pedreira, F.R.; Bento, N.A.; Novaes, R.D.; Dos Santos, E.G.; de Almeida Lima, G.D.; de Almeida, L.A.; Belo, T.C.A.; Vieira, F.V.; Mohammadi, N.; et al. Digested galactoglucomannan mitigates oxidative stress in human cells, restores gut bacterial diversity, and provides chemopreventive protection against colon cancer in rats. Int. J. Biol. Macromol. 2024, 277, 133986. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, M.A.V.; Fidelis, M.; Sanchez, C.A.; Castro, A.P.; Camps, I.; Colombo, F.A.; Marques, M.J.; Myoda, T.; Granato, D.; Azevedo, L. Camu-camu (Myrciaria dubia) seeds as a novel source of bioactive compounds with promising antimalarial and antischistosomicidal properties. Food Res. Int. 2020, 136, 109334. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Reshamwala, D.; Korpinen, R.; Azevedo, L.; Carmo, M.A.V.D.; Cruz, T.M.; Marques, M.B.; Wen, M.; Zhang, L.; Marjomäki, V.; et al. From the forest to the plate—Hemicelluloses, galactoglucomannan, glucuronoxylan, and phenolic-rich extracts from unconventional sources as functional food ingredients. Food Chem. 2022, 381, 132284. [Google Scholar] [CrossRef]
- de Athayde, A.E.; de Araujo, C.E.S.; Sandjo, L.P.; Biavatti, M.W. Metabolomic analysis among ten traditional “Arnica” (Asteraceae) from Brazil. J. Ethnopharmacol. 2021, 265, 113149. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wu, H.; Zhou, A. Rapid identification of absorbed components and metabolites of Gandou decoction in rat plasma and liver by UPLC-Q-TOF-MSE. J. Chromatogr. B 2020, 1137, 121934. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.; Dos Santos Lima, A.; Azevedo, L.; Granato, D. Bridging the gap in antioxidant activity of flavonoids: Correlating the oxidation of human plasma with chemical and cellular assays. Curr. Res. Food Sci. 2024, 8, 100714. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Mi, Y.; Cao, H.; Chen, L. Enzymatic acylation of raspberry anthocyanin: Evaluations on its stability and oxidative stress prevention. Food Chem. 2022, 372, 130766. [Google Scholar] [CrossRef]
- Fidelis, M.; Carmo, M.A.V.D.; Azevedo, L.; Cruz, T.M.; Marques, M.B.; Myoda, T.; Sant’ana, A.S.; Furtado, M.M.; Wen, M.; Zhang, L.; et al. Response surface optimization of phenolic compounds from jabuticaba (Myrciaria cauliflora [Mart.] O.Berg) seeds: Antioxidant, antimicrobial, antihyperglycemic, antihypertensive and cytotoxic assessments. Food Chem. Toxicol. 2020, 142, 111439. [Google Scholar] [CrossRef]
- Do Carmo, M.A.V.; Fidelis, M.; Pressete, C.G.; Marques, M.J.; Castro-Gamero, A.M.; Myoda, T.; Granato, D.; Azevedo, L. Hydroalcoholic Myrciaria dubia (camu-camu) seed extracts prevent chromosome damage and act as antioxidant and cytotoxic agents. Food Res. Int. 2019, 125, 108551. [Google Scholar] [CrossRef]
