Crystallization Kinetics of Oleogels Prepared with Essential Oils from Thirteen Spices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oleogels
2.3. Measurement of Oil Binding Capacity
2.4. Determination of Textural Properties
2.5. Determination of Thermal Behavior
2.6. Rheology Test
2.7. X-Ray Diffraction
2.8. Statistical Analysis of Data
3. Results and Discussion
3.1. Analysis of Gelling Properties
3.2. Oil Binding Capacity
3.3. Texture Analysis
3.4. Analysis of Thermodynamic Properties
3.5. Analysis of Rheological Properties
3.6. X-Ray Diffraction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciuffarin, F.; Alongi, M.; Peressini, D.; Barba, L.; Lucci, P.; Calligaris, S. Role of the polyphenol content on the structuring behavior of liposoluble gelators in extra virgin olive oil. Food Chem. 2023, 412, 135572. [Google Scholar] [CrossRef] [PubMed]
- Dhal, S.; Alhamidi, A.; Al-Zahrani, S.M.; Anis, A.; Pal, K. The Influence of Emulsifiers on the Physiochemical Behavior of Soy Wax/Rice Bran Oil-Based Oleogels and Their Application in Nutraceutical Delivery. Gels 2023, 9, 47. [Google Scholar] [CrossRef]
- CM, O.S.; Davidovich-Pinhas, M.; Wright, A.J.; Barbut, S.; Marangoni, A.G. Ethylcellulose oleogels for lipophilic bioactive delivery—Effect of oleogelation on in vitro bioaccessibility and stability of beta-carotene. Food Funct. 2017, 8, 1438–1451. