Application of Stable Isotopes and Multi Elemental Fingerprints to Verify the Origin of Premium Chinese Hainan Bananas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Pre-Treatment
2.3. Stable Isotope Analysis
- (1)
- Stable carbon and nitrogen isotopes
- (2)
- Stable hydrogen and oxygen isotopes
- (3)
- Isotope calculation and reference materials
2.4. Elemental Analysis
2.5. Statistical Analysis
2.6. Chemometric Analysis
3. Results and Discussion
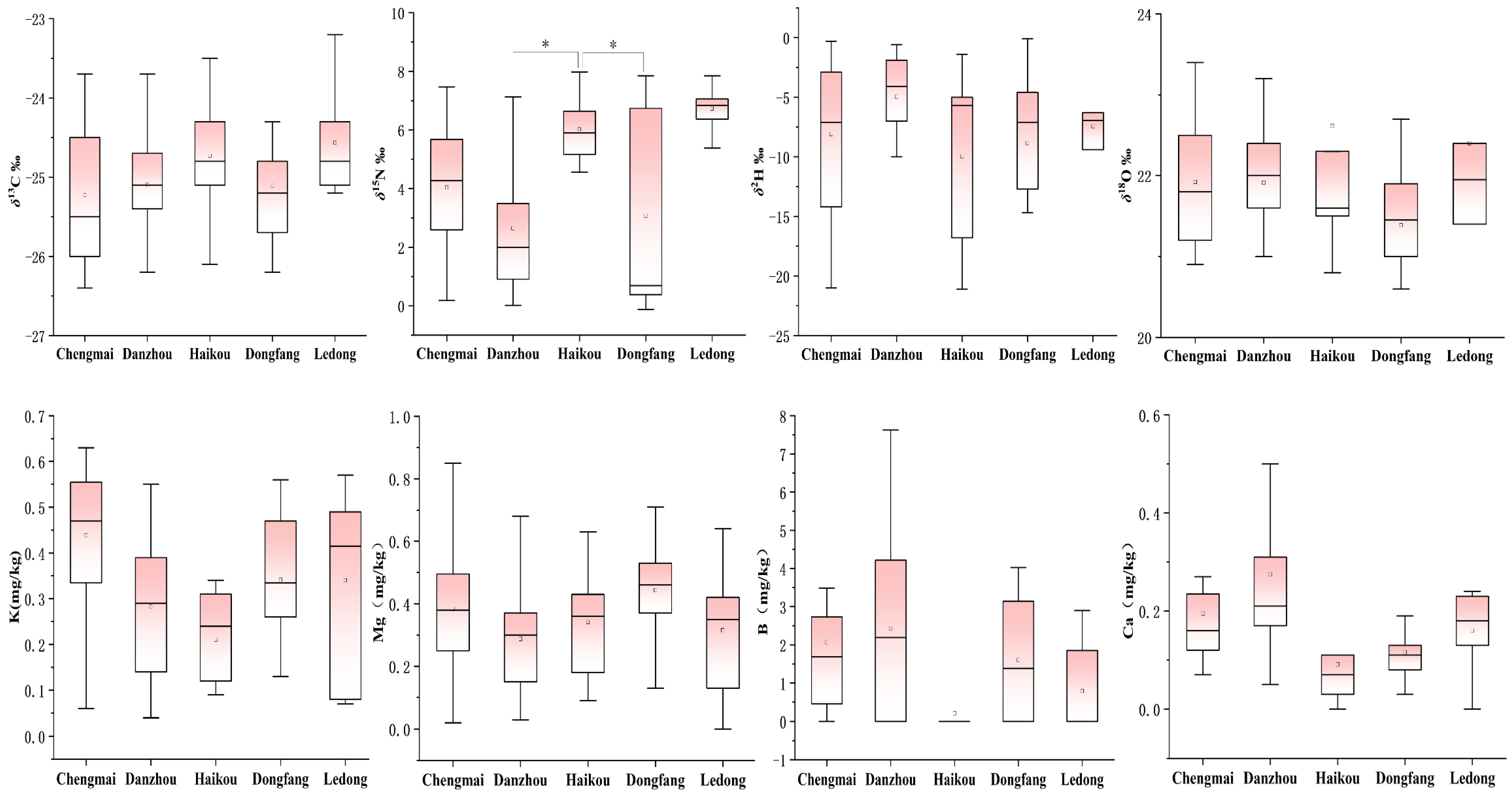
3.1. Stable Isotope and Multi-Elemental Characteristics of Hainan Bananas
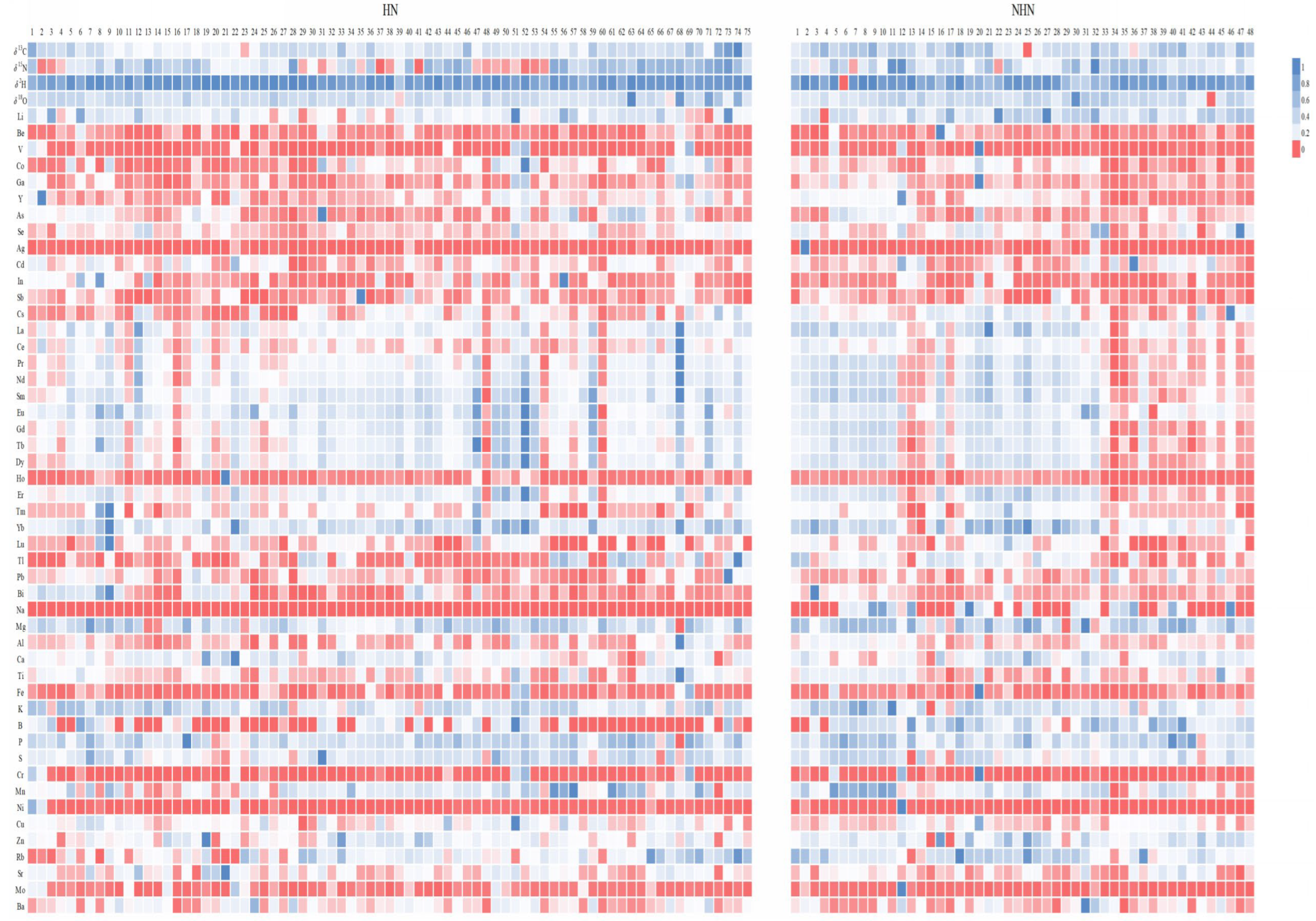
3.2. Differences Between Hainan and Non-Hainan Bananas
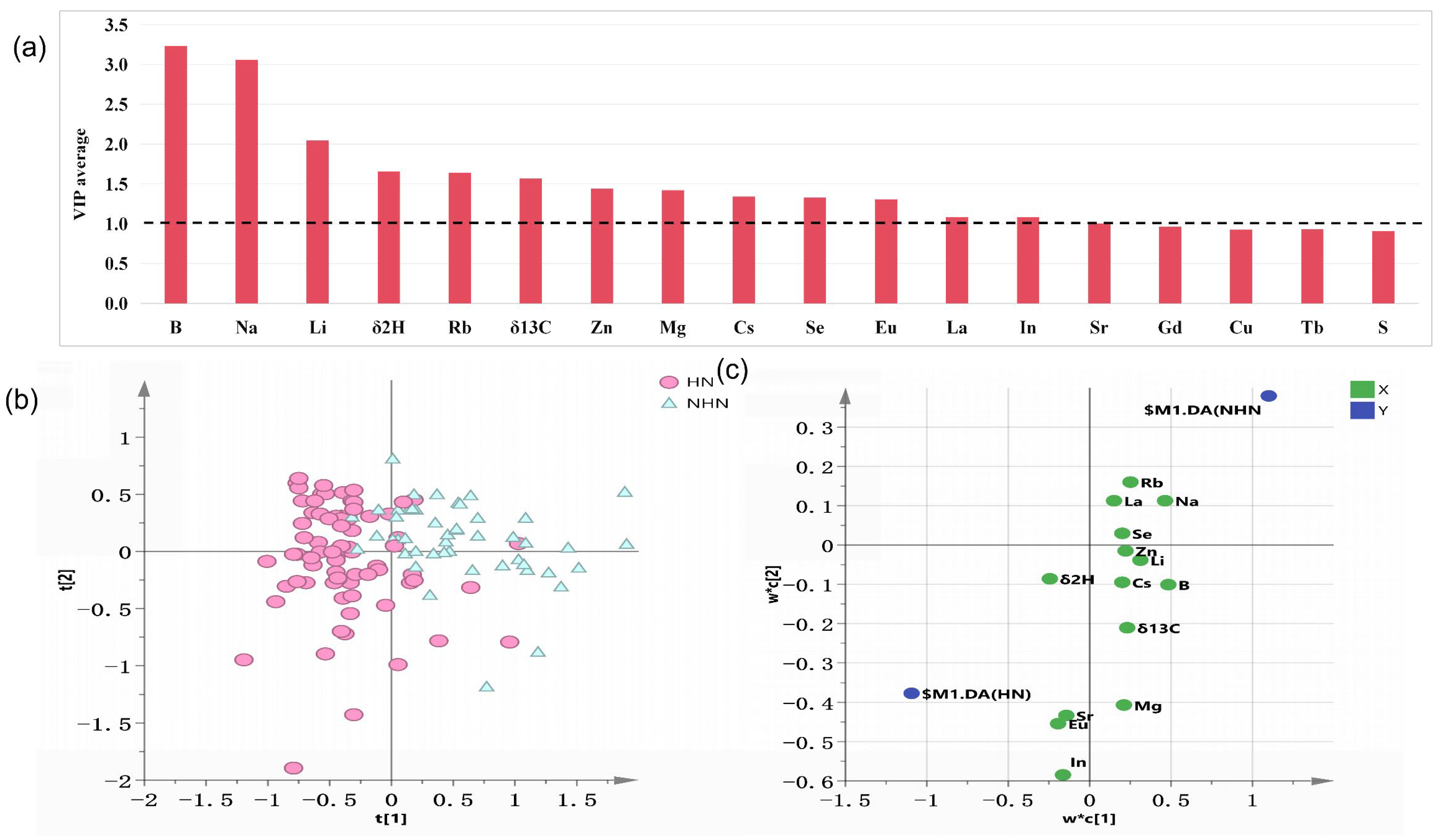
3.3. Model Performance and Key Variable Selection of PLS-DA Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, M.Q.; Liu, W.; Zhai, S.J.; Sui, G.X.; Cai, S.Y.; Cong, X.L. Characteristics, effect and application prospect of tropical fruit storage and preservation technology based on industrialization. J. Food Process. Preserv. 2024, 24, 70–79. [Google Scholar]
- Wei, J.D. Effect of Plant Cover on Soil Chemical Properties and Microbiological Characteristics of Banana Gardens; Yunnan Agricultural University: Kunming, China, 2022. [Google Scholar] [CrossRef]
- Luo, L.N.; Han, S.R.; Yi, Z.G.; Qi, X.G.; Wei, C.P.; Lu, Z.Y. Characteristics of banana bud absorption and bud removal technology. Mod. Agric. Sci. Technol. 2024, 3, 67–70. [Google Scholar]
- Chen, R.D. Study on the Influencing Factors of the Development Benefits of New Business Entities in Hainan Banana Industry; Hainan University: Haikou, China, 2022. [Google Scholar] [CrossRef]
- Huang, L.N.; Zhao, Z.X.; Cheng, S.M.; Wei, J.Y.; Wei, S.X. Analysis of the current situation and development countermeasures of Hainan Banana Industry. Trop. Agric. Sci. 2023, 43, 103–108. [Google Scholar]