- Cruz, T.M.; Lima, A.d.S.; Silva, A.O.; Mohammadi, N.; Zhang, L.; Azevedo, L.; Marques, M.B.; Granato, D. High-throughput synchronous erythrocyte cellular antioxidant activity and protection screening of phenolic-rich extracts: Protocol validation and applications. Food Chem. 2024, 440, 138281. [Google Scholar] [CrossRef] [PubMed]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418. [Google Scholar] [CrossRef] [PubMed]
- Alves, U.V.; Jardim e Silva, E.; dos Santos, J.G.; Santos, L.O.; Lanna, E.; de Souza Pinto, A.C.; da Fonseca, A.L.; Varotti, F.d.P.; Batista, R. Potent and selective antiplasmodial activity of marine sponges from Bahia state, Brazil. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound extraction conditions effect on antioxidant capacity of mango by-product extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Cruz, T.M.; Santos, J.S.; do Carmo, M.A.V.; Hellström, J.; Pihlava, J.-M.; Azevedo, L.; Granato, D.; Marques, M.B. Extraction optimization of bioactive compounds from ora-pro-nobis (Pereskia aculeata Miller) leaves and their in vitro antioxidant and antihemolytic activities. Food Chem. 2021, 361, 130078. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Pereira, Z.C.; Cruz, J.M.d.A.; Castro, D.R.G.; Ramos, A.S.; Rocha, D.d.Q.; Arruda, H.S.; Borsoi, F.T.; Neri-Numa, I.A.; Pastore, G.M.; Sanches, E.A.; et al. Passiflora nitida Kunth fruit: Chemical analysis, antioxidant capacity, and cytotoxicity. Food Sci. Technol. 2024, 44, 1–8. [Google Scholar] [CrossRef]
- Oliveira, E.S.C.; Pontes, F.L.D.; Acho, L.D.R.; da Silva, B.J.P.; Rosário, A.S.D.; Chaves, F.C.M.; Campos, F.R.; Bezerra, J.d.A.; Lima, E.S.; Machado, M.B. NMR and multivariate methods: Identification of chemical markers in extracts of pedra-ume-caá and their antiglycation, antioxidant, and enzymatic inhibition activities. Phytochem. Anal. 2024, 35, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.d.J.; DavidI, J.M.; da Silva, E.P.; David, J.P.; Lopes, L.M.X.; Guedes, M.L.S. Flavonóides, norisoprenóides e outros terpenos das folhas de Tapirira guianensis. Química Nova 2008, 31, 2056–2059. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Zhou, X.; Fu, J.; He, C.-Y.; Feng, R.; Huang, M.; Shou, J.-W.; Zhao, Z.-X.; Li, X.-Y.; Zhang, L.; et al. In Vivo Metabolite Profiling of a Purified Ellagitannin Isolated from Polygonum capitatum in Rats. Molecules 2016, 21, 1110. [Google Scholar] [CrossRef]
- Dubrow, G.A.; Tello, E.; Schwartz, E.; Forero, D.P.; Peterson, D.G. Identification of non-volatile compounds that impact consumer liking of strawberry preserves: Untargeted LC–MS analysis. Food Chem. 2022, 378, 132042. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.S.C.; Pontes, F.L.D.; Acho, L.D.R.; do Rosário, A.S.; da Silva, B.J.P.; de, A. Bezerra, J.; Campos, F.R.; Lima, E.S.; Machado, M.B. qNMR quantification of phenolic compounds in dry extract of Myrcia multiflora leaves and its antioxidant, anti-AGE, and enzymatic inhibition activities. J. Pharm. Biomed. Anal. 2021, 201, 114109. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Taylor, P.G.; Cesari, I.M.; Arsenak, M.; Ballen, D.; Abad, M.J.; Fernández, A.; Milano, B.; Ruiz, M.-C.; Williams, B.; Michelangeli, F. Evaluation of Venezuelan Medicinal Plant Extracts for Antitumor and Antiprotease Activities. Pharm. Biol. 2006, 44, 349–362. [Google Scholar] [CrossRef]