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.D.M.; Tur, J.A. Dietary fat intake and metabolic syndrome in adults: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Aiache, J.M.; Gauthier, P.; Aiache, S. New gelification method for vegetable oils I: Cosmetic application. Int. J. Cosmet. Sci. 1992, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, G.; Bogojevic, O.; Pedersen, J.N.; Guo, Z. Edible oleogels as solid fat alternatives: Composition and oleogelation mechanism implications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2077–2104. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Shabbir, M.A.; Tahir, F.; Khan, M.R.; Ahmed, W.; Yıkmış, S.; Manzoor, M.F.; Abdi, G.; Aadil, R.M. Development of oleogel by structuring the blend of corn oil and sunflower oil with beeswax to replace margarine in cookies. Food Chem. X 2024, 23, 101676. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M.; Yüceer, Y.K. Physical Properties, Volatiles Compositions and Sensory Descriptions of the Aromatized Hazelnut Oil-Wax Organogels. J. Food Sci. 2015, 80, S2035–S2044. [Google Scholar] [CrossRef]
- Kavya, M.; Udayarajan, C.; Fabra, M.J.; López-Rubio, A.; Nisha, P. Edible oleogels based on high molecular weight oleogelators and its prospects in food applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 4432–4455. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, C.; Wang, C.; Han, R.; Xie, S. Effects of hydrocolloids and oleogel on techno-functional properties of dairy foods. Food Chem. X 2024, 21, 101215. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.C.; de Souza Paglarini, C.; Rodrigues Pollonio, M.A.; Lopes Cunha, R. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2021, 174, 108424. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.C.; Okuro, P.K.; Badan, A.P.; Cunha, R.L. Role of the oil on glyceryl monostearate based oleogels. Food Res. Int. 2019, 120, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Ghan, S.Y.; Siow, L.F.; Tan, C.P.; Cheong, K.W.; Thoo, Y.Y. Palm Olein Organogelation Using Mixtures of Soy Lecithin and Glyceryl Monostearate. Gels 2022, 8, 30. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Fernandes, B.; Oliveira, M.C.; Marques, A.C.; Dos Santos, R.G.; Serrano, C. Microencapsulation of Essential Oils and Oleoresins: Applications in Food Products. Foods 2024, 13, 3873. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Ju, R.; Chen, K.; Bhandari, B.; Wang, H. Advances in efficient extraction of essential oils from spices and its application in food industry: A critical review. Crit. Rev. Food Sci. Nutr. 2023, 63, 11482–11503. [Google Scholar] [CrossRef]
- Asensio, C.M.; Nepote, V.; Grosso, N.R. Sensory attribute preservation in extra virgin olive oil with addition of oregano essential oil as natural antioxidant. J. Food Sci. 2012, 77, S294–S301. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, H.; Li, K. Litsea cubeba essential oil: Extraction, chemical composition, antioxidant and antimicrobial properties, and applications in the food industry. J. Food Sci. 2024, 89, 4583–4603. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.G. Antioxidant Activity of Essential Oils. Antioxidants 2023, 12, 383. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Yang, C.; Chen, Y.; Yang, Y.; Zhou, C.; Wang, L.; Xia, G.; Yu, X.; Yang, H. Preparation and characterization of oregano essential oil-loaded Dioscorea zingiberensis starch film with antioxidant and antibacterial activity and its application in chicken preservation. Int. J. Biol. Macromol. 