Application Progress of Immobilized Enzymes in the Catalytic Synthesis of 1,3-Dioleoyl-2-palmitoyltriglyceride Structured Lipids
Abstract
:1. Introduction
Overview and Synthesis Methods of OPO Structured Lipids
2. Research Progress of Lipase Catalysts in the Enzymatic Synthesis of OPO Structured Lipids
2.1. Overview of Lipase
2.2. Catalytic Mechanism of Lipase in Triglycerides
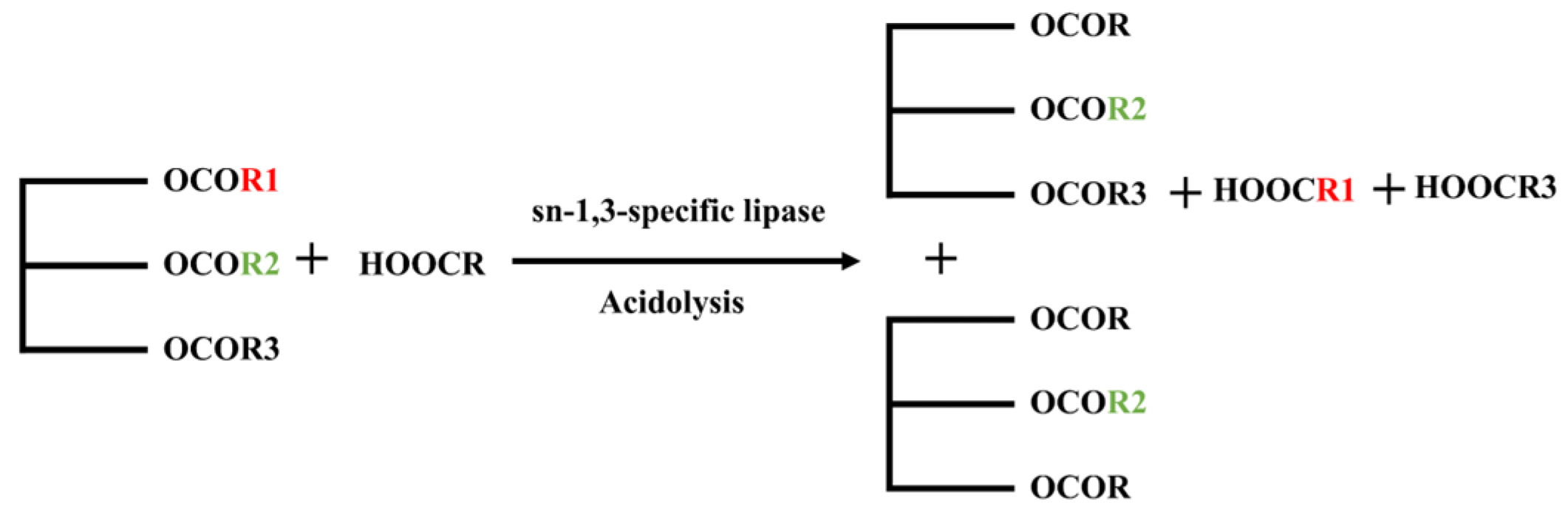
2.3. Application of Lipase in OPO Structured Lipids
3. Research Progress of Immobilized Lipase Catalysts in the Enzymatic Synthesis of OPO Structured Lipids
3.1. Lipase Immobilization Methods
3.2. Immobilized Enzyme Carrier for Synthesis of OPO Structured Lipids
4. Research Progress of Novel Enzyme Carrier Covalent Organic Framework Materials (COFs)
5. The Significance and Prospect of Research on the Synthesis of OPO Structured Lipids Catalyzed by Immobilized Enzymes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moltó-Puigmartí, C.; Permanyer, M.; Castellote, A.I.; López-Sabater, M.C. Effects of pasteurisation and high-pressure processing on vitamin C, tocopherols and fatty acids in mature human milk. Food Chem. 2011, 124, 697–702. [Google Scholar] [CrossRef]
- Bakry, I.A.; Korma, S.A.; Wei, W.; Nafea, A.E.; Mahdi, A.A.; Ziedan, N.I.; Wang, X.G. Changes in the fatty acid content of Egyptian human milk across the lactation stages and in comparison with Chinese human milk. Eur. Food Res. Technol. 2021, 247, 1035–1048. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Yang, T.K.; Xu, X.B.; Jacobsen, C. Production and oxidative stability of a human milk fat substitute produced from lard by enzyme technology in a pilot packed-bed reactor. Food Chem. 2006, 94, 53–60. [Google Scholar] [CrossRef]
- Wei, T.; Mueed, A.; Luo, T.; Sun, Y.; Zhang, B.; Zheng, L.; Deng, Z.; Li, J. 1,3-Dioleoyl-2-palmitoyl-glycerol and 1-oleoyl-2-palmitoyl-3-linoleoyl-glycerol: Structure-function relationship, triacylglycerols preparation, nutrition value. Food Chem. 2024, 443, 138560. [Google Scholar] [CrossRef]
- Tecelao, C.; Perrier, V.; Dubreucq, E.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes by Interesterification of Tripalmitin with Ethyl Oleate Catalyzed by Candida parapsilosis Lipase/Acyltransferase. J. Am. Oil Chem. Soc. 2019, 96, 777–787. [Google Scholar] [CrossRef]
- Ghide, M.K.; Yan, Y.J. 1,3-Dioleoyl-2-palmitoyl glycerol (OPO)-Enzymatic synthesis and use as an important supplement in infant formulas. J. Food Biochem. 2021, 45, e13799. [Google Scholar] [CrossRef]
- Liu, C.S.; Tian, J.J.; Zhang, R.W.; Xu, J.T.; Nie, K.L.; Deng, L.; Wang, F. Solvent-Free Alcoholysis of Tripalmitin to Produce 2-Monoglyceride as Precursor for 1, 3-Oleoyl-2-Palmitoylglycerol. Appl. Biochem. Biotechnol. 2020, 190, 867–879. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, J.M.; Akoh, C.C.; Kim, M.R.; Lee, K.T. Optimized synthesis of 1,3-dioleoyl-2-palmitoylglycerol-rich triacylglycerol via interesterification catalyzed by a lipase from Thermomyces lanuginosus. New Biotechnol. 2010, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Teichert, S.A.; Akoh, C.C. Characterization of Stearidonic Acid Soybean Oil Enriched with Palmitic Acid Produced by Solvent-free Enzymatic Interesterification. J. Agric. Food Chem. 2011, 59, 9588–9595. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, S.; Xiang, X.; Shi, J.; Huang, J.; Deng, Q.; Huang, F.; Xiao, J. Facile preparation of magnetic carbon nanotubes-immobilized lipase for highly efficient synthesis of 1,3-dioleoyl-2-palmitoylglycerol-rich human milk fat substitutes. Food Chem. 2017, 228, 476–483. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, K.L.; Zheng, Y.L.; Wang, F.; Deng, L.; Tan, T.W. Lipase Candida sp. 99-125Coupled with β-cyclodextrin as additive synthesized the human milk fat substitutes. J. Mol. Catal. B Enzym. 2016, 125, 1–5. [Google Scholar] [CrossRef]