- Silva-Oliveira, R.J.; Lopes, G.F.; Camargos, L.F.; Ribeiro, A.M.; dos Santos, F.V.; Severino, R.P.; Severino, V.G.P.; Terezan, A.P.; Thomé, R.G.; dos Santos, H.B.; et al. Tapirira guianensis Aubl. Extracts Inhibit Proliferation and Migration of Oral Cancer Cells Lines. Int. J. Mol. Sci. 2016, 17, 1839. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.N.; Stavis, V.K.; Boaretto, A.G.; Castro, D.T.H.; Alves, F.M.; Souza, K.d.P.; dos Santos, E.L.; Silva, D.B.; Carollo, C.A. Application of the metabolomics approach to the discovery of active compounds from Brazilian trees against resistant human melanoma cells. Phytochem. Anal. 2021, 32, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; Al-Qubaisi, M.; Rasedee, A.; Hussein, M.Z. Evaluation of the Cytotoxic Effect of Ellagic Acid Nanocomposite in Lung Cancer A549 Cell Line and RAW 264.9 Cells. J. Bionanosci. 2017, 11, 578–583. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, X.; Niu, C.; Wang, X. Ellagic acid promotes A549 cell apoptosis via regulating the phosphoinositide 3-kinase/protein kinase B pathway. Exp. Ther. Med. 2018, 16, 347–352. [Google Scholar] [CrossRef]
- Ko, E.-B.; Jang, Y.-G.; Kim, C.-W.; Go, R.-E.; Lee, H.K.; Choi, K.-C. Gallic Acid Hindered Lung Cancer Progression by Inducing Cell Cycle Arrest and Apoptosis in A549 Lung Cancer Cells via PI3K/Akt Pathway. Biomol. Ther. 2022, 30, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Tsantila, E.M.; Esslinger, N.; Christou, M.; Papageorgis, P.; Neophytou, C.M. Antioxidant and Anticancer Activity of Vitis vinifera Extracts in Breast Cell Lines. Life 2024, 14, 228. [Google Scholar] [CrossRef]
- Ghose, K.; Selvaraj, K.; McCallum, J.; Kirby, C.W.; Sweeney-Nixon, M.; Cloutier, S.J.; Deyholos, M.; Datla, R.; Fofana, B. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG). BMC Plant Biol. 2014, 14, 82. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gulcin, İ. Evaluation of the Antioxidant and Antiradical Properties of Some Phyto and Mammalian Lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef]
- Aqeel, T.; Gurumallu, S.C.; Bhaskar, A.; Hashimi, S.M.; Lohith, N.C.; Javaraiah, R. Protective role of flaxseed lignan secoisolariciresinol diglucoside against lead-acetate-induced oxidative-stress-mediated nephrotoxicity in rats. Phytomed. Plus 2021, 1, 100038. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Quezada-Maldonado, E.M.; Rivera-Pineda, A.; Chirino, Y.I.; García-Cuellar, C.M.; Sánchez-Pérez, Y. The Road to Malignant Cell Transformation after Particulate Matter Exposure: From Oxidative Stress to Genotoxicity. Int. J. Mol. Sci. 2023, 24, 1782. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, M.A.V.; Pressete, C.G.; Marques, M.J.; Granato, D.; Azevedo, L. Polyphenols as potential antiproliferative agents: Scientific trends. Curr. Opin. Food Sci. 2018, 24, 26–35. [Google Scholar] [CrossRef]
