Synergistic Antioxidant and Cytoprotective Effects of Thunbergia laurifolia Lindl and Zingiber officinale Extracts Against PM2.5-Induced Oxidative Stress in A549 and HepG2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. PM2.5 Preparation
2.3. Plant Preparation
2.4. Extract Preparation
2.5. Chromatography Conditions for Polyphenol Analysis
2.6. Cytotoxicity of PM2.5 Exposure A549 and HepG2 Cells
2.7. Cytoprotective Effect of RWE, GEE, and CRGE
2.8. Intracellular ROS Generation
2.9. Changes in Gene Expression of the Antioxidant Enzymes
2.10. RNA Isolation and cDNA Synthesis
2.11. qRT-PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. Phytochemical Profiling of RWE and GEE
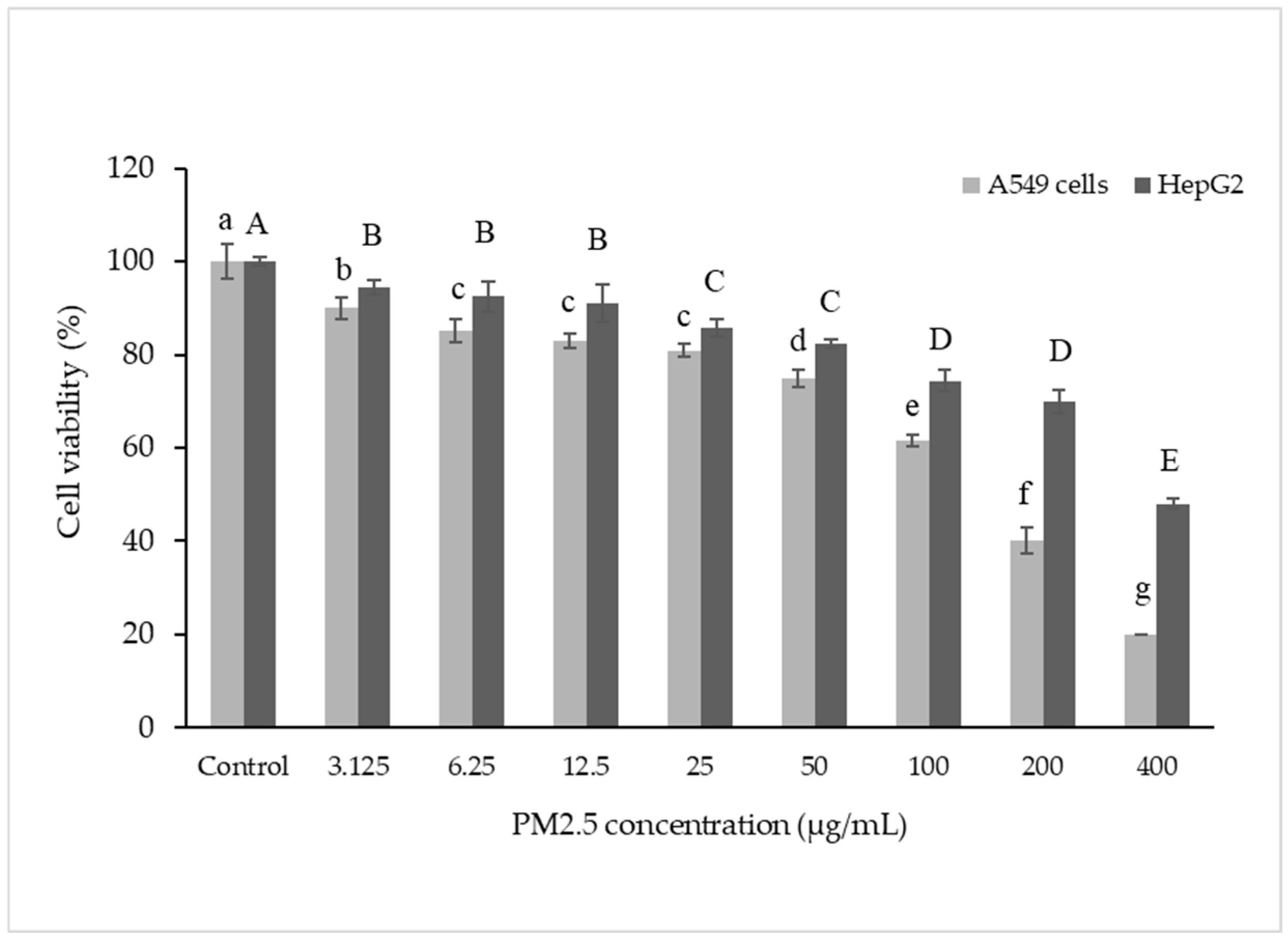
3.2. Cytotoxicity of PM2.5 Exposure A549 and HepG2 Cells
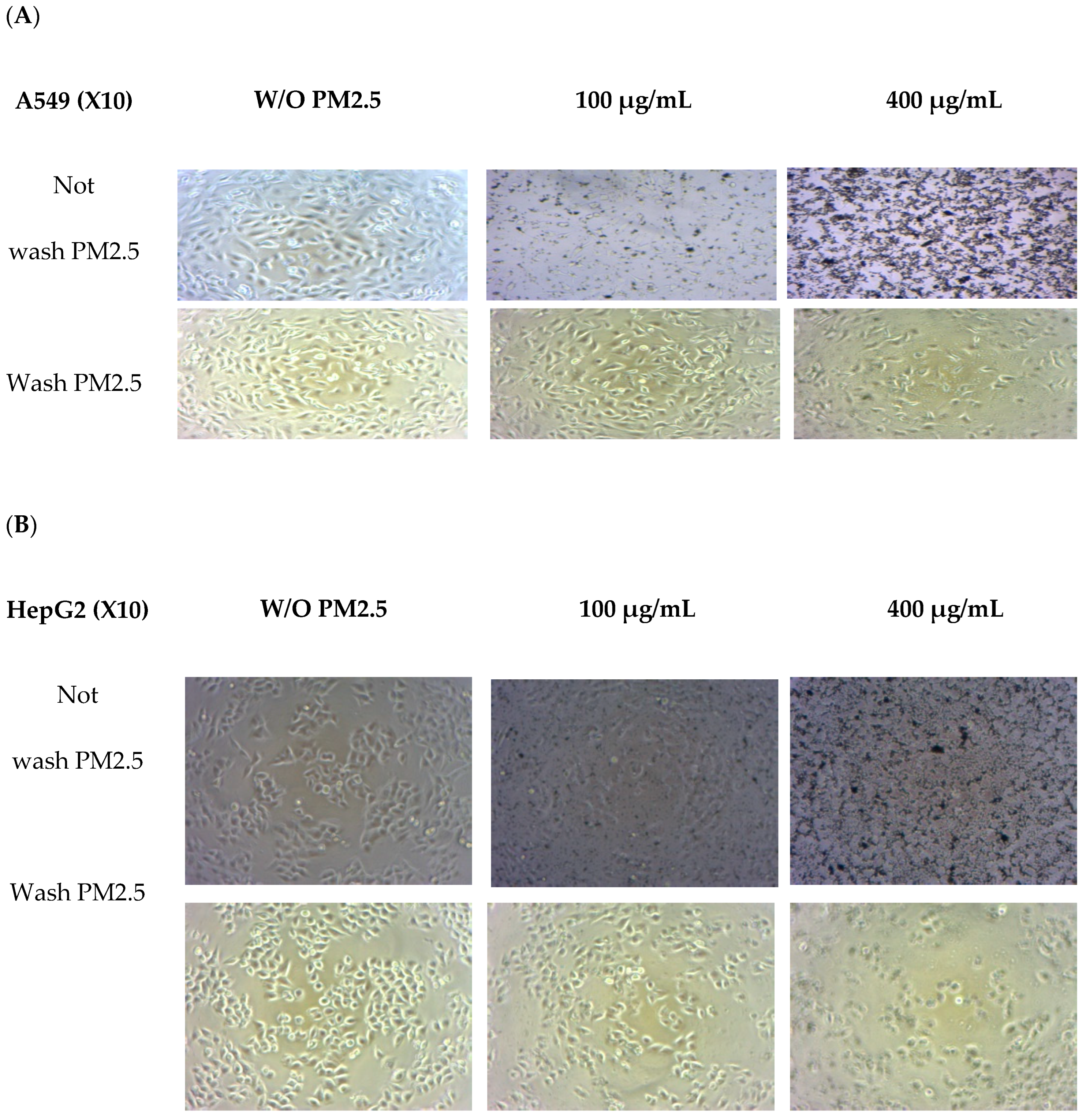
3.3. Cell Morphology
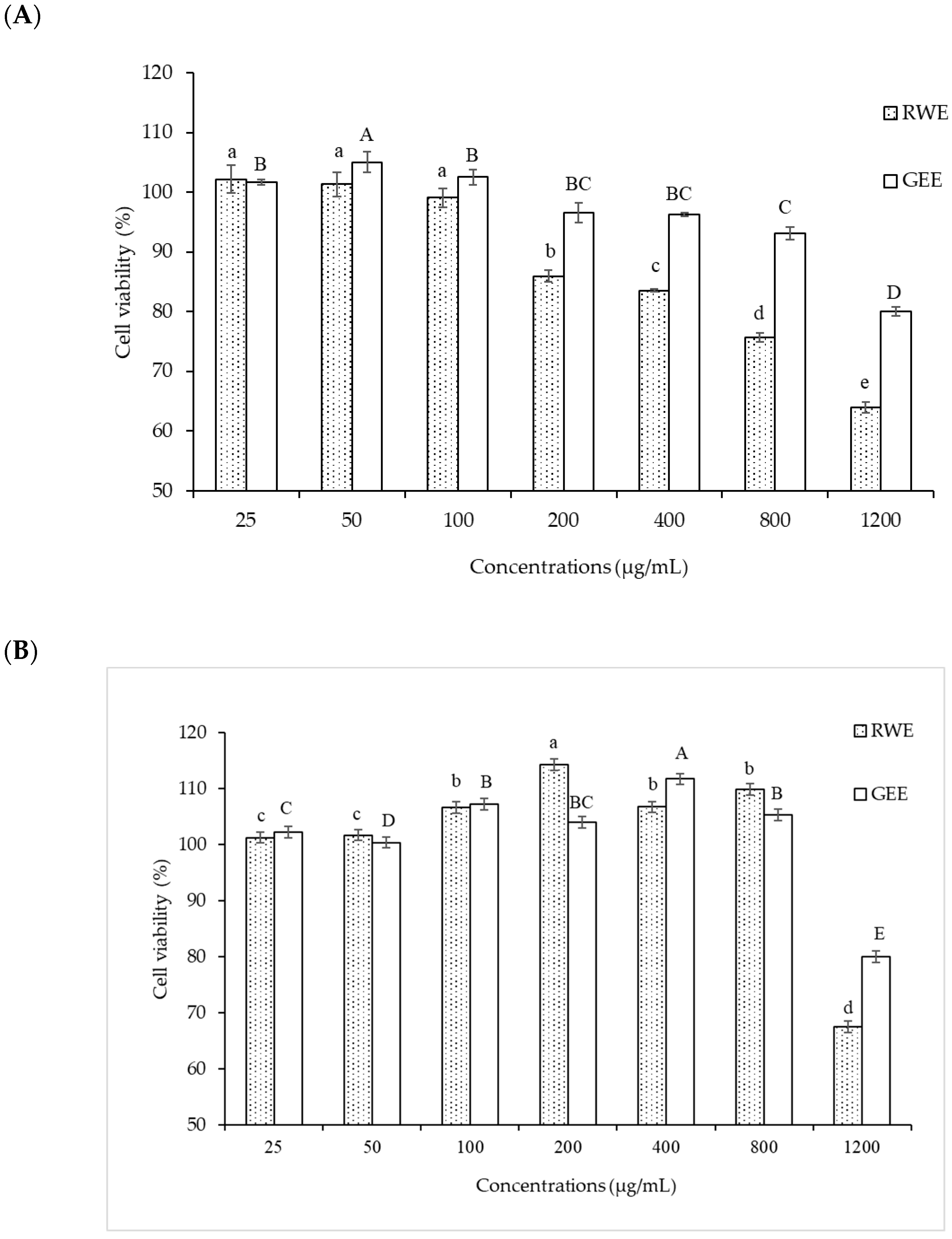
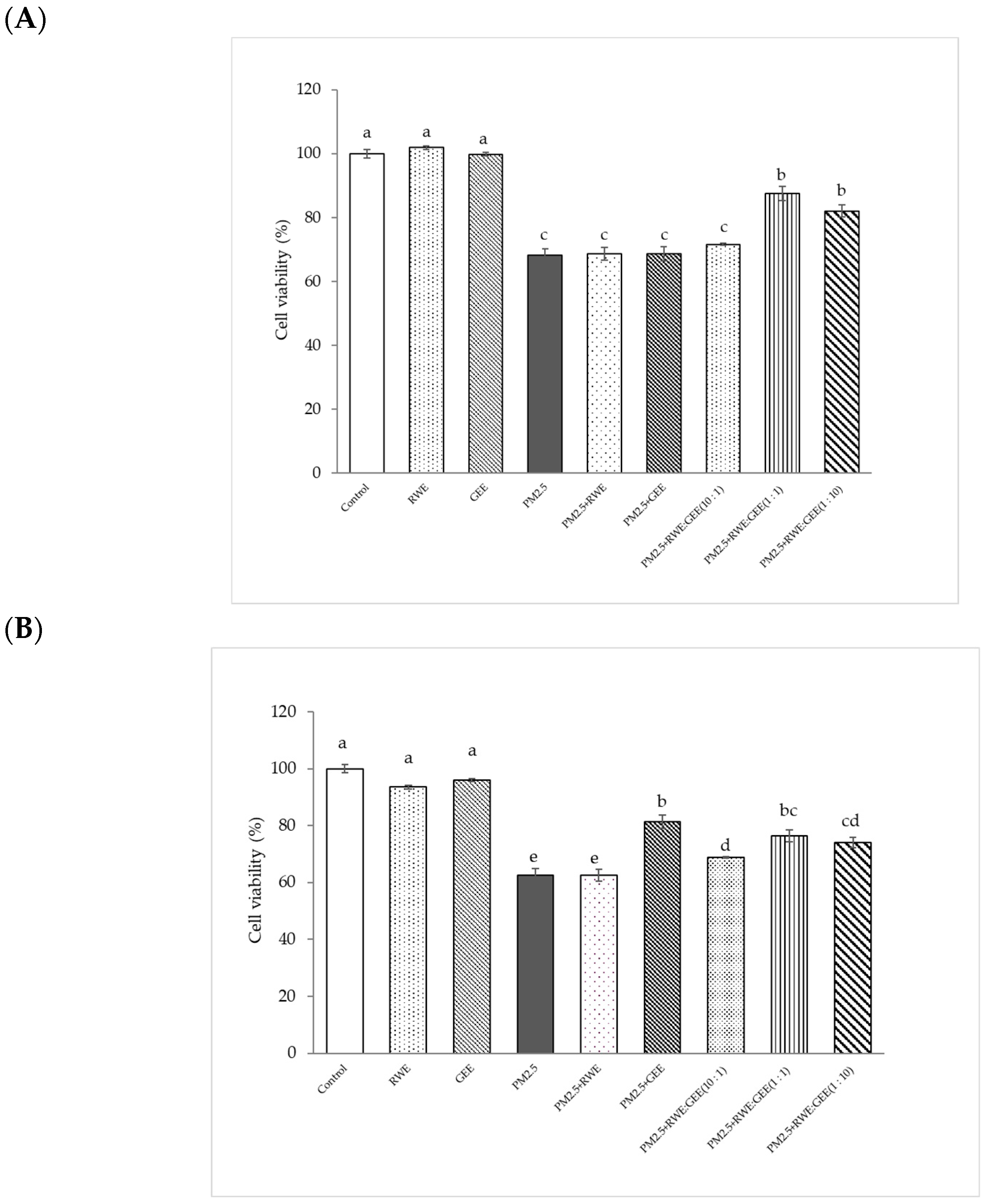
3.4. Cytoprotective Effect of RWE, GEE, and CRGE
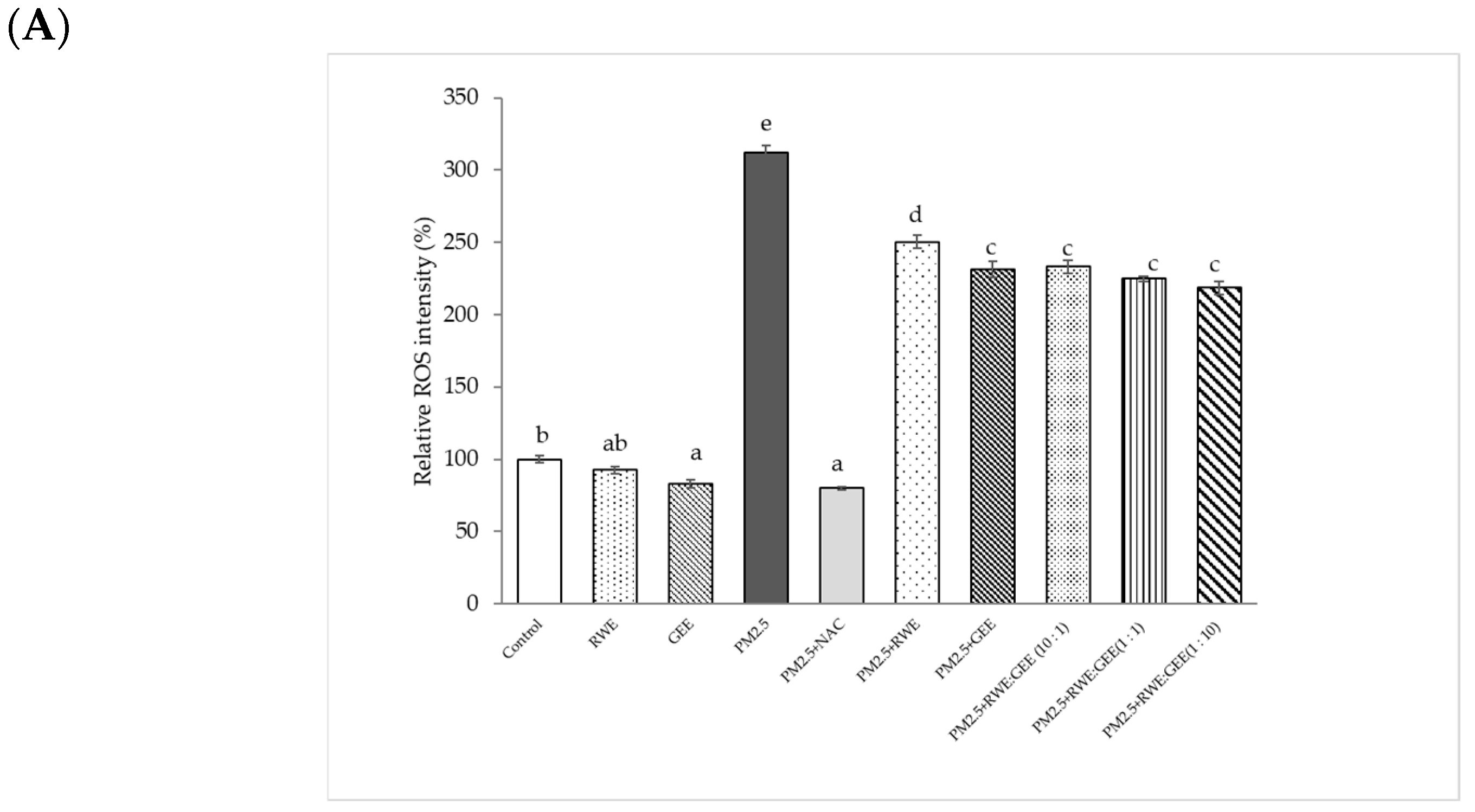
3.5. Intracellular ROS Scavenging of Extracts from PM2.5
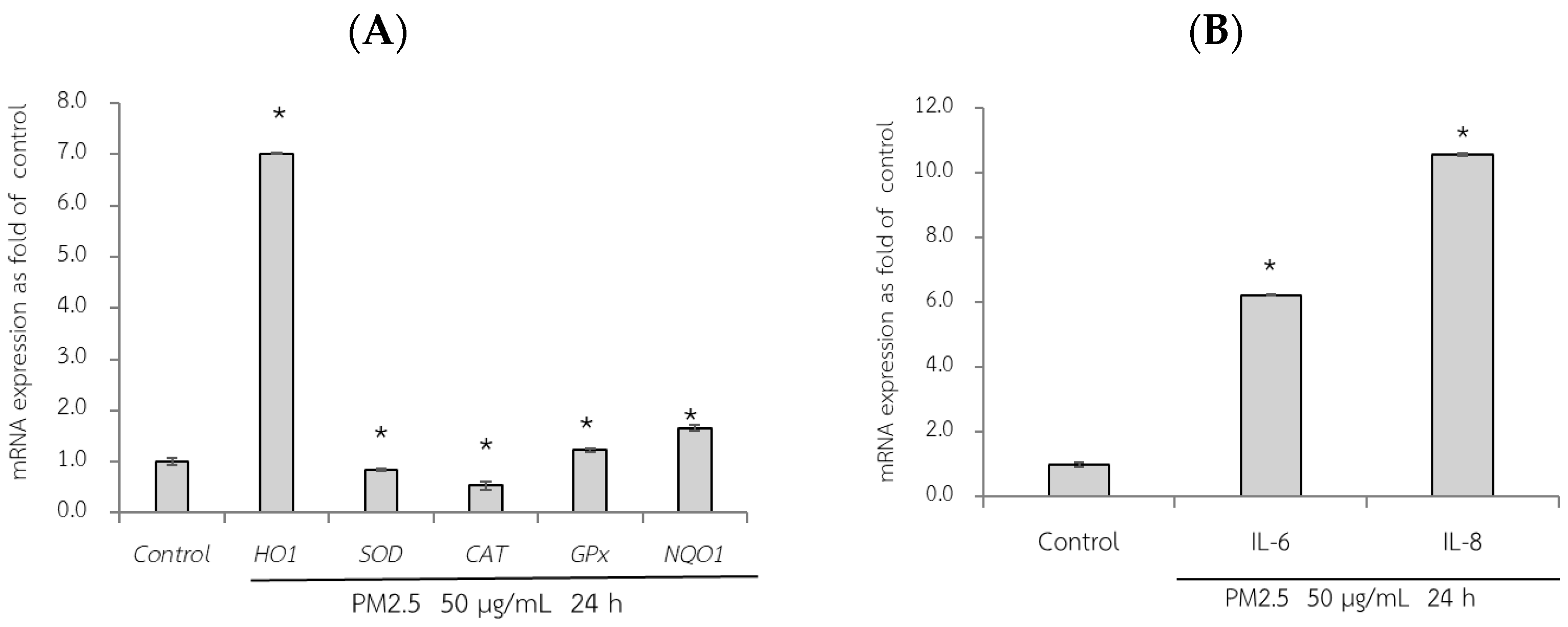
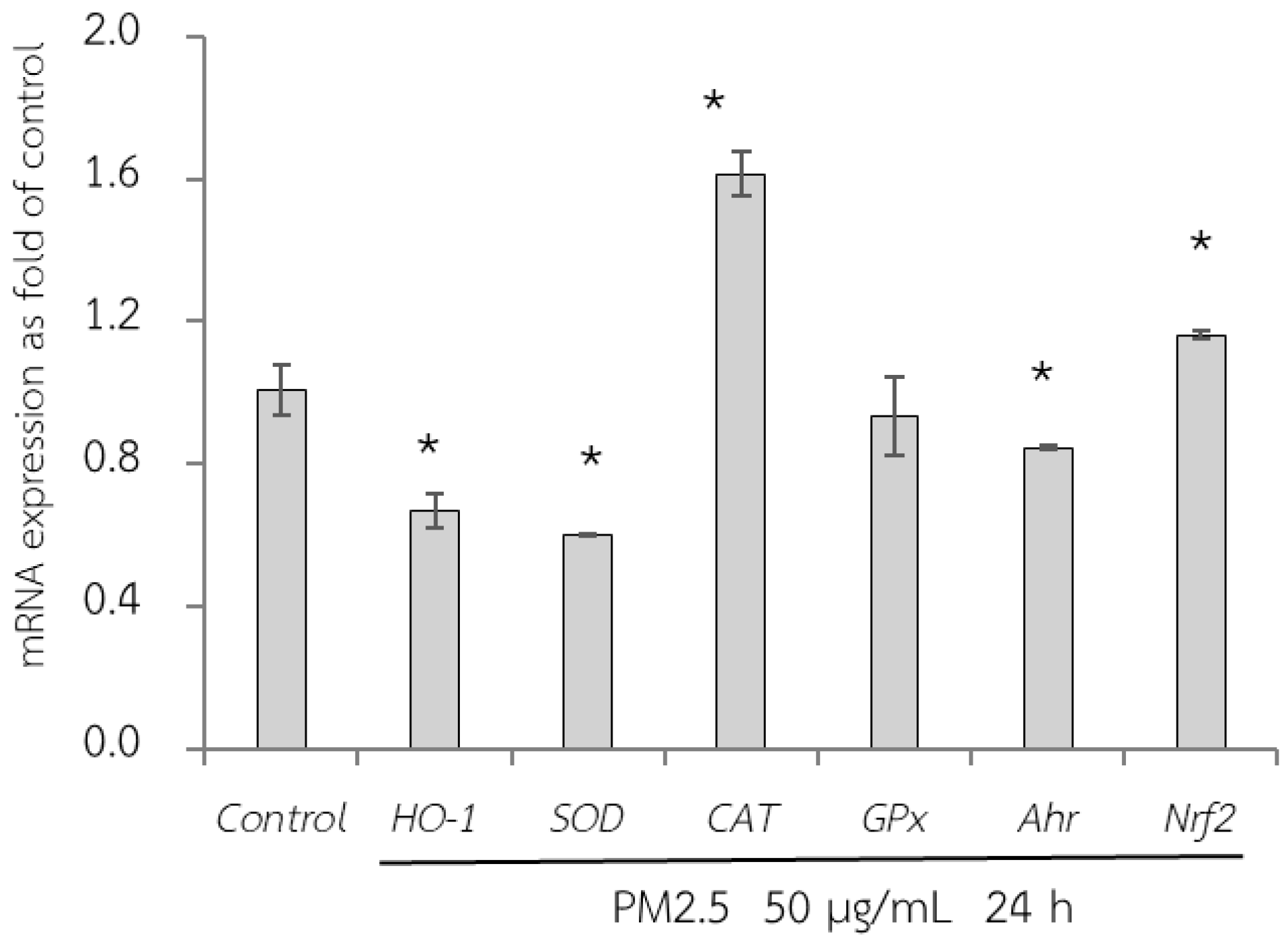
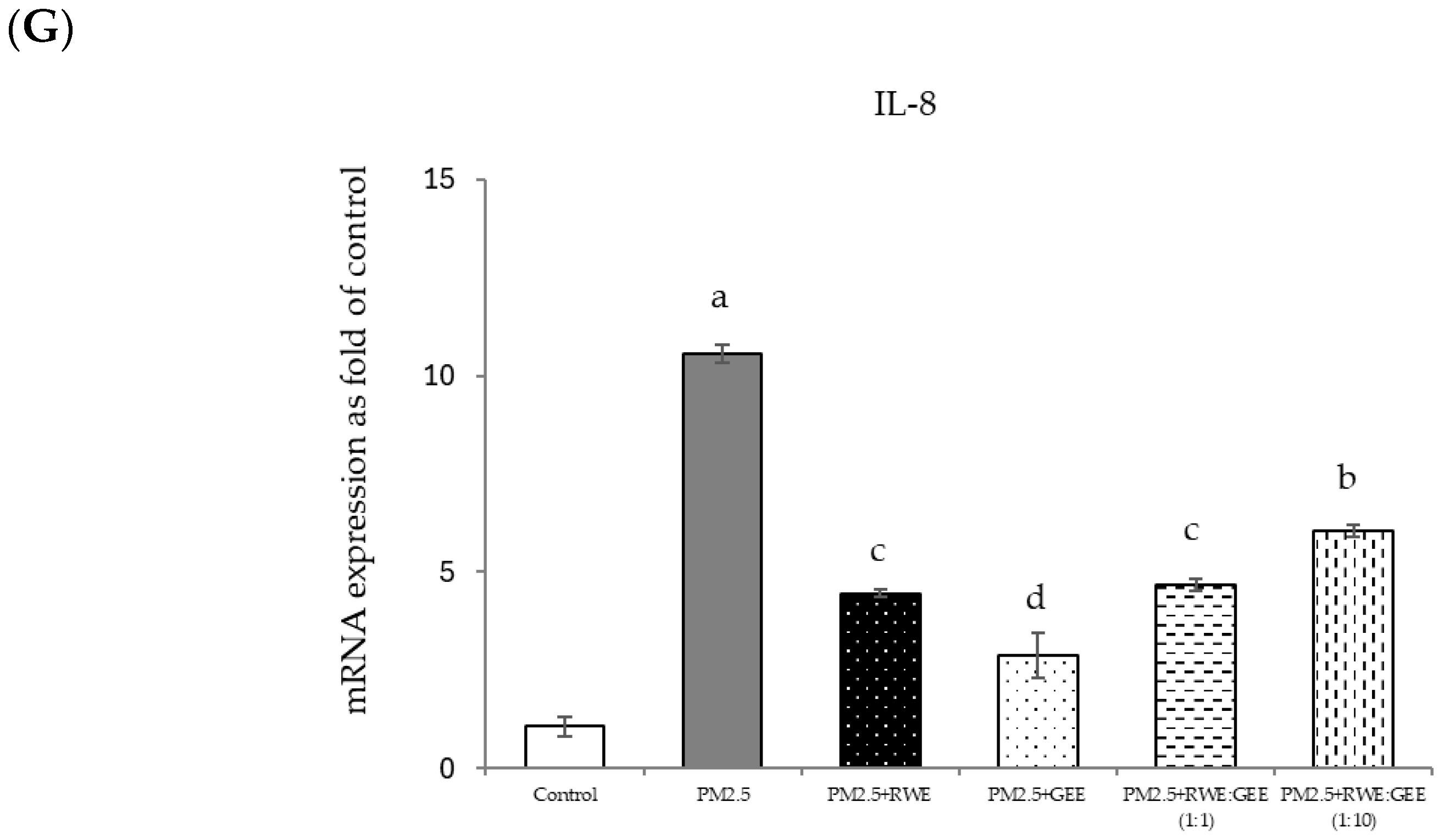
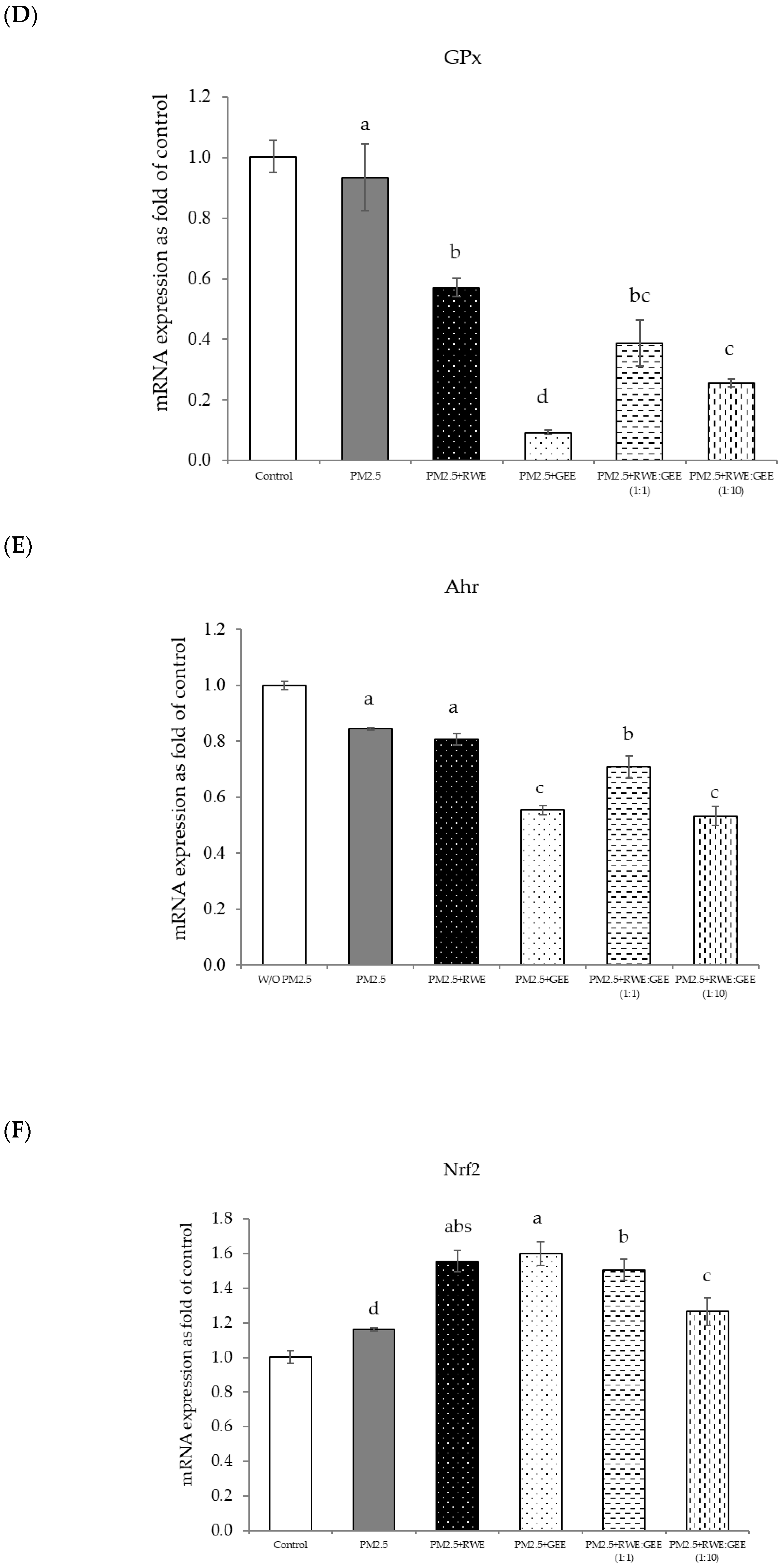
3.6. Protective Effects of RWE, GEE, and CRGE Against PM2.5-Induced Adverse Effects in Gene Expression of A549 and HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Batool, R.; Khan, M.R.; Sajid, M.; Ali, S.; Zahra, Z. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R.Br. BMC Chem. 2019, 13, 32. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Patanè, G.T.; Barreca, D.; Straface, E.; Gambardella, L.; Bozzuto, G.; Caruso, D.; Falliti, G.; Dossena, S.; et al. AAPH-induced oxidative damage reduced anion exchanger 1 (SLC4A1/AE1) activity in human red blood cells: Protective effect of an anthocyanin-rich extract. Front. Physiol. 