- Li, S.; Huang, F. Research on the development of “smart Orchard” in Zhanjiang city. Sci. Exp. Ctry. 2018, 10, 93–94+124. [Google Scholar]
- Wang, H. Food Safety News Report and its development research—Review of The Audience Psychology Research of Food Safety Report. Food Ind. 2021, 42, 407–408. [Google Scholar]
- Zhang, X.D.; Ke, Y.P.; Wang, F. Empirical Analysis of Hainan Banana brand image from the perspective of consumers. Manag. Adm. 2021, 12, 57–62. [Google Scholar] [CrossRef]
- Kabir, A.N.M.F.; Alam, M.J.; Begum, I.A.; McKenzie, A.M. Consumers’ interest and willingness to pay for traceable vegetables—An empirical evidence from Bangladesh. Future Foods 2023, 7, 2666–8335. [Google Scholar] [CrossRef]
- Song, T.T.; Xia, Z.Z.; Liu, C.L.; Nie, J.; Zhou, Y.X.; Syed Abdul Wadood; Zhang, Y.Z.; Li, C.L.; Karyne, M.R.; Yuan, Y.W. Model optimization for geographical discrimination of Lentinula edodes based stable isotopes and multi-elements in China. J. Food Compost. Anal. 2023, 118, 105–160. [Google Scholar] [CrossRef]
- Peng, K.X.; Liu, H.; Liu, G.; Zhang, X.Z.; Li, F.; Tian, X.H.; Gong, X.H.; Wang, B.; Sun, C.X.; Xu, Y.J.; et al. Application research of stable isotope technology in food traceability of animal and plant sources. Sci. Technol. Food Ind. 2021, 42, 338–345. [Google Scholar] [CrossRef]
- Lan, X.; Jia, W.; Zhu, G.; Zhang, Y.; Yu, Z.; Luo, H. Spatial and Temporal Variation Characteristics of Stable Isotopes in Precipitation and Their Relationships with Meteorological Factors in the Shiyang River Basin in China. Water 2023, 15, 3836. [Google Scholar] [CrossRef]
- Ding, J.X.; Gu, Z.R.; Wang, Y.L.; Zhang, Y.Y.; Wang, Y.P.; Sun, Y.J. Correlation Analysis Between Common Peaks of Angelica sinensis HPLC Fingerprint and Mineral Elements in Its Growing Soil. Zhong Yao Cai 2015, 38, 1595–1599. [Google Scholar] [PubMed]