- Yang, Y.F.; Zhou, C.S.; Ma, H.L.; Dong, Y.T.; Fu, J.Y.; Lai, X.Y.; Yagoub, A.A.; Peng, W.J.; Ni, H. Antioxidant and lipase inhibitory activities of Camellia pollen extracts: The effect of composition and extraction solvents. All Life 2022, 15, 1304–1314. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Q.Z.; Liu, C.; Yang, H.A.; Jia, L.; Zhao, L.T.; Gong, F.Y.; Tan, C.; Tao, H.X.; He, W.S. Improved antioxidant activity of rutin via lipase-mediated esterification with oleic acid. J. Sci. Food Agric. 2023, 103, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Liu, D.; Huang, M.G.; Huang, W.Y.; Li, Y.; Feng, J. Interfacial engineering strategy to improve the stabilizing effect of curcumin-loaded nanostructured lipid carriers. Food Hydrocoll. 2022, 127, 107552. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 35–54. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Rani, K.Y.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Owusu-Fordjour, M.; Xu, L.; Ding, Z.Y.; Gu, Z.H. Immobilization of Laccase on Magnetic Chelator Nanoparticles for Apple Juice Clarification in Magnetically Stabilized Fluidized Bed. Front. Bioeng. Biotechnol. 2020, 8, 589. [Google Scholar] [CrossRef]
- Mala, J.G.S.; Takeuchi, S. Understanding Structural Features of Microbial Lipases—An Overview. Anal. Chem. Insights 2012, 3, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.H.; Zhou, Z.J.; Wang, Y.; Han, J.; Li, C.M.; Zhang, W.L.; Ni, L. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite: Improvement of Loading Amount and Catalytic Activity. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Straathof, A.; Jongejan, J. The enantiomeric ratio: Origin, determination and prediction. Enzym. Microb. Technol. 1997, 21, 559–571. [Google Scholar] [CrossRef]
- Gu, S.Y.; Feng, F.Q.; Li, Y.; Wei, W.; Lu, R.R. Aspergillus oryzae Lipase-Catalyzed Synthesis of Dioleoyl; Palmitoyl-Rich Triacylglycerols in Two Reactors. J. Am. Oil Chem. Soc. 2016, 93, 1347–1354. [Google Scholar] [CrossRef]
- Faustino, A.R.; Osório, N.M.; Tecelao, C.; Canet, A.; Valero, F.; Ferreira-Dias, S. Camelina oil as a source of polyunsaturated fatty acids for the production of human milk fat substitutes catalyzed by a heterologous Rhizopus oryzae lipase. Eur. J. Lipid Sci. Technol. 2016, 118, 532–544. [Google Scholar] [CrossRef]
- Ghide, M.K.; Li, K.; Wang, J.H.; Abdulmalek, S.A.; Yan, Y.J. Effective Production of Human Milk Fat Substitutes Rich in 1,3-Dioleoyl-2-palmitoyl Glycerol (OPO) viaa New Strategy. Food Biophys. 2022, 17, 495–507. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, M.; Huang, X.; Yang, F.; Shi, Y.; Liao, C.; Yu, D. Investigation into the magnetic immobilization of lipase and its application in the synthesis of structured triacylglycerols. LWT 2023, 174, 114466. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, X.; Yu, X.; Wang, X.; Jin, Q.; Wang, X. Lipase-mediated production of 1-oleoyl-2-palmitoyl-3-linoleoylglycerol by a two-step method. Food Biosci. 2020, 36, 100678. [Google Scholar] [CrossRef]
- Huang, C.Q.; Zhou, Y.H.; Li, L.; Lin, L.; Li, C.Z.; Ye, Y. Insight into the medium-long-medium structured lipids made from Camellia oil: Composition-structure relationship. J. Food Sci. 2023, 88, 3384–3397. [Google Scholar] [CrossRef]
- Fan, L.L.; Xie, P.J.; Wang, Y.; Liu, X.L.; Li, Y.; Zhou, J.Z. Influences of mannosylerythritol lipid-A on the self-assembling structure formation and functional properties of heat-induced β-lactoglobulin aggregates. Food Hydrocoll. 2019, 96, 310–321. [Google Scholar] [CrossRef]
- Cao, Q.W.; Mei, S.H.; Mehmood, A.; Sun, Y.; Chen, X.M. Inhibition of pancreatic lipase by coffee leaves-derived polyphenols: A mechanistic study. Food Chem. 2024, 444, 138514. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santo, J.C.S.; Berenguer-Murcia, A.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.P.; Zhang, Y.; Sun, Q.; Guo, Z.M.; Zhang, D.; Zou, X.B. Enzyme-assisted patulin detoxification: Recent applications and perspectives. Trends Food Sci. Technol. 2024, 146, 104383. [Google Scholar] [CrossRef]