2022, 212, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Geng, S.; Liu, C.; Jiang, J.; Liu, B. Preparation and characterization of lutein ester-loaded oleogels developed by monostearin and sunflower oil. J. Food Biochem. 2019, 43, e12992. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Y.; Zhang, Y.; Gao, Y.; Mao, L. Surfactant addition to modify the structures of ethylcellulose oleogels for higher solubility and stability of curcumin. Int. J. Biol. Macromol. 2020, 165, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Yu, J.; Gao, Y.; Mao, L. Rheology and Tribology of Ethylcellulose-Based Oleogels and W/O Emulsions as Fat Substitutes: Role of Glycerol Monostearate. Foods 2022, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Ramírez, D.; Lobato-Calleros, C.; Jaime Vernon-Carter, E.; Alvarez-Ramirez, J. Cooling rate, sorbitan and glyceryl monostearate gelators elicit different microstructural, viscoelastic and textural properties in chia seed oleogels. Food Res. Int. 2019, 119, 829–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Tang, C.; Li, Y. Crystallization Behavior and Physical Properties of Monoglycerides-Based Oleogels as Function of Oleogelator Concentration. Foods 2023, 12, 345. [Google Scholar] [CrossRef]
- Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8, 520. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öğütcü, M. Comparative analysis of olive oil organogels containing beeswax and sunflower wax with breakfast margarine. J. Food Sci. 2014, 79, E1732–E1738. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Kim, M.J.; Lee, S.Y.; Lee, J. Physicochemical properties and oxidative stability of oleogels made of carnauba wax with canola oil or beeswax with grapeseed oil. Food Sci. Biotechnol. 2017, 26, 79–87. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fasolin, L.H.; Picone, C.S.F.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Structural and mechanical properties of organogels: Role of oil and gelator molecular structure. Food Res. Int. 2017, 96, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gerlei, M.; Pierson, H.; Ponçot, M.; Kahn, C.J.F.; Linder, M. Chemical Composition and Crystallization Behavior of Oil and Fat Blends for Spreadable Fat Applications. Foods 2024, 13, 3305. [Google Scholar] [CrossRef]
- Palla, C.A.; Dominguez, M.; Carrín, M.E. An overview of structure engineering to tailor the functionality of monoglyceride oleogels. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2587–2614. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Morales-Rueda, J.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Peña-Gil, A.; Toro-Vazquez, J.F. The Effect of Shearing in the Thermo-mechanical Properties of Candelilla Wax and Candelilla Wax–Tripalmitin Organogels. Food Biophys. 2011, 6, 359–376. [Google Scholar] [CrossRef]
- Han, L.; Li, L.; Li, B.; Zhao, L.; Liu, G.; Liu, X.; Wang, X. Effect of high pressure microfluidization on the crystallization behavior of palm stearin—Palm olein blends. Molecules 2014, 19, 5348–5359. [Google Scholar] [CrossRef]