2023, 14, 1303815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, H.; Kwon, Y. Development of a database of capsaicinoid contents in foods commonly consumed in Korea. Food Sci. Nutr. 2020, 8, 4611–4624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, W.; Zhao, C.; Shang, X.; Li, B.; Guo, J.; Wang, J.; Wu, B.; Fu, Y. Ameliorative Effects of Raisin Polyphenol Extract on Oxidative Stress and Aging In Vitro and In Vivo via Regulation of Sirt1-Nrf2 Signaling Pathway. Foods 2024, 14, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaiyana, W.; Chansakaow, S.; Intasai, N.; Kiattisin, K.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Leelapornpisid, P. Chemical Constituents, Antioxidant, Anti-MMPs, and Anti-Hyaluronidase Activities of Thunbergia laurifolia Lindl. Leaf Extracts for Skin Aging and Skin Damage Prevention. Molecules 2020, 25, 1923. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Eng, S.Y.; Tan, Y.P.; Wong, Z.C.; Lye, P.Y.; Tan, L.N. Antioxidant and Sensory Properties of Thai Herbal Teas with Emphasis on Thunbergia laurifolia Lindl. Chiang Mai J. Sci. 2012, 39, 599–609. [Google Scholar]
- Junsi, M.; Siripongvutikorn, S.; Yupanqui, C.T.; Usawakesmanee, W. Phenolic and flavonoid compounds in aqueous extracts of thunbergia laurifolia leaves and their effect on the toxicity of the carbamate insecticide methomyl to murine macrophage cells. Funct. Foods Health Dis. 2017, 7, 529–544. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Cheng, C.; Bomser, J.; Ferruzzi, M.G.; Ningsanond, S. Phytochemical profiling and phase II enzyme-inducing properties of Thunbergia laurifolia Lindl. (RC) extracts. J. Ethnopharmacol. 2007, 114, 300–306. [Google Scholar] [CrossRef]
- Preechasuk, L.; Akarasereenont, P.; Boonrak, R.; Thamsermsang, O.; Pratumvinit, B.; Thongtang, N. Effect of Thunbergia laurifolia Herbal Tea on Glucose Homeostasis in Healthy Volunteers: A Single-Arm Phase I Study. J. Evid. Based Complement. Altern. Med. 2020, 2020, 3212546. [Google Scholar] [CrossRef]
- Aung, M.M.; Han, B.H.; Ko, S.O. Phytochemical and free radical scavenging activity of extracts from ginger (Zingiber officinale Roscoe) root. J. Funct. Agric. 2015, 1, 32–42. [Google Scholar]