- Le Anh Cao, K.; Rahmatika, A.M.; Kitamoto, Y.; Nguyen, M.T.T.; Ogi, T. Controllable synthesis of spherical carbon particles transition from dense to hollow structure derived from Kraft lignin. J. Colloid Interface Sci. 2021, 589, 252–263. [Google Scholar] [CrossRef]
- Brombin, V.; Mistri, E.; Bianchini, G. Multi stable isotope ratio analysis for the traceability of northern Italian apples. Food Chem. X 2022, 16, 100514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, H.; Pan, J.; Chen, H.; Jin, B.; Yan, Z.; Xie, L.; Rogers, K.M. Geographical traceability of south-east Asian durian: A chemometric study using stable isotopes and elemental compositions. J. Food Compost. Anal. 2021, 101, 103940. [Google Scholar] [CrossRef]
- Coelho, I.; Matos, A.S.; Teixeira, R.; Nascimento, A.; Bordado, J.; Donard, O.; Castanheira, I. Combining multielement analysis and chemometrics to trace the geographical origin of Rocha Pear. J. Food Compost. Anal. 2019, 77, 889–1575. [Google Scholar] [CrossRef]
- Wen, J.; Li, J.; Wang, D.; Li, C.; Robbat, A.; Xia, L. Identification of geographical origin of winter jujube based on GC–MS coupled with machine-learning algorithms. J. Integr. Agric. 2024, 124, 105710. [Google Scholar] [CrossRef]
- Zhang, Y. Source of Origin Study of Sweet Cherry Based on Stability Isotope. Master’s Thesis, Yantai University, Yantai, China, 2020. [Google Scholar] [CrossRef]
- Li, Q.H. Analysis of Blueberry Origin Traceability Based on Strontium Isotopes and Mineral Elements. Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar] [CrossRef]
- Bin, L.; Wang, C.; Liu, Z.; He, W.; Zhao, D.; Fang, Y.; Li, Y.; Zhang, Z.H.; Chen, P.; Liu, W. Geographical origin traceability of muskmelon from Xinjiang province using stable isotopes and multi-elements with chemometrics. J. Food Compost. Anal. 2022, 106, 104320. [Google Scholar] [CrossRef]