- Gao, S.P.; Zhou, R.Y.; Zhang, D.; Zheng, X.Y.; El-Seedi, H.R.; Chen, S.Q.; Niu, L.D.; Li, X.; Guo, Z.M.; Zou, X.B. Magnetic nanoparticle-based immunosensors and aptasensors for mycotoxin detection in foodstuffs: An update. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13266. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, H.; Wang, M.M.; Yu, X.L.; Cui, Y.; Xu, L.; Ma, A.Z.; Ding, Z.Y.; Huo, S.H.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Chen, J.J.; Ni, D.W.; Xu, W.; Zhang, W.L.; Mu, W.M. Microbial dextran-hydrolyzing enzyme: Properties, structural features, and versatile applications. Food Chem. 2024, 437, 137951. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Khan, I.M.; He, W.S.; Li, Y.X.; Jin, P.; Campanella, O.H.; Zhang, H.H.; Huo, Y.R.; Chen, Y.; Yang, H.Q.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Y.; Xing, C.R.; Xue, M.; Fang, Y.; Li, P. Selective removal of Pb(II) from yellow rice wine using magnetic carbon-based adsorbent. J. Sci. Food Agric. 2023, 103, 6929–6939. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.B.; Peng, L.L.; Wang, X.H.; Han, S.Q.; Yang, L.Z.; Wang, H.L.; Hao, C. Efficient capture of lead ion and methylene blue by functionalized biomass carbon-based adsorbent for wastewater treatment. Ind. Crops Prod. 2022, 183, 114966. [Google Scholar] [CrossRef]
- Ma, S.; Pan, L.G.; You, T.Y.; Wang, K. g-C3N4/Fe3O4 Nanocomposites as Adsorbents Analyzed by UPLC-MS/MS for Highly Sensitive Simultaneous Determination of 27 Mycotoxins in Maize: Aiming at Increasing Purification Efficiency and Reducing Time. J. Agric. Food Chem. 2021, 69, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, M.; You, T.Y.; Wang, K. Using Magnetic Multiwalled Carbon Nanotubes as Modified QuEChERS Adsorbent for Simultaneous Determination of Multiple Mycotoxins in Grains by UPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, N.; Najafpour-Darzi, G. Preparation of immobilized lipase on Co2+ chelated carboxymethyl cellulose based MnFe2O4 magnetic nanocomposite particles. Mol. Catal. 2022, 519, 112118. [Google Scholar] [CrossRef]
- Aghaei, H.; Mohammadbagheri, Z.; Hemasi, A.; Taghizadeh, A. Efficient hydrolysis of starch by α-amylase immobilized on cloisite 30B and modified forms of cloisite 30B by adsorption and covalent methods. Food Chem. 2022, 373, 131425. [Google Scholar] [CrossRef]
- Goncalves, D.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.Q.; Li, C.Z.; Ye, Y.; Cui, H.Y.; Lin, L. Preparation and physicochemical effects of zein nanofiber membrane encapsulated with citral/HP-β-CD inclusion complex and its application on cheese. Food Biosci. 2022, 50, 101990. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.H.; Zhu, S.; Jiang, B.; Gao, S.; Zhang, Y.F.; Wang, H.L.; Xu, S.M.; Liu, Y.F.; An, Y.F. Embedding inulin fructotransferase from Arthrobacter aurescens into novel curdlan-based mesoporous silica microspheres for efficient production of Difructose Anhydride III. Food Chem. 2019, 299, 125128. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.R.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sanders, J.P.M. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Bibi, Z.; Rehman, H.U.; Aman, A.; Ul Qader, S.A.; Rashid, M.H. Single step immobilization of CMCase within agarose gel matrix: Kinetics and thermodynamic studies. Colloids Surf. B-Biointerfaces 2021, 200, 111583. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.Y.; Chen, Y.; Li, B.Q.; Tian, S.P. Highly efficient removal of patulin using immobilized enzymes of Pseudomonas aeruginosa TF-06 entrapped in calcium alginate beads. Food Chem. 2022, 377, 131973. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Shi, L.L.; Huang, Y.; Gao, J.; Zhang, X.; Zhou, L.Y. Preparation of Robust Biocatalyst Based on Cross-Linked Enzyme Aggregates Entrapped in Three-Dimensionally Ordered Macroporous Silica. ACS Appl. Mater. Interfaces 2014, 6, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Khodaiyan, F. Green synthesis of chitosan magnetic nanoparticles and their application with poly-aldehyde kefiran cross-linker to immobilize pectinase enzyme. Biocatal. Agric. Biotechnol. 2020, 29, 101681. [Google Scholar] [CrossRef]