- Salehi, B.; Sharifi-Rad, M.; Anil Kumar, N.V.; Selvakumar, P.; Venugopal, V.; Farbood, Y. The role of antioxidative properties of ginger (Zingiber officinale Roscoe) in health benefits. Biomed. Pharmacother. 2019, 117, 114469. [Google Scholar]
- Haniadka, R.; Saldanha, E.; Sunita, V.; Palatty, P.L.; Fayad, R.; Baliga, M.S. A review of the gastroprotective effects of ginger (Zingiber officinale Roscoe). Food Funct. 2013, 4, 845–855. [Google Scholar] [CrossRef]
- Li, F.; Nitteranon, V.; Tang, X.; Liang, J.; Zhang, G.; Parkin, K.L.; Hu, Q. In vitro antioxidant and anti-inflammatory activities of 1-dehydro-[6]-gingerdione, 6-shogaol, 6-dehydroshogaol and hexahydrocurcumin. Food Chem. 2012, 135, 332–337. [Google Scholar] [CrossRef]
- Polasa, H.; Krishna, T.L.; Padmasree, K.; Rao, T.P. GC-MS analysis and evaluation of biological activities of different solvent extracts of ginger (Zingiber officinale Roscoe). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2567–2572. [Google Scholar]
- Nakatani, N.; Ohta, H.; Nakae, Y.; Imai, S.; Ohnishi, S. Scavenging effects of antioxidants from ginger against DPPH radical. Nat. Prod. Commun. 2008, 3, 709–712. [Google Scholar]
- Kim, H.J.; Kim, Y.M.; Kwon, H.Y.; Han, J.H.; Park, K.J. 6-Gingerol protects human keratinocytes from UVB-induced oxidative stress through activation of Nrf2 and PI3K/Akt signaling. J. Agric. Food Chem. 2016, 64, 8415–8423. [Google Scholar]
- Wu, C.; Sun, M.; Xiao, W. Antioxidant and anti-proliferative activity of ginger (Zingiber officinale Roscoe) extracts and their phenolic and flavonoid constituents. J. Food Biochem. 2019, 43, e12859. [Google Scholar]