- Mei, H.Y.; Nie, J.; Pan, M.Z.; Rogers, K.M.; Guo, Z.C.; Li, C.L.; Shao, S.Z.; Zhang, Y.Z.; Yuan, Y.W. Geographical origin identification of Pujiang Kyoho grapes in China using stable isotope and elemental profiles. Food Control 2024, 162, 110454. [Google Scholar] [CrossRef]
- Rogers, K.M. Nitrogen isotopes as a screening tool to determine the growing regimen of some organic and nonorganic supermarket produce from New Zealand. J. Agric. Food. Chem. 2008, 56, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; He, Y.; Li, S.; Deng, J.; Avula, B.; Zhang, J.; Pugh, N.D.; Solís-Sáinz, J.C.; Wang, M.; Katragunta, K. Proximate composition and nutritional analysis of selected bananas cultivated in Hainan, China. J. Food Compost. Anal. 2024, 125, 105798. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, Z.H.; Zhang, L.D. Evaluation of differential nutritional characteristics of Chinese banana 9 and Brazilian banana ears. South China Fruits 2024, 53, 123–128. [Google Scholar] [CrossRef]
- Wang, X.T.; Shen, Q.; Guleng, B.J.; Kang, Q.; Pan, J.X.; He, W.Z.; Wang, C. Based on OPLS-DA analysis of five provinces melon mineral element content characteristics and RNI dietary evaluation. J. Mod. Food Technol. 2024, 41, 1–9. [Google Scholar] [CrossRef]
- Vantyghem, M.; Merckx, R.; Stevens, B.; Hood-Nowotny, R.; Swennen, R.; Dercon, G. The potential of stable carbon isotope ratios and leaf temperature as proxies for drought stress in banana under field conditions. Agric. Water Manag. 2021, 260, 107247. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Chai, Y.H.; Lim, J.W.; Ho, Y.C.; Tan, I.S.; Lau, S.Y.; Show, P.L.; Lee, K.T. Unlocking the potential of microalgae bio-factories for carbon dioxide mitigation: A comprehensive exploration of recent advances, key challenges, and energy-economic insights. Bioresour. Technol. 2023, 380, 129094. [Google Scholar] [CrossRef]