- Perța-Crișan, S.; Ursachi, C.; Chereji, B.D.; Munteanu, F.D. Oleogels-Innovative Technological Solution for the Nutritional Improvement of Meat Products. Foods 2022, 12, 131. [Google Scholar] [CrossRef]
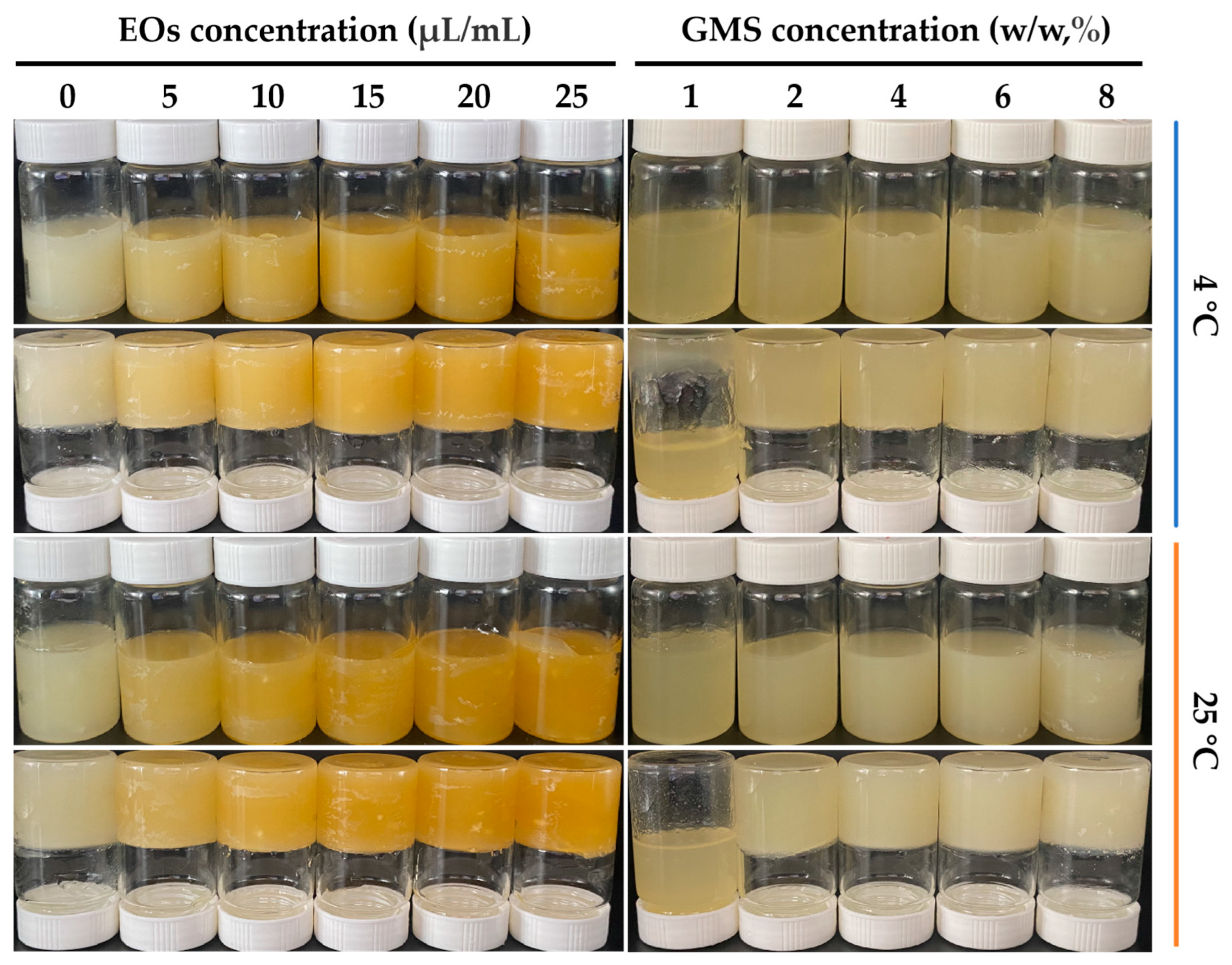
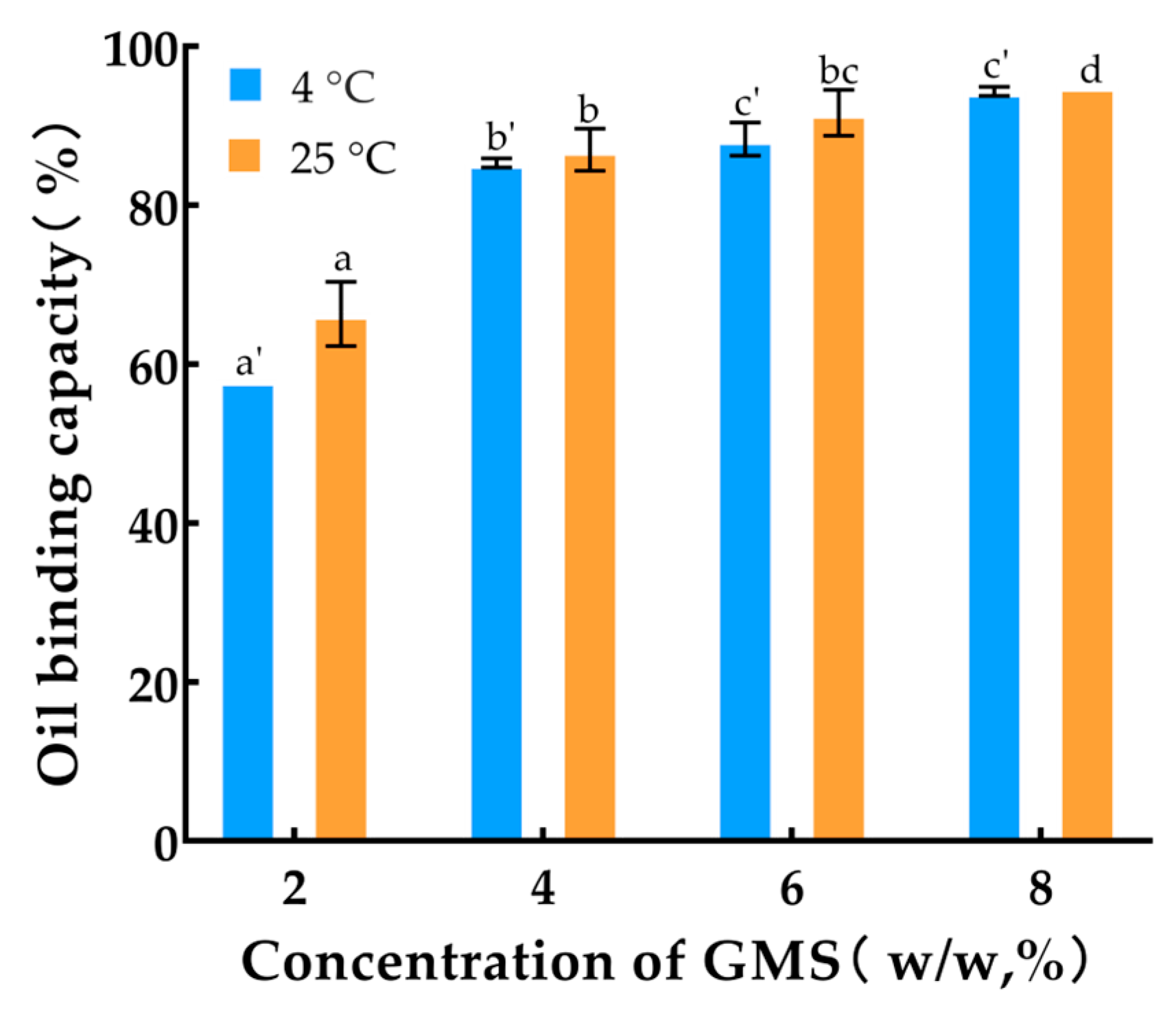
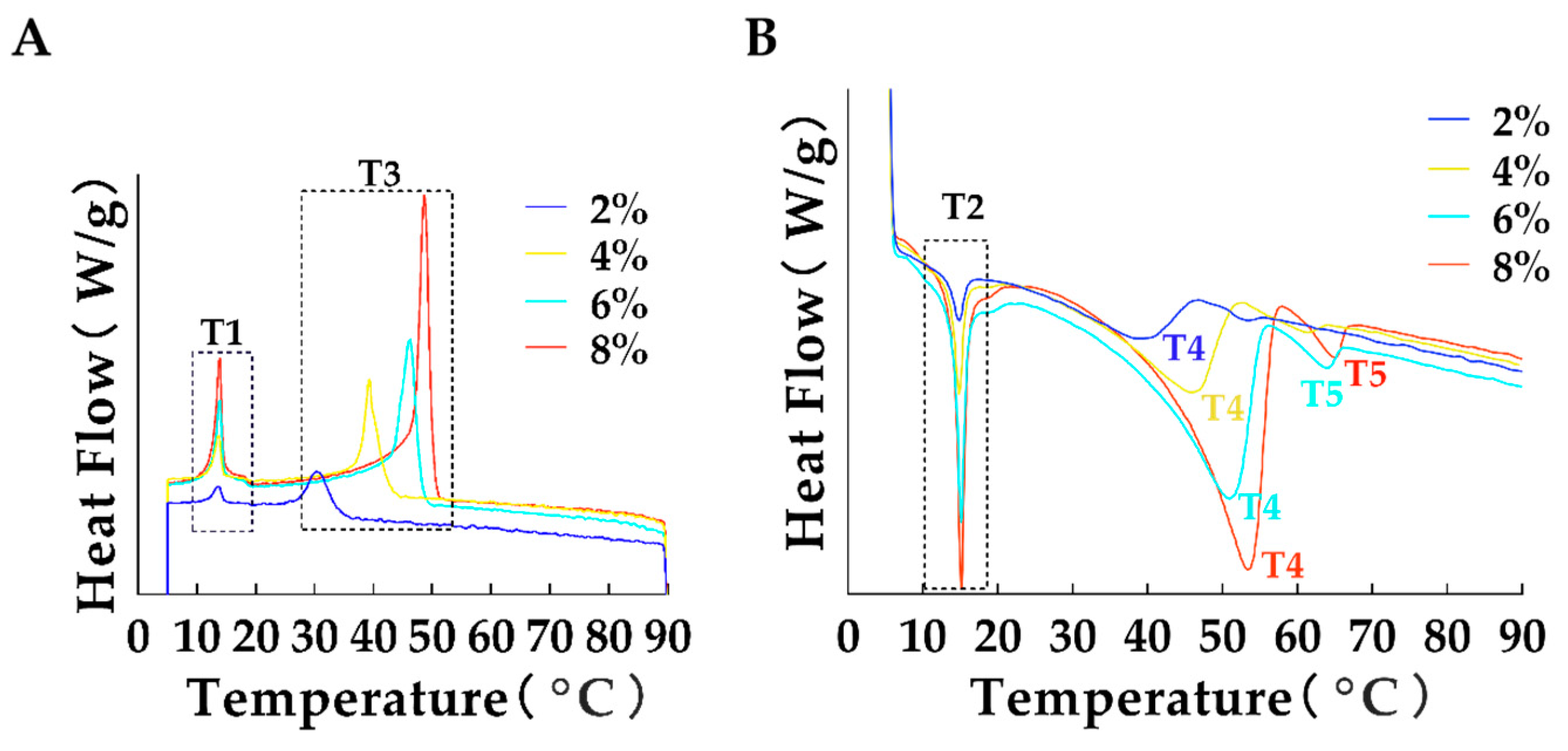
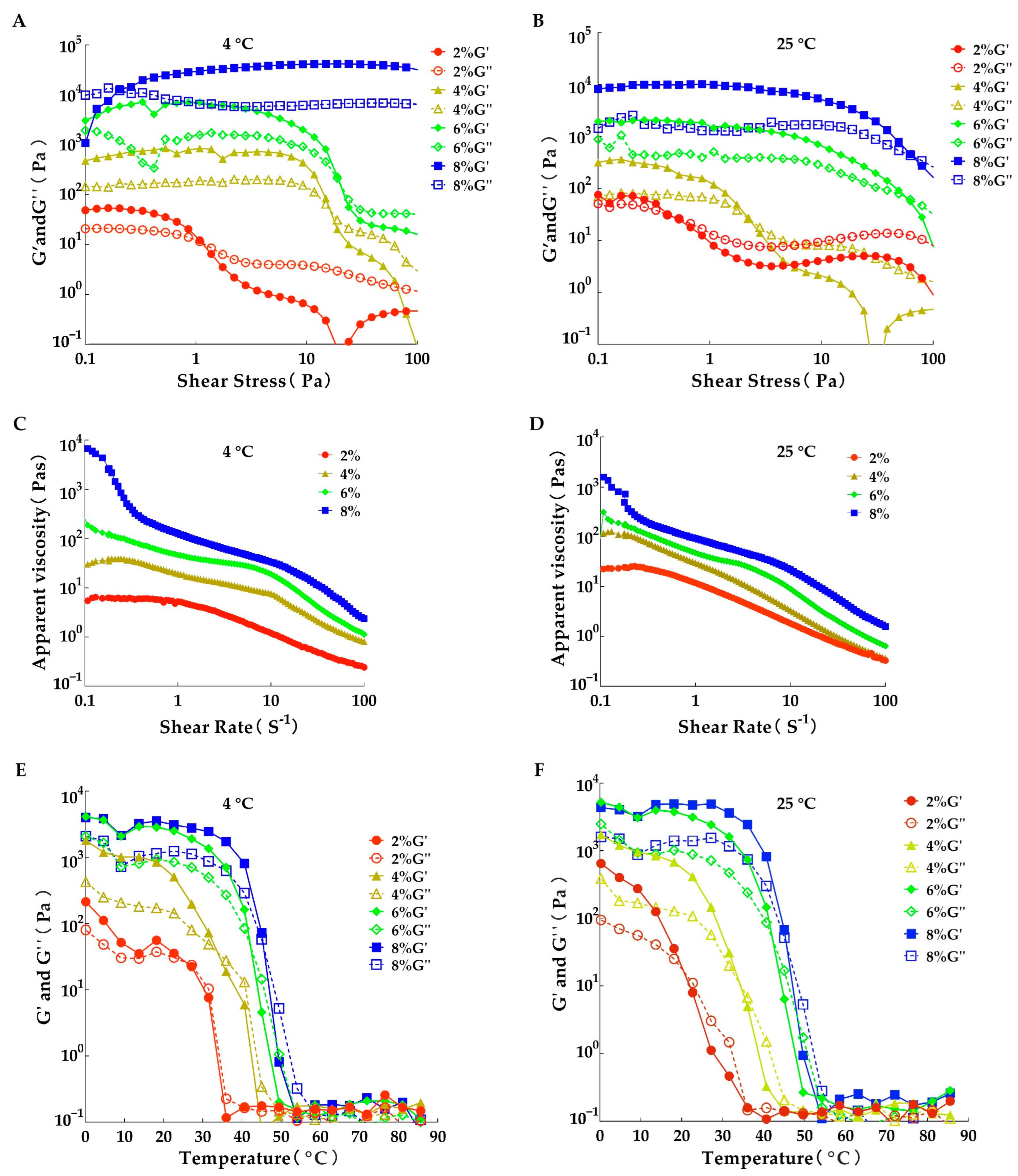

| Temperature (°C) | Concentration (μL/mL) | L* | a* | b* |
|---|---|---|---|---|
| 4 | 0 | 11.07 ± 0.70 b | −0.53 ± 0.15 d | 3.57 ± 0.64 b |
| 5 | 12.73 ± 2.20 ab | 0.87 ± 0.21 c | 5.23 ± 0.42 a | |
| 10 | 12.87 ± 0.12 ab | 1.17 ± 0.06 bc | 5.43 ± 0.23 a | |
| 15 | 12.97 ± 0.68 ab | 1.33 ± 0.21 b | 5.47 ± 0.15 a | |
| 20 | 13.33 ± 0.71 ab | 1.50 ± 0.30 b | 5.7 ± 0.10 a | |
| 25 | 14.23 ± 1.24 a | 2.10 ± 0.01 a | 5.90 ± 0.44 a | |
| 25 | 0 | 13.17 ± 0.59 a | −0.53 ± 0.06 e | 3.5 ± 0.20 b |
| 5 | 13.37 ± 0.94 a | 0.47 ± 0.15 d | 5.97 ± 0.50 a | |
| 10 | 13.57 ± 0.40 a | 0.97 ± 0.15 c | 6.00 ± 0.40 a | |
| 15 | 13.63 ± 0.90 a | 1.40 ± 0.17 b | 6.57 ± 0.21 a | |
| 20 | 14.10 ± 0.10 a | 1.70 ± 0.10 b | 6.63 ± 0.31 a | |
| 25 | 14.73 ± 1.00 a | 2.20 ± 0.20 a | 6.67 ± 0.38 a |
| Temperature (°C) | Concentration (w/w,%) | Hardness (N) | Brittleness (N) | Viscosity (N.ges) | Chewiness (N) |
|---|---|---|---|---|---|
| 4 | 2 | 35.83 ± 7.21 a | 31.9 ± 4.39 a | 15.51 ± 3.65 a | 15.40 ± 3.74 a |
| 4 | 128.97 ± 27.13 a | 125.70 ± 25.61 a | 33.01 ± 12.36 a | 32.37 ± 12.05 ab | |
| 6 | 323.77 ± 33.03 b | 325.67 ± 21.41 b | 64.12 ± 10.19 ab | 63.84 ± 10.09 ab | |
| 8 | 437.66 ± 79.50 c | 427.20 ± 75.88 b | 110.56 ± 39.77 b | 95.04 ± 54.52 b | |
