Effect of Food Matrix on Regulation of Intestinal Barrier and Microbiota Homeostasis by Polysaccharides Sulfated Carrageenan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
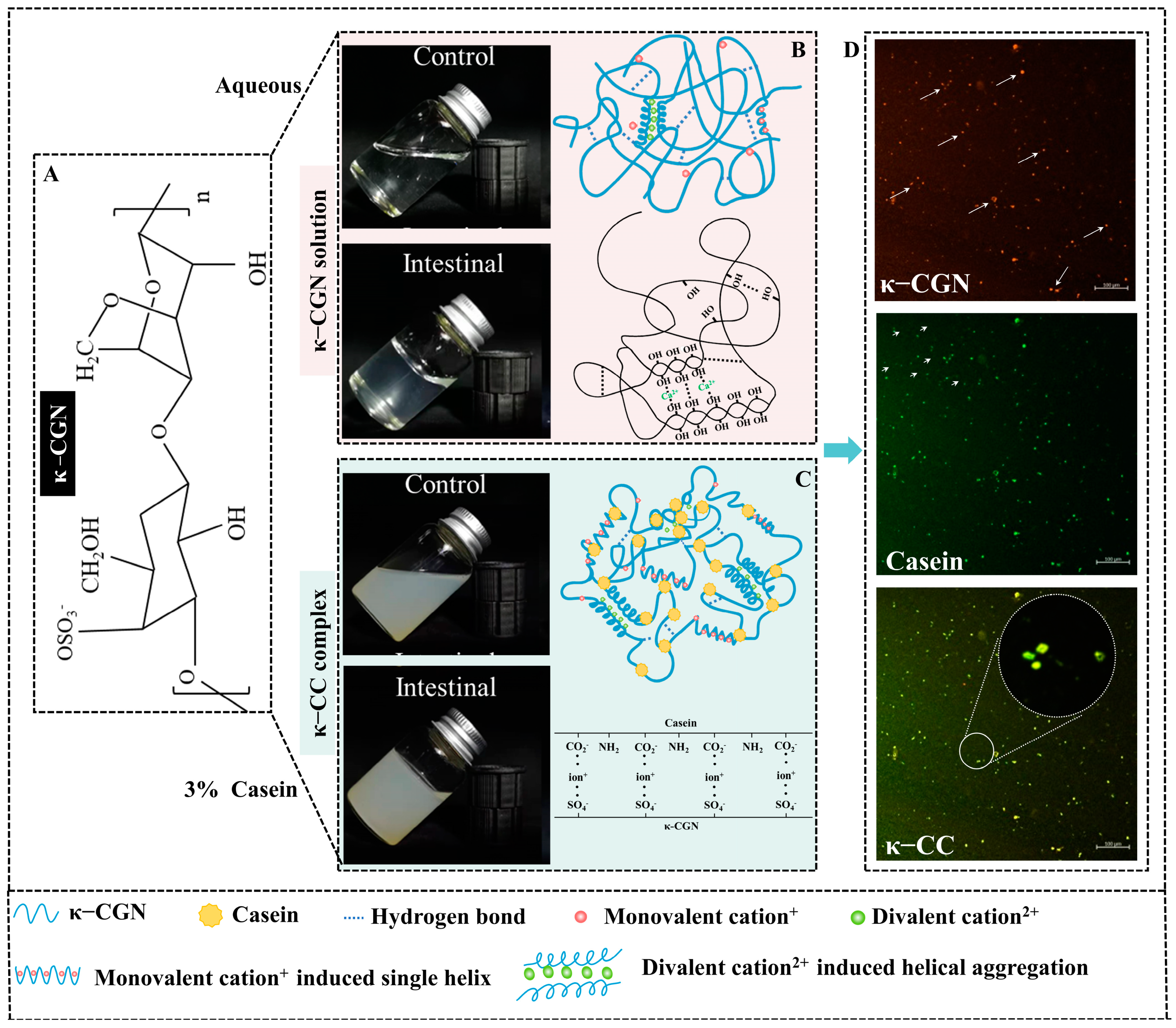
2.2. Preparation of κ-CGN and κ-CC
2.3. Animals and Experimental Design