- Szpak, P.; Longstaffe, F.J.; Millaire, J.F.; White, C.D. Large variation in nitrogen isotopic composition of a fertilized legume. J. Archaeol. Sci. 2014, 45, 72–79. [Google Scholar] [CrossRef]
- Liao, P.; Liu, L.; Chen, J.; Sun, Y.N.; Huang, S.; Zeng, Y.J.; Van Groenigen, K.J. Liming reduces nitrogen uptake from chemical fertilizer but increases that from straw in a double rice cropping system. Soil Till. Res. 2023, 235, 105873. [Google Scholar] [CrossRef]
- Meng, Y.C.; Jin, B.H.; Rogers, K.M.; Zhou, H.C.; Song, X.; Zhang, Y.H.; Lin, G.H.; Wu, H. Hydrogen and oxygen isotope fractionation effects in different organ tissues of grapes under drought conditions. J. Agric. Food Chem. 2023, 71, 13662–13671. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Serret, M.D.; Marimon, R.M.; Bort, J.; Araus, J.L. The hydrogen isotope composition δ2H reflects plant performance. Plant Physiol. 2019, 180, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.M. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J. Food Compost. Anal. 2006, 19, 434–445. [Google Scholar] [CrossRef]
- Tescione, I.; Marchionni, S.; Casalini, M.; Vignozzi, N.; Mattei, M.; Conticelli, S. 87Sr/86Sr isotopes in grapes of different cultivars: A geochemical tool for geographic traceability of agriculture products. Food Chem. 2018, 258, 374–380. [Google Scholar] [CrossRef]
- Tommasini, S.; Marchionni, S.; Tescione, I.; Casalini, M.; Braschi, E.; Avanzinelli, R.; Conticelli, S. Strontium Isotopes in Biological Material: A Key Tool for the Geographic Traceability of Foods and Humans Beings. In Behaviour of Strontium in Plants and the Environment; Gupta, D., Walther, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 145–166. [Google Scholar] [CrossRef]
- Tixier, P.; Loeillet, D.; Coulis, M.; Lescot, T.; de Lapeyre de Bellaire, L. Isotopes Don’t Lie, differentiating organic from conventional banana (Musa AAA, Cavendish subgroup) fruits using C and N stable isotopes. Food Chem. 2022, 394, 133491. [Google Scholar] [CrossRef]