- Bonassi, S.; Norppa, H.; Ceppi, M.; Strömberg, U.; Vermeulen, R.; Znaor, A.; Cebulska-Wasilewska, A.; Fabianova, E.; Fucic, A.; Gundy, S.; et al. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: Results from a pooled cohort study of 22 358 subjects in 11 countries. Carcinogenesis 2008, 29, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.; Alonso, P.; Ringwald, P. Current and emerging strategies to combat antimalarial resistance. Expert Rev. Anti-Infect. Ther. 2022, 20, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, W.; Castro, W.; González, S.; Madamet, M.; Amalvict, R.; Pradines, B.; Navarro, M. Copper (I)-Chloroquine Complexes: Interactions with DNA and Ferriprotoporphyrin, Inhibition of β-Hematin Formation and Relation to Antimalarial Activity. Pharmaceuticals 2022, 15, 921. [Google Scholar] [CrossRef]
- Jansen, O.; Tits, M.; Angenot, L.; Nicolas, J.-P.; De Mol, P.; Nikiema, J.-B.; Frédérich, M. Anti-plasmodial activity of Dicoma tomentosa (Asteraceae) and identification of urospermal A-15-O-acetate as the main active compound. Malar. J. 2012, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, W.S.A.; Yasin, K.; Mahgoub, N.S.; Abdel Hamid, M.M. Cross sectional study to determine chloroquine resistance among Plasmodium falciparum clinical isolates from Khartoum, Sudan. F1000Research 2018, 7, 208. [Google Scholar] [CrossRef]
- Foguim, F.T.; Bogreau, H.; Gendrot, M.; Mosnier, J.; Fonta, I.; Benoit, N.; Amalvict, R.; Madamet, M.; Wein, S.; Pradines, B. Prevalence of mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, and association with ex vivo susceptibility to common anti-malarial drugs against African Plasmodium falciparum isolates. Malar. J. 2020, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Agustar, H.K.; Hassan, N.I.; Latip, J.; Embi, N.; Sidek, H.M. Data on antiplasmodial and stage-specific inhibitory effects of Aromatic (Ar)-Turmerone against Plasmodium falciparum 3D7. Data Brief 2020, 33, 106592. [Google Scholar] [CrossRef]
- Kennedy, K.; Cobbold, S.A.; Hanssen, E.; Birnbaum, J.; Spillman, N.J.; McHugh, E.; Brown, H.; Tilley, L.; Spielmann, T.; McConville, M.J.; et al. Delayed death in the malaria parasite Plasmodium falciparum is caused by disruption of prenylation-dependent intracellular trafficking. PLoS Biol. 2019, 17, e3000376. [Google Scholar] [CrossRef] [PubMed]
- Painter, H.J.; Morrisey, J.M.; Vaidya, A.B. Mitochondrial Electron Transport Inhibition and Viability of Intraerythrocytic Plasmodium falciparum. Antimicrob. Agents Chemother. 2010, 54, 5281–5287. [Google Scholar] [CrossRef]