- Ghafoor, A.; Ahmed, I.; Aslam, F. Ginger: A potential nutraceutical with anti-inflammatory and anti-oxidant properties. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 218–229. [Google Scholar]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Keyvani, F.; Pezeshki, A.; Gholian, M.M. Phytosome and liposome: The benificial encapsulation systems in drug delivery and food application. Appl. Food Biotechnol. 2015, 2, 17–27. [Google Scholar]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Tian, Y.; Zhang, X.; Du, M.; Li, F.; Xiao, M.; Zhang, W. Synergistic Antioxidant Effects of Araloside A and L-Ascorbic Acid on H2O2 Induced HEK293 Cells: Regulation of Cellular Antioxidant Status. Oxid. Med. Cell. Longev. 2021, 2021, 9996040. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, Additive, and Antagonistic Effects of Food Mixtures on Total Antioxidant Capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, F.; Zhang, X.; Li, P.; Zhang, X.; Wu, Z.; Li, D. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef] [PubMed]
- Posridee, K.; Oonsivilai, A.; Oonsivilai, R. Acute and sub-chronic toxicity study of Rang Chuet (Thunbergia laurifolia Lindl.) extracts and its antioxidant activities. Toxicol. Rep. 2022, 9, 2000–2017. [Google Scholar] [CrossRef]
- Sunthrarak, C.; Oonsivilai, R. Chemical Antioxidant Activity of Thunbergia Laurifolia Linn. (Rang Chuet) Leaves and its Combined Extracts. Food Appl. Biosci. J. 2022, 10, 13–29. [Google Scholar]
- Deligiannidou, G.-E.; Philippou, E.; Vasiari, E.; de Andrade, V.L.; Massaro, M.; Chervenkov, M.; Ivanova, T.; Jorge, R.; Dimitrova, D.; Ruskovska, T.; et al. Exploring the Relationship between Mediterranean Diet Adherence and Subjective Well-Being among Greek and Cypriot Adults. Nutrients 2024, 16, 1238. [Google Scholar] [CrossRef]
- Bitari, A.; Oualdi, I.; Touzani, R.; Elachouri, M.; Legssyer, A. Zingiber officinale Roscoe: A comprehensive review of clinical properties. Mater. Today Proc. 2023, 72, 3757–3767. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Longhin, E.; Mattioli, M.; Mantecca, P.; Tinaglia, V.; Mangano, E.; Battaglia, C. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol. Lett. 2012, 209, 136–145. [Google Scholar] [CrossRef]