- Wang, Z.; Erasmus, S.W.; Dekker, P.; Guo, B.; Stoorvogel, J.J.; van Ruth, S.M. Linking growing conditions to stable isotope ratios and elemental compositions of Costa Rican bananas (Musa spp.). Food Res. Int. 2019, 129, 108882. [Google Scholar] [CrossRef]
| Variables | Hainan Samples | Non-Hainan Samples | p | ||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | ||
| δ13C (‰) | −26.7 | −22.2 | −25.1 | −27.0 | −22.3 | −24.3 | 0.001 |
| δ15N (‰) | −0.1 | 8.8 | 3.6 | 0.4 | 10.7 | 3.9 | 0.019 |
| δ2H (‰) | −16.5 | 30.0 | 6.1 | −11.3 | 62.1 | 10.8 | 0.073 |
| δ18O (‰) | 18.9 | 28.8 | 22.0 | 18.2 | 28.3 | 22.0 | 0.627 |
| Na (mg/kg) | 0.00 | 0.09 | 0.01 | 0.00 | 0.07 | 0.01 | 0.010 |
| Mg (mg/kg) | 2.22 | 6.14 | 3.98 | 2.29 | 6.84 | 4.07 | 0.189 |
| Al (mg/kg) | 0.00 | 0.10 | 0.01 | 0.00 | 0.02 | 0.01 | 0.033 |
| Ca (mg/kg) | 0.48 | 3.22 | 1.18 | 0.56 | 4.26 | 1.33 | 0.018 |
| K (mg/kg) | 2.76 | 10.54 | 5.28 | 3.08 | 7.65 | 5.38 | 0.332 |
| Ti (mg/kg) | 0.02 | 0.29 | 0.06 | 0.02 | 0.11 | 0.04 | 0.036 |
| Fe (mg/kg) | 0.03 | 2.39 | 0.17 | 0.03 | 0.22 | 0.08 | 0.085 |
| B (mg/kg) | 0.00 | 9.70 | 1.87 | 0.00 | 8.50 | 2.16 | 0.031 |
| P (mg/kg) | 0.40 | 1.06 | 0.74 | 0.43 | 1.27 | 0.71 | 0.369 |
| S (mg/kg) | 0.23 | 0.85 | 0.41 | 0.22 | 0.68 | 0.35 | <0.001 |
| V (mg/kg) | 0.00 | 0.53 | 0.03 | 0.00 | 0.18 | 0.01 | 0.388 |
| Li (µg/kg) | 0.00 | 258.45 | 74.04 | 10.53 | 197.70 | 74.89 | 0.113 |
| Be (µg/kg) | 0.15 | 49.55 | 2.52 | 0.15 | 10.68 | 1.66 | 0.735 |
| Cr (µg/kg) | 0.00 | 67.33 | 3.02 | 0.00 | 41.19 | 2.23 | 0.764 |
| Co (µg/kg) | 3.41 | 169.72 | 58.31 | 3.08 | 103.76 | 32.84 | 0.001 |
| Mn (µg/kg) | 3.41 | 169.72 | 47.73 | 3.08 | 143.06 | 49.38 | 0.045 |
| Ni (µg/kg) | 0.00 | 6.18 | 0.61 | 0.00 | 150.26 | 7.26 | 0.099 |