- Aminake, M.N.; Schoof, S.; Sologub, L.; Leubner, M.; Kirschner, M.; Arndt, H.-D.; Pradel, G. Thiostrepton and Derivatives Exhibit Antimalarial and Gametocytocidal Activity by Dually Targeting Parasite Proteasome and Apicoplast. Antimicrob. Agents Chemother. 2011, 55, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Biosca, A.; Ramírez, M.; Gomez-Gomez, A.; Lafuente, A.; Iglesias, V.; Pozo, O.J.; Imperial, S.; Fernàndez-Busquets, X. Characterization of Domiphen Bromide as a New Fast-Acting Antiplasmodial Agent Inhibiting the Apicoplastidic Methyl Erythritol Phosphate Pathway. Pharmaceutics 2022, 14, 1320. [Google Scholar] [CrossRef]


| Compounds | HE50 | HE80 | EE100 | AE100 |
|---|---|---|---|---|
| TPC (mg GAE/g) | 53 ± 1 a | 23 ± 1 c | 14 ± 0.6 d | 32 ± 0.7 b |
| TFC (mg CE/g) | 158 ± 11 a | 113 ± 2 c | 32 ± 4 d | 132 ± 2 b |
| Total flavonol content (mg QE/g) | 16 ± 1 a | 4 ± 0.3 b | 6 ± 0.7 b | 5 ± 1 b |
| Ortho-diphenols (mg CAE/g) | 33 ± 1 a | 15 ± 0.5 b | 7 ± 0.4 c | 15 ± 1 b |
| Antioxidant activity | ||||
| DPPH (mg AAE/g) | 103 ± 3 b | 78 ± 1 c | 27 ± 2 d | 119 ± 1 a |
| FRAP (mg AAE/g) | 87 ± 2 a | 54 ± 1 b | 24 ± 1 d | 50 ± 0.3 c |
| Hydroxyl radical-scavenging activity (mg GAE/g) | 16 ± 0.4 d | 44 ± 1 b | 23 ± 0.5 c | 53 ± 2 a |
| No | Retention Time | Adduct | m/z | Identified Mass | Calculated Mass | Fragmentations (m/z) | Compound (Empirical Formula, Error in ppm) | δ 1H in ppm (J, Hz) | δ 13C in ppm | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | - | - | - | α-glucose | δ 5.08 (d; J = 3.7 Hz, H-1) | 93.8 (C-1), 71.8 (C-2), 73.5 (C3). | [30] |
| 2 | - | - | - | - | - | - | β-glucose | δ 4.45 (d; J = 7.7 Hz, H-1) δ 3.31 (dd; J = 9.2; 7.8 Hz, H-3) | 98.0 (C-1), 77.8 (C-3), 70.3 (C-4). | [30] |
| 3 | - | - | - | - | - | - | 4,6,2′-trihydroxi-6-[10′(Z)-heptadecenyl]-1-cyclohexene-2-one (C23H40O4) | 5.88 (dd, J = 10.2; 2.0 Hz, H-2); 6.92 (m, H-3); 1.90 (m, H-1′), 4.02 (m, H-2′); 5.31 (t, J = 5.0 Hz, H-10′, H-11′); 2.0 (m, H-12′), 0.88 (t, J = 7.0 Hz, H-17′). | 126.2 (C-2), 153.6 (C-3), 64.7 (C-4), 43.3 (C-1′), 70.7 (C-2′), 130.5 (C10′, C11′), 27.8 (C12′), 14.0 (C-17′). | [4] |
| 4 | 1.8 | [M−H]− | 169.0133 | 170.0211 | 170.0215 | 125.0245 | Gallic acid (C7H5O5, -2.35) | 7.03 (s, H-2,6) | 108.8 (C-2, 6), 145.0 (C-3,5), 138.5 (C-4), 168.8 (C-7). | [1,31] |
| 5 | 8.7 | [M+formic acid-H]- | 385.1892 | 386.1970 | 386.1940 | 431.1931 223.1338 205.1258 | (6S,7E,9S)-6,9-dihydroxy-megastigma-4,7-dien-3-one 9-O-β-glucopyranoside (C19H30O8, 7.77) | 2.44 (m, H-2ax) 2.56 (m, H-2eq) | 50.5 (C-2), 200.0 (C-3), 131.3 (C-7), 71.8 (C-4′). | [32] |
| 6 | 10.8 | [M−H]− | 300.9990 | 302.00683 | 302.00626 | 283.9960 229.0141 185.0220 | Ellagic acid (C14H5O8, 1.88) | 7.53 (s, H-5′, H-5′). | 113.0 (C-1, C-1′), 139.7 (C-3, C-3′), 148.0 (C-4, C-4′), 110.5 (C5, C-5′), 108.1 (C6, C-6′), 160.0 (C-7, C-7′). | [31,33] |