- Xia, J.J.; Yan, Y.; Bin, Z.; Feng, L. Improved catalytic performance of carrier-free immobilized lipase by advanced cross-linked enzyme aggregates technology. Bioprocess Biosyst. Eng. 2022, 45, 147–158. [Google Scholar] [CrossRef]
- Xu, K.L.; Chen, X.X.; Zheng, R.C.; Zheng, Y.G. Immobilization of Multi-Enzymes on Support Materials for Efficient Biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liang, N.N.; Hu, X.T.; Li, W.T.; Guo, Z.; Zhang, X.A.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Xia, J.J.; Liu, F.; Yan, L.S.; Suo, H.B.; Qian, J.Y.; Zou, B. Simultaneous determination of tert-butylhydroquinone, butylated hydroxyanisole and phenol in plant oil by metalloporphyrin-based covalent organic framework electrochemical sensor. J. Food Compos. Anal. 2023, 122, 105486. [Google Scholar] [CrossRef]
- Aghaei, H.; Yasinian, A.; Taghizadeh, A. Covalent immobilization of lipase from Candida rugosa on epoxy-activated cloisite 30B as a new heterofunctional carrier and its application in the synthesis of banana flavor and production of biodiesel. Int. J. Biol. Macromol. 2021, 178, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Gan, J.S.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review. Int. J. Biol. Macromol. 2021, 167, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Han, J.; Li, Y.; Wang, Y.; Wang, L.; Ni, L. Preparation of dendritic polymer-based magnetic carrier for application of bromelain separation and purification. J. Food Biochem. 2019, 43, e12976. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.S.; Ma, L.X.; Li, X.H.; Zhang, H.Y.; Xiao, R. Preparation of activated carbon with heavy fraction of bio-oil from rape straw pyrolysis as carbon source and its performance in the aldol condensation for aviation fuel as carrier. Ind. Crops Prod. 2023, 192, 115912. [Google Scholar] [CrossRef]
- Wen, C.T.; Zhang, J.X.; Zhang, H.H.; Duan, Y.Q. New Perspective on Natural Plant Protein-Based Nanocarriers for Bioactive Ingredients Delivery. Foods 2022, 11, 1701. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, S.H.; Manivel, P.; Ma, H.L.; Chen, X.M. Zinc oxide nanoparticles synthesized using coffee leaf extract assisted with ultrasound as nanocarriers for mangiferin. Curr. Res. Food Sci. 2022, 5, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Chai, Z.; Cui, L.; Zhao, X.Y.; Zhao, X.; Li, B.; Yang, Y.Y.; Huang, W.Y. Gastrointestinal fate of blueberry anthocyanins in ferritin-based nanocarriers. Food Res. Int. 2024, 176, 113811. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.L.; França, A.D.; de Castro, A.M.; de Souza, R.; Esteves, P.M.; Goncalves, R.S.B. Enzyme Immobilization in Covalent Organic Frameworks: Strategies and Applications in Biocatalysis. Chempluschem 2020, 85, 2051–2066. [Google Scholar] [CrossRef]
- Zhou, C.K.; Huang, C.Q.; Li, L.; Tian, Y.N.; Zhang, J.; Lin, L.; Li, C.Z.; Ye, Y. Apricot polysaccharides as new carriers to make curcumin nanoparticles and improve its stability and antibacterial activity. J. Food Sci. 2024, 89, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Jafari, S.M.; Karim, A.; Rasheed, H.A.; Assadpour, E.; Virk, M.S.; Liang, Q.F.; Suleria, H.A.R.; Gan, R.Y.; Ren, X.F. Ultrasonically Prepared Biopolymeric Multifunctional Nanocarriers for Encapsulating Dietary Oils: Recent Developments and Food Applications. Food Bioprocess Technol. 2024, 17, 4537–4574. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Chen, W.Q.; Liu, Q.; Wang, L.F. Encapsulation of phenolic acids within food-grade carriers systems: A systematic review. Crit. Rev. Food Sci. Nutr. 2024, 64, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Shishir, M.R.I.; Marappan, G.; Khan, S.; Hashim, S.B.H.; Aalim, H.; Arslan, M.; Tahir, H.E.; Zhihua, L.; Zhai, X.D.; et al. Recent advances in delivering mangosteen-based phytochemicals using promising micro/nanocarriers: Formulation, outcomes, and perspectives. Trends Food Sci. Technol. 2024, 153, 104734. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.; Huang, W.Y.; Zhao, X.Y.; Xu, L.J.; Teng, C.; Li, Y. Hyaluronic acid-decorated lipid nanocarriers as novel vehicles for curcumin: Improved stability, cellular absorption, and anti-inflammatory effects. Food Chem. 2025, 463, 141420. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, W.J.; Xie, X.; Ye, J.; Tan, C.P.; Lai, O.M.; Li, A.; Wang, Y. Enzymatic Interesterification of Palm Stearin and Palm Olein Blend Catalyzed by sn-1,3-Specific Lipase: Interesterification Degree, Acyl Migration, and Physical Properties. J. Agric. Food Chem. 2021, 69, 9056–9066. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, C.; Xu, W.; Miu, Z.; Jin, Q.; Wang, X. Enzymatic synthesis of structured triacylglycerols rich in 1,3-dioleoyl-2-palmitoylglycerol and 1-oleoyl-2-palmitoyl-3-linoleoylglycerol in a solvent-free system. LWT—Food Sci. Technol. 2020, 118, 108798. [Google Scholar] [CrossRef]
- Peng, B.; Chen, F.; Liu, X.B.; Hu, J.N.; Zheng, L.F.; Li, J.; Deng, Z.Y. Trace water activity could improve the formation of 1,3-oleic-2-medium chain-rich triacylglycerols by promoting acyl migration in the lipase RM IM catalyzed interesterification. Food Chem. 2020, 313, 126130. [Google Scholar] [CrossRef] [PubMed]
- Ghide, M.K.; Li, K.; Wang, J.; Abdulmalek, S.A.; Yan, Y. Immobilization of Rhizomucor miehei lipase on magnetic multiwalled carbon nanotubes towards the synthesis of structured lipids rich in sn-2 palmitic acid and sn-1,3 oleic acid (OPO) for infant formula use. Food Chem. 2022, 390, 133171. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zeng, C.; Wei, L.; Xu, L.; Song, F.; Huang, J.; Zhong, N. Fabrication of immobilized lipases for efficient preparation of 1,3-dioleoyl-2-palmitoylglycerol. Food Chem. 2023, 408, 135236. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.X.; Wei, Y.; Wu, M.; Guo, Y.L.; Xie, Y.P.; Tao, R.; Li, R.Q.; Wang, P.G.; Ma, A.Q.; Zhang, H.B. Lipase Immobilized on Layer-by-Layer Polysaccharide-Coated Fe3O4@SiO2 Microspheres as a Reusable Biocatalyst for the Production of Structured Lipids. ACS Sustain. Chem. Eng. 2019, 7, 6685–6695. [Google Scholar] [CrossRef]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wu, J.Y. Biocompatible Polyelectrolyte Complex Nanoparticles from Lactoferrin and Pectin as Potential Vehicles for Antioxidative Curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.M.; Yu, F.F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Ahmad, W.; Rong, Y.W.; Chen, Q.S.; Zuo, M.; Ouyang, Q.; Guo, Z.M. Designing an aptamer based magnetic and upconversion nanoparticles conjugated fluorescence sensor for screening Escherichia coli in food. Food Control 2020, 107, 106761. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, Y.; Cai, Y.F.; Mao, Y.L.; Ni, L.; Xie, X.Q. Preparation of temperature-sensitive magnetic microspheres for separation and purification of bromelain. Food Bioprod. Process. 2019, 114, 253–262. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.M.; Mao, Y.L.; Wang, Y. Fabrication of a core-shell-shell magnetic polymeric microsphere with excellent performance for separation and purification of bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rong, N.; He, S.Y.; Li, B.; Lin, X.G.; Liu, X.L.; Yu, Y.J.; Feng, Y.Z. Coupled magnetic nanoparticle-mediated isolation and single-cell image recognition to detect Bacillus’ cell size in soil. Eur. J. Soil Sci. 2022, 73, e13236. [Google Scholar] [CrossRef]