- Hu, R.; Xie, X.-Y.; Xu, S.-K.; Wang, Y.-N.; Jiang, M.; Wen, L.-R.; Guan, L. PM (2.5) Exposure Elicits Oxidative Stress Responses and Mitochondrial Apoptosis Pathway Activation in HaCaT Keratinocytes. Chin. Med. J. 2017, 130, 2205–2214. [Google Scholar] [CrossRef]
- Li, S.G.; Ding, Y.S.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Guo, S.X. Grape Seed Proanthocyanidin Extract Alleviates Arsenic-induced Oxidative Reproductive Toxicity in Male Mice. Biomed. Environ. Sci. 2015, 28, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, I.; Sankareswaran, S.K.; Palanisamy, R. Morin Protects Human Respiratory Cells from PM(2.5) Induced Genotoxicity by Mitigating ROS and Reverting Altered mRNA Expression. Int. J. Environ. Res. Public Health 2019, 16, 2389. [Google Scholar] [CrossRef] [PubMed]
- Woottisin, N.; Sukprasert, S.; Kulsirirat, T.; Tharavanij, T.; Sathirakul, K. Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model. Molecules 2022, 27, 3884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, Q.; Ru, Q.; Chen, L.; Yue, K.; Tian, X.; Ma, B.; Li, C. Combined Effects of Fine Particulate Matter and Lipopolysaccharide on Apoptotic Responses in NR8383 Macrophages. J. Toxicol. Environ. Health Part A 2015, 78, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Prasongdee, P.; Posridee, K.; Oonsivilai, A.; Oonsivilai, R. A Culinary and Medicinal Gem: Exploring the Phytochemical and Functional Properties of Thai Basil. Foods 2024, 13, 632. [Google Scholar] [CrossRef]
- Moeurng, S.; Posridee, K.; Kamkaew, A.; Thaiudom, S.; Oonsivilai, A.; Oonsivilai, R. Identification of Pheophytin a and Hydroxy Pheophytin a from Rang Chuet (Thunbergia laurifolia Linn.) as Potent NQO-1 Inducers in Liver Cells. Foods 2024, 13, 1443. [Google Scholar] [CrossRef]
- Kachenpukdee, N.; Santerre, C.R.; Ferruzzi, M.; Oonsivilai, R. Modified dietary fiber from cassava pulp and assessment of mercury bioaccessibility and intestinal uptake using an in vitro digestion/Caco-2 model system. J. Food Sci. 2016, 81, T1854–T1863. [Google Scholar] [CrossRef] [PubMed]