| Cu (µg/kg) | 0.85 | 31.52 | 4.74 | 1.95 | 12.41 | 5.06 | 0.846 |
| Zn (µg/kg) | 5.46 | 33.78 | 13.05 | 5.87 | 38.44 | 11.52 | 0.699 |
| Ga (µg/kg) | 0.50 | 24.60 | 2.36 | 0.28 | 7.15 | 1.42 | 0.117 |
| Rb (µg/kg) | 1.66 | 46.27 | 15.79 | 0.66 | 41.78 | 7.31 | <0.001 |
| Sr (µg/kg) | 0.27 | 2.76 | 1.03 | 0.32 | 8.57 | 1.64 | 0.033 |
| Y (µg/kg) | 3.08 | 35.10 | 11.34 | 1.85 | 102.18 | 10.46 | 0.675 |
| As (µg/kg) | 0.45 | 62.43 | 7.20 | 0.00 | 18.60 | 5.10 | 0.163 |
| Se (µg/kg) | 1.15 | 60.25 | 7.21 | 0.00 | 42.23 | 7.21 | 0.221 |
| Mo (µg/kg) | 0.02 | 10.18 | 0.78 | 0.01 | 45.31 | 2.49 | 0.199 |
| Ag (µg/kg) | 0.00 | 0.42 | 0.01 | 0.00 | 0.08 | 0.00 | 0.401 |
| Cd (µg/kg) | 0.30 | 3.58 | 1.37 | 0.28 | 15.88 | 2.95 | 0.001 |
| In (µg/kg) | 0.00 | 12.75 | 0.71 | 0.05 | 10.75 | 1.18 | 0.459 |
| Sb (µg/kg) | 0.00 | 62.10 | 4.16 | 0.00 | 6.78 | 2.27 | 0.051 |
| Cs (µg/kg) | 2.10 | 141.53 | 22.30 | 0.28 | 66.58 | 9.10 | 0.002 |
| Ba (µg/kg) | 0.39 | 5.08 | 1.40 | 0.30 | 12.75 | 2.17 | 0.066 |
| La (µg/kg) | 1.48 | 24.98 | 7.03 | 0.73 | 20.88 | 4.77 | 0.082 |
| Ce (µg/kg) | 1.95 | 44.95 | 6.65 | 0.95 | 23.33 | 5.54 | 0.412 |
| Pr (µg/kg) | 0.20 | 4.23 | 1.08 | 0.18 | 2.30 | 0.65 | 0.005 |
| Nd (µg/kg) | 1.13 | 16.13 | 4.61 | 0.70 | 11.50 | 2.73 | 0.001 |
| Sm (µg/kg) | 0.18 | 2.90 | 0.94 | 0.08 | 2.33 | 0.54 | <0.001 |
| Eu (µg/kg) | 0.10 | 1.05 | 0.30 | 0.03 | 0.83 | 0.29 | 0.425 |
| Gd (µg/kg) | 0.25 | 4.53 | 1.27 | 0.15 | 3.15 | 0.74 | <0.001 |
| Tb (µg/kg) | 0.05 | 0.68 | 0.18 | 0.03 | 0.50 | 0.11 | 0.005 |
| Dy (µg/kg) | 0.15 | 3.43 | 0.92 | 0.10 | 1.98 | 0.51 | <0.001 |
| Ho (µg/kg) | 0.03 | 0.63 | 0.19 | 0.03 | 6.38 | 0.25 | 0.655 |
| Er (µg/kg) | 0.10 | 1.73 | 0.48 | 0.05 | 0.90 | 0.28 | 0.002 |
| Tm (µg/kg) | 0.00 | 0.33 | 0.06 | 0.00 | 0.48 | 0.05 | 1.000 |
| Yb (µg/kg) | 0.03 | 0.70 | 0.30 | 0.00 | 0.73 | 0.20 | 0.002 |
| Lu (µg/kg) | 0.00 | 0.30 | 0.05 | 0.00 | 0.53 | 0.05 | 0.638 |