- Yin, L.M.; Cai, J.R.; Jayan, H.; Yosri, N.; Majeed, U.; Guo, Z.M.; Zou, X.B. Assembly of stabilized dendritic magnetic ferric-silver nanocomposite with gold nanoparticles for sensitive detection of ochratoxin A using SERS aptasensor. Food Control 2024, 165, 110704. [Google Scholar] [CrossRef]
- Shao, P.J.; Chen, H.; Ying, Q.; Zhang, S.H. Structure-Activity Relationship of Carbonic Anhydrase Enzyme Immobilized on Various Silica-Based Mesoporous Molecular Sieves for CO2 Absorption into a Potassium Carbonate Solution. Energy Fuels 2020, 34, 2089–2096. [Google Scholar] [CrossRef]
- Shi, Y.H.; Rong, X.S.; Chen, C.; Wu, M.; Takai, Y.; Qiu, X.C.; Wang, C.C.; Shimasaki, Y.; Oshima, Y. Effects of ZIF-8 Nanoparticles on the Survival, Development, and Locomotor Activity of Early-Life-Stages of Zebrafish (Danio rerio). J. Fac. Agric. Kyushu Univ. 2021, 66, 211–216. [Google Scholar] [CrossRef]
- Matsuura, S.; Chiba, M.; Tsunoda, T.; Yamaguchi, A. Enzyme Immobilization in Mesoporous Silica for Enhancement of Thermostability. J. Nanosci. Nanotechnol. 2018, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Huang, T.; Wang, S.O.; Yan, Y.S. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 104427. [Google Scholar] [CrossRef]
- Chao, Y.H.; Pang, J.Y.; Bai, Y.; Wu, P.W.; Luo, J.; He, J.; Jin, Y.; Li, X.W.; Xiong, J.; Li, H.M.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.F.; Wang, J.X.; Rashid, A.; Hou, T.; Ma, H.L.; Liang, Q.F. Characterization of Nano-SiO2/Zein Film Prepared Using Ultrasonic Treatment and the Ability of the Prepared Film to Resist Different Storage Environments. Foods 2023, 12, 3056. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.Y.; Li, L.B.; Liu, X.H.; Luo, L.J.; Cheng, Z.L.; Sun, J.Y.; Cai, Z.B.; Liu, J.M.; You, T.Y. Inner filter effect-modulated ratiometric fluorescence aptasensor based on competition strategy for zearalenone detection in cereal crops: Using mitoxantrone as quencher of CdTe QDs@SiO2. Food Chem. 2021, 349, 129171. [Google Scholar] [CrossRef]
- Luo, L.J.; Ma, S.; Li, L.B.; Liu, X.H.; Zhang, J.Y.; Li, X.; Liu, D.; You, T.Y. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019, 292, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.T.; Zhai, X.D.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y. A simple and sensitive electrochemical sensing based on amine-functionalized metal-organic framework and polypyrrole composite for detection of lead ions in meat samples. J. Food Meas. Charact. 2024, 18, 5813–5825. [Google Scholar] [CrossRef]
- Shi, B.Q.; Zhang, X.A.; Li, W.T.; Liang, N.N.; Hu, X.T.; Xiao, J.B.; Wang, D.Y.; Zou, X.B.; Shi, J.Y. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.W.; Li, Y.H.; Li, Y.X.; Li, Z.H.; Zhang, W.; Zou, X.B.; Shi, J.Y.; Huang, X.W.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.N.; Hu, X.T.; Zhang, X.A.; Li, W.T.; Guo, Z.; Huang, X.W.; Li, Z.H.; Zhang, R.J.; Shen, T.T.; Zou, X.B.; et al. Ratiometric Sensing for Ultratrace Tetracycline Using Electrochemically Active Metal-Organic Frameworks as Response Signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef]
- Gan, Z.Y.; Zhang, W.; Arslan, M.; Hu, X.T.; Zhang, X.A.; Li, Z.H.; Shi, J.Y.; Zou, X.B. Ratiometric Fluorescent Metal-Organic Framework Biosensor for Ultrasensitive Detection of Acrylamide. J. Agric. Food Chem. 2022, 70, 10065–10074. [Google Scholar] [CrossRef]
- Chaparro Sosa, A.F.; Bednar, R.M.; Mehl, R.A.; Schwartz, D.K.; Kaar, J.L. Faster Surface Ligation Reactions Improve Immobilized Enzyme Structure and Activity. J. Am. Chem. Soc. 2021, 143, 7154–7163. [Google Scholar] [CrossRef]
- Qin, X.L.; Wang, Y.M.; Wang, Y.H.; Huang, H.H.; Yang, B. Preparation and Characterization of 1,3-Dioleoyl-2-palmitoylglycerol. J. Agric. Food Chem. 2011, 59, 5714–5719. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Jiménez, M.J.; Esteban, L.; González, P.A.; Martín, L.; Rodríguez, A.; Molina, E. Enzymatic production of human milk fat substitutes containing palmitic and docosahexaenoic acids at sn-2 position and oleic acid at sn-1,3 positions. LWT—Food Sci. Technol. 2011, 44, 1986–1992. [Google Scholar] [CrossRef]