- Kachenpukdee, N.; Santerre, C.R.; Ferruzzi, M.; Oonsivilai, R. Enzymatic digestion optimization of dietary fiber from cassava pulp and their effect on mercury bioaccessibility and intestinal uptake from fish using an in vitro digestion/Caco-2 model. Int. Food Res. J. 2016, 23, 660–666. [Google Scholar]
- Posridee, K.; Oonsivilai, A.; Oonsivilai, R. Maltodextrin from Sweet Cassava: A Promising Endurance Enhancer. Foods 2024, 13, 766. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Lee, T.-L.; Chen, Y.-C.; Liang, C.-J.; Wang, S.-H.; Lue, J.-H.; Chen, Y.-L. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef]
- Shim, I.; Kim, W.; Kim, H.; Lim, Y.M.; Shin, H.; Park, K.S.; Yu, S.D. Comparative Cytotoxicity Study of PM2.5 and TSP Collected from Urban Areas. Toxics 2021, 9, 167. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, J.; Sun, J.; Xin, L. PM2.5 induces inflammatory responses via oxidative stress-mediated mitophagy in human bronchial epithelial cells. Toxicol. Res. 2022, 11, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, C.; Qiao, Y.; Lu, C.; Li, J.; Song, W.; Wen, A. Safflower yellow B suppresses HepG2 cell injury induced by oxidative stress through the AKT/Nrf2 pathway. Int. J. Mol. Med. 2016, 37, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signaling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, W.; Guo, C. Tetrahydroxy stilbene glucoside ameliorates H2O2-induced human brain microvascular endothelial cell dysfunction in vitro by inhibiting oxidative stress and inflammatory responses. Mol. Med. Rep. 2017, 16, 5219–5224. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Zhang, X.J.; Yang, Y.; Zhang, C.; Zhu, C.H.; Miao, J.Y.; Chen, R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018, 13, 2119–2128. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Ding, W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. Vitr. 2013, 27, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hua, S.; Song, L. PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress. Oxidative Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.Y.; Liu, X.; Li, W.; Zu, Y.Y.; Zhou, F.M.; Shou, Q.Y.; Ding, Z.S. PM2.5 Exposure Induces Inflammatory Response in Macrophages via the TLR4/COX-2/NF-kappa B Pathway. Inflammation 2020, 43, 1948–1958. [Google Scholar] [CrossRef]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme oxygenase-1 in kidney health and disease. Antioxid. Redox Signal. 2016, 25, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Eramo, S.L.M.; Mancuso, C.; Troiani, D.; Paludetti, G. Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radic. Biol. Med. 2015, 85, 269–281. [Google Scholar] [CrossRef]
- Lv, Q.-Z.; Long, J.-T.; Gong, Z.-F.; Nong, K.-Y.; Liang, X.-M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Ge, C.; Tan, J.; Zhong, S.; Lai, L.; Chen, G.; Zhao, J.; Xu, M. Nrf2 mitigates prolonged PM2.5 exposure-triggered liver inflammation by positively regulating SIKE activity: Protection by Juglanin. Redox Biol. 2020, 36, 101645. [Google Scholar] [CrossRef] [PubMed]





| Treatments | Pre-Treatments | Activation |
|---|---|---|
| Control | Media (24 h) | Media (24 h) |
| PM2.5 (Negative control) | Media (24 h) | PM2.5 (24 h) |
| RWE | RWE 50 µg/mL (24 h) | Media (24 h) |
| GEE | GEE 50 µg/mL (24 h) | Media (24 h) |
| RWE + PM2.5 | RWE 50 µg/mL (24 h) | PM2.5 50 µg/mL (24 h) |
| GEE + PM2.5 | GEE 50 µg/mL (24 h) | PM2.5 50 µg/mL (24 h) |
| RWE: GEE (1:1) + PM2.5 | RWE: GEE (1:1) 50 µg/mL (24 h) | PM2.5 50 µg/mL (24 h) |
| RWE: GEE (1:10) + PM2.5 | RWE: GEE (1:10) 50 µg/mL (24 h) | PM2.5 50 µg/mL (24 h) |
| Target Gene | Forward Primer Sequence (5′ to 3′) | Reverse Primer Sequence (3′ to 5′) |
|---|---|---|
| HO-1 | ATGGCCTCCCTGTACCACATC | TGTTGCGCTCAATCTCCTCCT |
| SOD1 | GCAGGTCCTCACTTTAATCCTCT | ATCGGCCACACCATCTTTGT |
| CAT | TGAAGATGCGGCGAGACTTT | TGGATGTAAAAAGTCCAGGAGGG |
| NQO1 | GGATTGGACCGAGCTGGAA | AATTGCAGTGAAGATGAAGGCAAC |
| GAPDH | TCCAAAATCAAGTGGGGCGA | TGATGACCCTTTTGGCTCCC |