| 7 | 13.3 | [M−H]− | 523.2188 | 524.2266 | 524.2257 | 361.1647 | (−)-Secoisolariciresinol-9′-O-β-d-glucopyranoside (C26H36O11, 1.71) | 6.56 (d, J = 1.8 Hz, H-2); 6.63 (d, J = 8.0 Hz, H-6); 3.49 (d, J = 5.1 Hz, H-9); 2.49–2.54 (m, H-7, H-7′); 1.91 (m, H-8); 6.58 (d, J = 1.8 Hz, H-2′); 6.63 (d, J = 8.0 Hz, H-5′); 6.53 (m, H-6′); 4.20 (d, J = 7.8 Hz, H-1′’); 3.84 (d, J = 1.8 Hz, H-6’’); 3.63 (m, H-6’’); 3.82 (m, OCH3-3); 3.71 (s, OCH3-3′). | 113.3 (C-2), 147.2 (C-4), 122.4 (C-6), 61.1 (C9), 36.5 (C-7, C-7′); 43.5 (C-8), 132.7 (C-1′), 115.8 (C-2′), 147.4 (C-3′), 144.2 (C-4′), 115.5 (C-5′), 122.4 (C-6′), 104.3 (C-1″); 62.5 (C-6″), 56.4 (OCH3-3); 56.0 (OCH3-3′) | [34] |
| Extracts | HUVEC/P. falciparum | HUVEC/Cancer Cells | ||
|---|---|---|---|---|
| 3D7 | W2 | A549 | HCT-8 | |
| HE50 | 28.4 | 15.5 | 1.7 | 2.2 |
| HE80 | 70.5 | 60.2 | 2.6 | 2.7 |
| EE100 | 20 | 21.3 | 0.6 | 1.2 |
| AE100 | 17.6 | 18 | 0.3 | 0.7 |
| HE50 (µg GAE/mL) | CIS | TC | Aberrant Type | TNCA | CA Rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DC | FR | CB | CEB | TC | QC | RE | |||||
| NC | - | 4315 | 2 | 31 | 0 | 0 | 0 | 1 | 1 | 7 | 42 b | 1 |
| PC | 4 μM | 5239 | 8 | 40 | 0 | 0 | 0 | 3 | 5 | 22 | 78 a | 1.5 |
| 20 | - | 4343 | 3 | 19 | 1 | 0 | 0 | 1 | 7 | 9 | 40 b | 1 |
| 50 | - | 4367 | 0 | 15 | 1 | 0 | 0 | 1 | 2 | 2 | 21 c | 0.5 |
| 5 | 4 μM | 4045 | 7 | 29 | 1 | 1 | 1 | 7 | 2 | 6 | 41 b | 1 |
| 10 | 4 μM | 4038 | 5 | 22 | 3 | 4 | 1 | 2 | 6 | 4 | 34 b | 1 |
| 20 | 4 μM | 4269 | 3 | 30 | 3 | 1 | 1 | 2 | 4 | 4 | 35 bc | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crispim, M.; Silva, T.C.; Lima, A.d.S.; Cruz, L.d.S.; Bento, N.A.; Cruz, T.M.; Stelle, Y.; Mar, J.M.; Rocha, D.d.Q.; Bezerra, J.d.A.; et al. From Traditional Amazon Use to Food Applications: Tapirira guianensis Seed Extracts as a Triad of Antiproliferative Effect, Oxidative Defense, and Antimalarial Activity. Foods 2025, 14, 467. https://doi.org/10.3390/foods14030467
Crispim M, Silva TC, Lima AdS, Cruz LdS, Bento NA, Cruz TM, Stelle Y, Mar JM, Rocha DdQ, Bezerra JdA, et al. From Traditional Amazon Use to Food Applications: Tapirira guianensis Seed Extracts as a Triad of Antiproliferative Effect, Oxidative Defense, and Antimalarial Activity. Foods. 2025; 14(3):467. https://doi.org/10.3390/foods14030467
Chicago/Turabian StyleCrispim, Marcell, Thaise Caputo Silva, Amanda dos Santos Lima, Laura da Silva Cruz, Nathalia Alves Bento, Thiago Mendanha Cruz, Yasmin Stelle, Josiana Moreira Mar, Daniel de Queiroz Rocha, Jaqueline de Araújo Bezerra, and et al. 2025. "From Traditional Amazon Use to Food Applications: Tapirira guianensis Seed Extracts as a Triad of Antiproliferative Effect, Oxidative Defense, and Antimalarial Activity" Foods 14, no. 3: 467. https://doi.org/10.3390/foods14030467
APA StyleCrispim, M., Silva, T. C., Lima, A. d. S., Cruz, L. d. S., Bento, N. A., Cruz, T. M., Stelle, Y., Mar, J. M., Rocha, D. d. Q., Bezerra, J. d. A., & Azevedo, L. (2025). From Traditional Amazon Use to Food Applications: Tapirira guianensis Seed Extracts as a Triad of Antiproliferative Effect, Oxidative Defense, and Antimalarial Activity. Foods, 14(3), 467. https://doi.org/10.3390/foods14030467