- Wei, W.; Feng, Y.; Zhang, X.; Cao, X.; Feng, F. Synthesis of structured lipid 1,3-dioleoyl-2-palmitoylglycerol in both solvent and solvent-free system. LWT—Food Sci. Technol. 2015, 60, 1187–1194. [Google Scholar] [CrossRef]
- Liu, S.-l.; Dong, X.-y.; Wei, F.; Wang, X.; Lv, X.; Zhong, J.; Wu, L.; Quek, S.-y.; Chen, H. Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content. Ultrason. Sonochemistry 2015, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Oh, S.W.; Kwon, D.Y.; Yoon, S.H. Production of 1, 3-dioleoyl-2-palmitoyl glycerol as a human milk fat substitute using enzymatic interesterification of natural fats and oils. Food Sci. Biotechnol. 2015, 24, 433–437. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Zhang, X.; Nie, K.; Deng, L.; Wang, F. The two-step synthesis of 1,3-oleoyl-2-palmitoylglycerol by Candida sp. 99–125 lipase. J. Mol. Catal. B Enzym. 2016, 133, S1–S5. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Liu, L.; Liu, T.; Li, C.; Sun, L. 1,3-Dioleoyl-2-palmitoylglycerol-rich triacylglycerol characterization by three processing methods. Int. J. Food Prop. 2019, 22, 1156–1171. [Google Scholar] [CrossRef]
- Agapay, R.C.; Ju, Y.H.; Tran-Nguyen, P.L.; Ismadji, S.; Angkawijaya, A.E.; Go, A.W. Process evaluation of solvent-free lipase-catalyzed esterification schemes in the synthesis of structured triglycerides from oleic and palmitic acids. Asia-Pac. J. Chem. Eng. 2020, 16, e2606. [Google Scholar] [CrossRef]
- Sun, D.; Li, S.; Shang, J.; You, L.; Wang, M.; Sun, C.; Wang, X. Process optimization of simultaneous enzymatic production of 1,3-dioleoyl-2-palmitoylglycerol and 1-oleoyl-2-palmitoyl-3-linoleoylglycerol. J. Am. Oil Chem. Soc. 2021, 98, 1167–1176. [Google Scholar] [CrossRef]
- Cao, X.; Pan, Y.; Qiao, M.; Yuan, Y. Synthesis of human milk fat substitutes based on enzymatic preparation of low erucic acid acyl-donors from rapeseed oil. Food Chem. 2022, 387, 132907. [Google Scholar] [CrossRef]
- Feng, T.; Shi, J.; Xia, J.; Ren, X.; Adesanya, O.I.; Suo, H.; Zou, B. Lipase in-situ immobilized in covalent organic framework: Enzymatic properties and application in the preparation of 1, 3-dioleoyl-2-palmitoylglycerol. Colloids Surf. B Biointerfaces 2024, 238, 113873. [Google Scholar] [CrossRef]
- Jiaojiao, X.; Ting, F.; Hongbo, S.; Lishi, Y.; Bin, Z. Ultrasound assisted enzymatic synthesis of OPO structured lipids by covalent organic framework immobilized lipase. Colloids Surf. B Biointerfaces 2025, 245, 114256. [Google Scholar] [CrossRef]
- Cheng, H.; Wei, Y.Q.; Han, J.Y.; Wang, X.; Ji, W.H.; Jin, X.H. Catalytic hydrolysis of ginsenosides by pectinase immobilized on a covalent organic framework material. Process Biochem. 2022, 118, 317–322. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Zheng, Y.L.; An, H.D.; Aguila, B.; Yang, C.X.; Dong, Y.Y.; Xie, W.; Cheng, P.; Zhang, Z.J.; Chen, Y.; et al. Covalent Organic Frameworks with Chirality Enriched by Biomolecules for Efficient Chiral Separation. Angew. Chem. Int. Ed. 2018, 57, 16754–16759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Li, X.L.; Liao, Q.B.; Liu, Y.F.; Xi, K.; Huang, W.Y.; Jia, X.D. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018, 9, 2785. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, C.W.; Aguila, B.; Perman, J.; Wang, S.; Huang, H.Y.; Xiao, F.S.; Ma, S.Q. Pore Environment Control and Enhanced Performance of Enzymes Infiltrated in Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Samui, A.; Happy; Sahu, S.K. Integration of α-amylase into covalent organic framework for highly efficient biocatalyst. Microporous Mesoporous Mater. 2020, 291, 109700. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Lan, P.C.; Ma, S.Q. Tuning Pore Heterogeneity in Covalent Organic Frameworks for Enhanced Enzyme Accessibility and Resistance against Denaturants. Adv. Mater. 2019, 31, e1900008. [Google Scholar] [CrossRef]