| Photochemical | RWE (µg/g of Extract) | GEE (µg/g of Extract) | |
|---|---|---|---|
| Phenolic compounds | Gallic acid | 128 ± 11 | ND |
| Proto-catechuic acid | 418 ± 14 | ND | |
| Caffeic acid | 919 ± 20 | 125 ± 0.58 | |
| Coumaric acid | 1025 ± 49 | 35 ± 0.81 | |
| Ferulic acid | ND | 72 ± 0.89 | |
| Sinapic acid | ND | ND | |
| Rosmarinic acid | 374 ± 14 | ND | |
| Flavonoids | Quercetin | ND | ND |
| Apigenin | 107 ± 2.9 | ND | |
| Total phenolic compounds and flavonoids | 2976 ± 19 | 232 ± 0.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunthrarak, C.; Posridee, K.; Noisa, P.; Shim, S.-M.; Thaiudom, S.; Oonsivilai, A.; Oonsivilai, R. Synergistic Antioxidant and Cytoprotective Effects of Thunbergia laurifolia Lindl and Zingiber officinale Extracts Against PM2.5-Induced Oxidative Stress in A549 and HepG2 Cells. Foods 2025, 14, 517. https://doi.org/10.3390/foods14030517
Sunthrarak C, Posridee K, Noisa P, Shim S-M, Thaiudom S, Oonsivilai A, Oonsivilai R. Synergistic Antioxidant and Cytoprotective Effects of Thunbergia laurifolia Lindl and Zingiber officinale Extracts Against PM2.5-Induced Oxidative Stress in A549 and HepG2 Cells. Foods. 2025; 14(3):517. https://doi.org/10.3390/foods14030517
Chicago/Turabian StyleSunthrarak, Chattip, Kakanang Posridee, Parinya Noisa, Soon-Mi Shim, Siwatt Thaiudom, Anant Oonsivilai, and Ratchadaporn Oonsivilai. 2025. "Synergistic Antioxidant and Cytoprotective Effects of Thunbergia laurifolia Lindl and Zingiber officinale Extracts Against PM2.5-Induced Oxidative Stress in A549 and HepG2 Cells" Foods 14, no. 3: 517. https://doi.org/10.3390/foods14030517
APA StyleSunthrarak, C., Posridee, K., Noisa, P., Shim, S.-M., Thaiudom, S., Oonsivilai, A., & Oonsivilai, R. (2025). Synergistic Antioxidant and Cytoprotective Effects of Thunbergia laurifolia Lindl and Zingiber officinale Extracts Against PM2.5-Induced Oxidative Stress in A549 and HepG2 Cells. Foods, 14(3), 517. https://doi.org/10.3390/foods14030517