| 25 | 2 | 20.32 ± 1.05 a | 18.05 ± 3.09 a | 11.86 ± 1.96 a | 11.75 ± 2.01 a |
| 4 | 62.36 ± 2.92 b | 49.06 ± 10.75 a | 29.29 ± 2.80 b | 29.11 ± 2.89 b | |
| 6 | 211.79 ± 31.90 c | 197.40 ± 28.63 b | 45.96 ± 3.04 c | 45.79 ± 2.88 c | |
| 8 | 332.00 ± 22.07 d | 262.24 ± 112.88 c | 99.48 ± 9.60 d | 94.50 ± 13.01 d |
| GMS Concentration (w/w,%) | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | T5 (°C) |
|---|---|---|---|---|---|
| 2 | 13.48 ± 1.29 a | 14.60 ± 1.36 a | 30.76 ± 3.27 b | 38.58 ± 7.68 b | -- |
| 4 | 13.50 ± 1.07 a | 14.73 ± 1.09 a | 39.26 ± 1.59 b | 44.06 ± 8.12 b | -- |
| 6 | 13.72 ± 0.93 a | 14.95 ± 0.99 a | 45.91 ± 2.45 b | 48.99 ± 7.14 b | 62.85 ± 3.48 c |
| 8 | 13.75 ± 0.92 a | 14.97 ± 0.97 a | 48.69 ± 1.55 b | 51.01 ± 6.99 b | 63.96 ± 2.96 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yu, L.; Wei, Z. Crystallization Kinetics of Oleogels Prepared with Essential Oils from Thirteen Spices. Foods 2025, 14, 542. https://doi.org/10.3390/foods14030542
Zhou W, Yu L, Wei Z. Crystallization Kinetics of Oleogels Prepared with Essential Oils from Thirteen Spices. Foods. 2025; 14(3):542. https://doi.org/10.3390/foods14030542
Chicago/Turabian StyleZhou, Wei, Lin Yu, and Zihao Wei. 2025. "Crystallization Kinetics of Oleogels Prepared with Essential Oils from Thirteen Spices" Foods 14, no. 3: 542. https://doi.org/10.3390/foods14030542
APA StyleZhou, W., Yu, L., & Wei, Z. (2025). Crystallization Kinetics of Oleogels Prepared with Essential Oils from Thirteen Spices. Foods, 14(3), 542. https://doi.org/10.3390/foods14030542