| Tl (µg/kg) | 0.43 | 83.38 | 15.69 | 0.00 | 19.03 | 3.43 | <0.001 |
| Pb (µg/kg) | 8.98 | 559.23 | 52.28 | 9.50 | 252.48 | 61.49 | 0.696 |
| Bi (µg/kg) | 0.00 | 5.90 | 0.43 | 0.00 | 5.68 | 0.80 | 0.133 |
| Data | Index | Model 1 (53 Variables) | Model 2 (14 Variables) |
|---|---|---|---|
| Training set | HN samples | 90.6 | 92.7 |
| NHN samples | 72.5 | 78.8 | |
| Overall accuracy | 83.7 | 87.3 | |
| Test set | HN samples | 91.8 | 96.6 |
| NHN samples | 71.8 | 82.4 | |
| Overall accuracy | 84.6 | 90.9 | |
| All | HN samples | 90.9 | 93.7 |
| NHN samples | 72.3 | 79.7 | |
| Overall accuracy | 83.9 | 88.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Mei, H.; Zhang, Y.; Wang, M.; Huan, Z.; Nie, J.; Rogers, K.M.; Nuralykyzy, B.; Li, C.; Yuan, Y. Application of Stable Isotopes and Multi Elemental Fingerprints to Verify the Origin of Premium Chinese Hainan Bananas. Foods 2025, 14, 554. https://doi.org/10.3390/foods14040554
Huang Y, Mei H, Zhang Y, Wang M, Huan Z, Nie J, Rogers KM, Nuralykyzy B, Li C, Yuan Y. Application of Stable Isotopes and Multi Elemental Fingerprints to Verify the Origin of Premium Chinese Hainan Bananas. Foods. 2025; 14(4):554. https://doi.org/10.3390/foods14040554
Chicago/Turabian StyleHuang, Yurong, Hanyi Mei, Yongzhi Zhang, Mingyue Wang, Zhibo Huan, Jing Nie, Karyne M. Rogers, Bayan Nuralykyzy, Chunlin Li, and Yuwei Yuan. 2025. "Application of Stable Isotopes and Multi Elemental Fingerprints to Verify the Origin of Premium Chinese Hainan Bananas" Foods 14, no. 4: 554. https://doi.org/10.3390/foods14040554
APA StyleHuang, Y., Mei, H., Zhang, Y., Wang, M., Huan, Z., Nie, J., Rogers, K. M., Nuralykyzy, B., Li, C., & Yuan, Y. (2025). Application of Stable Isotopes and Multi Elemental Fingerprints to Verify the Origin of Premium Chinese Hainan Bananas. Foods, 14(4), 554. https://doi.org/10.3390/foods14040554