- Wang, H.P.; Jiao, F.L.; Gao, F.Y.; Zhao, X.Y.; Zhao, Y.; Shen, Y.H.; Zhang, Y.J.; Qian, X.H. Covalent organic framework-coated magnetic graphene as a novel support for trypsin immobilization. Anal. Bioanal. Chem. 2017, 409, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Ma, W.D.; He, Y.T.; Ouyang, D.; Li, G.R.; Yang, Y.X.; Zheng, Q.; Huang, H.; Cai, Z.W.; Lin, Z.A. Controllable Synthesis of Hollow Microtubular Covalent Organic Frameworks as an Enzyme-Immobilized Platform for Enhancing Catalytic Activity. ACS Appl. Mater. Interfaces 2021, 13, 52417–52424. [Google Scholar] [CrossRef] [PubMed]
- Elmerhi, N.; Al-Maqdi, K.; Athamneh, K.; Mohammed, A.K.; Skorjanc, T.; Gándara, F.; Raya, J.; Pascal, S.; Siri, O.; Trabolsi, A.; et al. Enzyme-immobilized hierarchically porous covalent organic framework biocomposite for catalytic degradation of broad-range emerging pollutants in water. J. Hazard. Mater. 2023, 459, 132261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, Z.Y.; Wang, Y.; Jiang, Z.Q.; Yan, Q.J.; Yang, S.Q. Directed Evolution of an Alginate Lyase from Flammeovirga sp. for Seaweed Fertilizer Production from the Brown Seaweed Laminaria japonica. J. Agric. Food Chem. 2025, 73, 1468–1477. [Google Scholar] [CrossRef]



| Type | Lipase | Materials | Enzyme Dosage (%) | Temperature (℃) | Time (h) | OPO Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| Interesterification | Lipozyme TL IM | PS/ethyl oleate | 10 | 50 | 3 | 31.43 | [8] |
| Acidolysis | Lipozyme RM IM | 34L-leaf lard/camellia oil FAs | 6 | 45 | 6 | 43.72 | [95] |
| Acidolysis | Novozym 435 | Tuna oil/ PPP/OA | 10 | 37 | 1 | 52.1 | [96] |
| Acidolysis | Lipozyme RM IM | PPP/OA | 12 | 60 | 4 | 40.23 | [97] |
| Acidolysis | Lipozyme RM IM | PPP/OA | 12 | 50 | 4 | 51.8 | [98] |
| Interesterification | Lipozyme IM-20 | PPP/OA | 8 | 40 | 1 | 55.2 | [99] |
| Alcoholysis | Candida sp. 99-125 | PPP/OA | 15 | 45 | 1.5 | 65 | [100] |
| Acidolysis | CLL@mMWCNTs | PPP/OA | 20 | 50 | 2 | 46.5 | [10] |
| Acidolysis | Novozym 435 | PPP/OA | 8 | 60 | 6 | 71.22 | [101] |
| Acidolysis | lipase NS 40086 | PS/OA/LA | 8 | 60 | 4 | 39.2 | [69] |
| Acidolysis | Novozyme 435 | Glycerol/PA/OA | 4 | 60 | 24 | 19.3 | [102] |
| Interesterification | Lipozyme RM IM | soy oil/palm kernel stearin/PS | 10 | 56 | 5 | 23.1 | [103] |
| Acidolysis | Novozym 40086 | PPP/FFA | 10 | 40 | 2 | 17.96 | [104] |
| Acidolysis | TLL@R-SBA-15 | PPP/OA | 10 | 50 | 8 | 73.15 | [72] |
| Acidolysis | RML@COF-1 | PPP/OA | 10 | 45 | 5 | 47.35 | [105] |
| Acidolysis | COF@RML | PPP/OA | 10 | 45 | 6 | 51.27 | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Feng, T.; Zhang, Y.; Lin, Z.; Kong, F.; Zhang, X.; Lu, Q.; Zhao, Y.; Zou, B. Application Progress of Immobilized Enzymes in the Catalytic Synthesis of 1,3-Dioleoyl-2-palmitoyltriglyceride Structured Lipids. Foods 2025, 14, 475. https://doi.org/10.3390/foods14030475
Ni X, Feng T, Zhang Y, Lin Z, Kong F, Zhang X, Lu Q, Zhao Y, Zou B. Application Progress of Immobilized Enzymes in the Catalytic Synthesis of 1,3-Dioleoyl-2-palmitoyltriglyceride Structured Lipids. Foods. 2025; 14(3):475. https://doi.org/10.3390/foods14030475
Chicago/Turabian StyleNi, Xing, Ting Feng, Yuyang Zhang, Zhiyuan Lin, Fanzhuo Kong, Xue Zhang, Qiongya Lu, Yani Zhao, and Bin Zou. 2025. "Application Progress of Immobilized Enzymes in the Catalytic Synthesis of 1,3-Dioleoyl-2-palmitoyltriglyceride Structured Lipids" Foods 14, no. 3: 475. https://doi.org/10.3390/foods14030475
APA StyleNi, X., Feng, T., Zhang, Y., Lin, Z., Kong, F., Zhang, X., Lu, Q., Zhao, Y., & Zou, B. (2025). Application Progress of Immobilized Enzymes in the Catalytic Synthesis of 1,3-Dioleoyl-2-palmitoyltriglyceride Structured Lipids. Foods, 14(3), 475. https://doi.org/10.3390/foods14030475