2.4. Sample Collection
2.5. Disease Activity Index (DAI) Assessment
2.6. Organ Index and Colon Length
2.7. Serum Analysis
2.8. Histological Analysis
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Immunohistochemistry Staining
2.11. Western Blot Analysis
2.12. Fecal Microbial Community Analysis
2.13. Statistical Analysis
3. Results
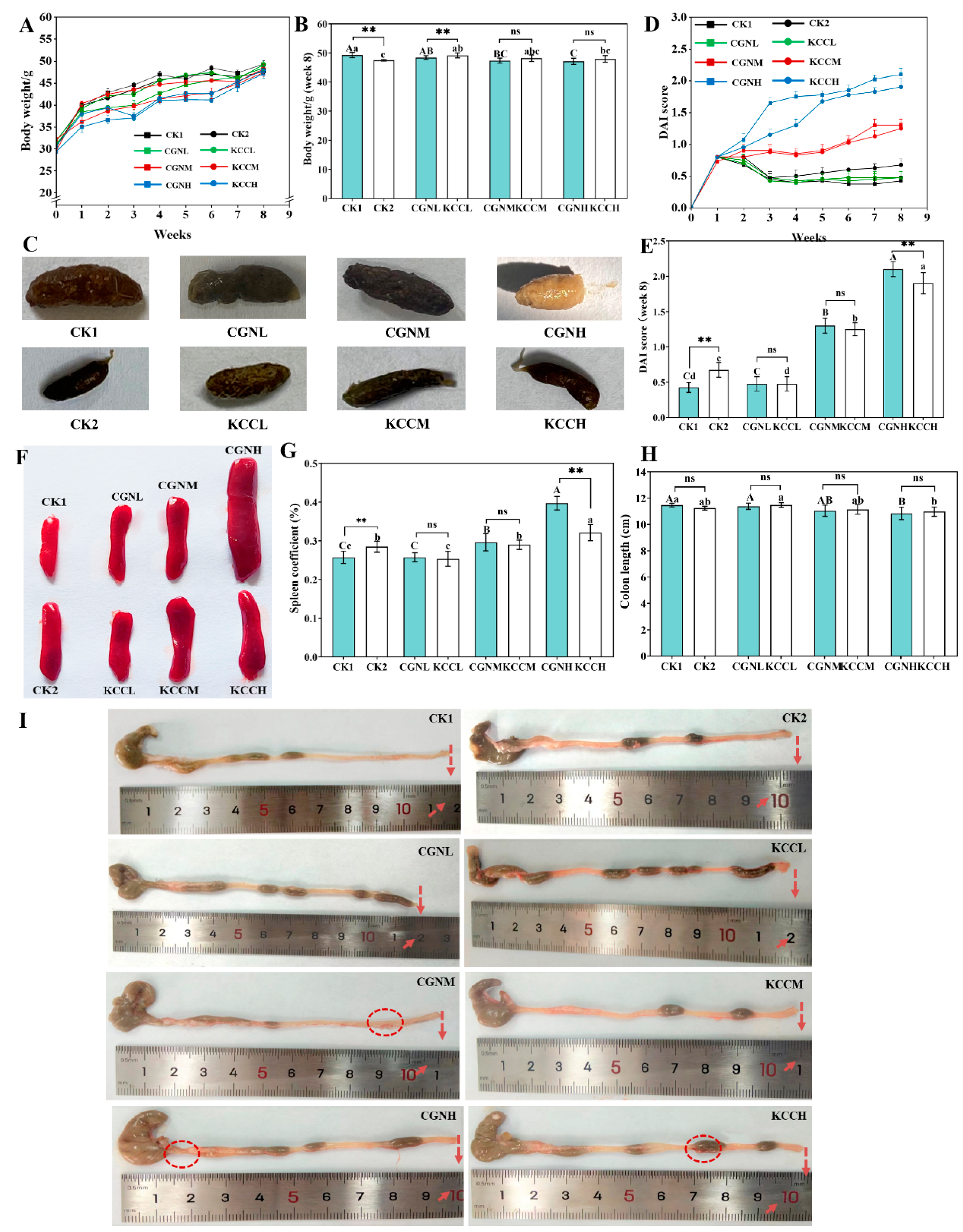
3.1. Effects of κ-CGN and κ-CC on Clinical Symptom in Mice
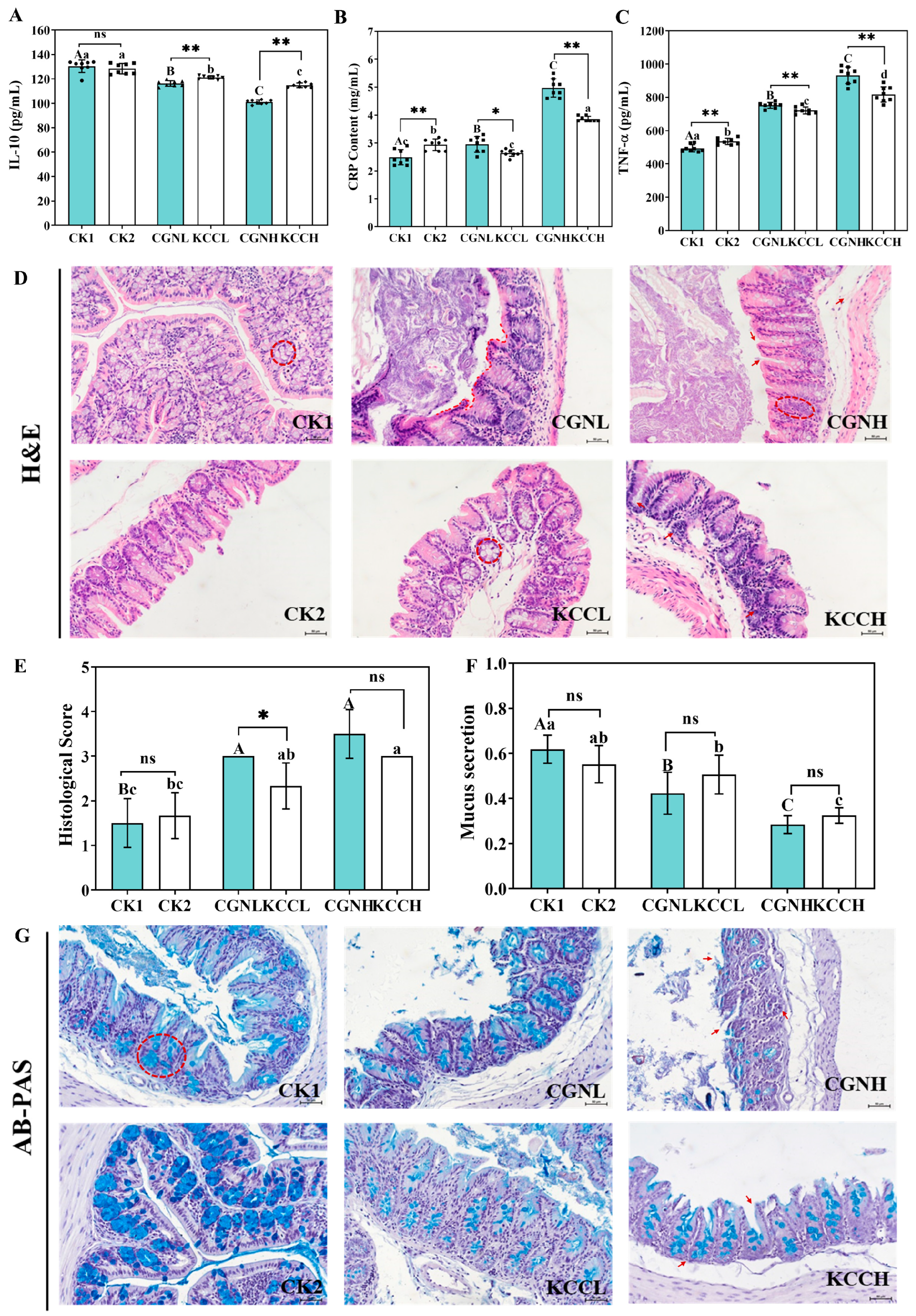
3.2. Effects of κ-CGN and κ-CC on Inflammatory Cytokines in Mice
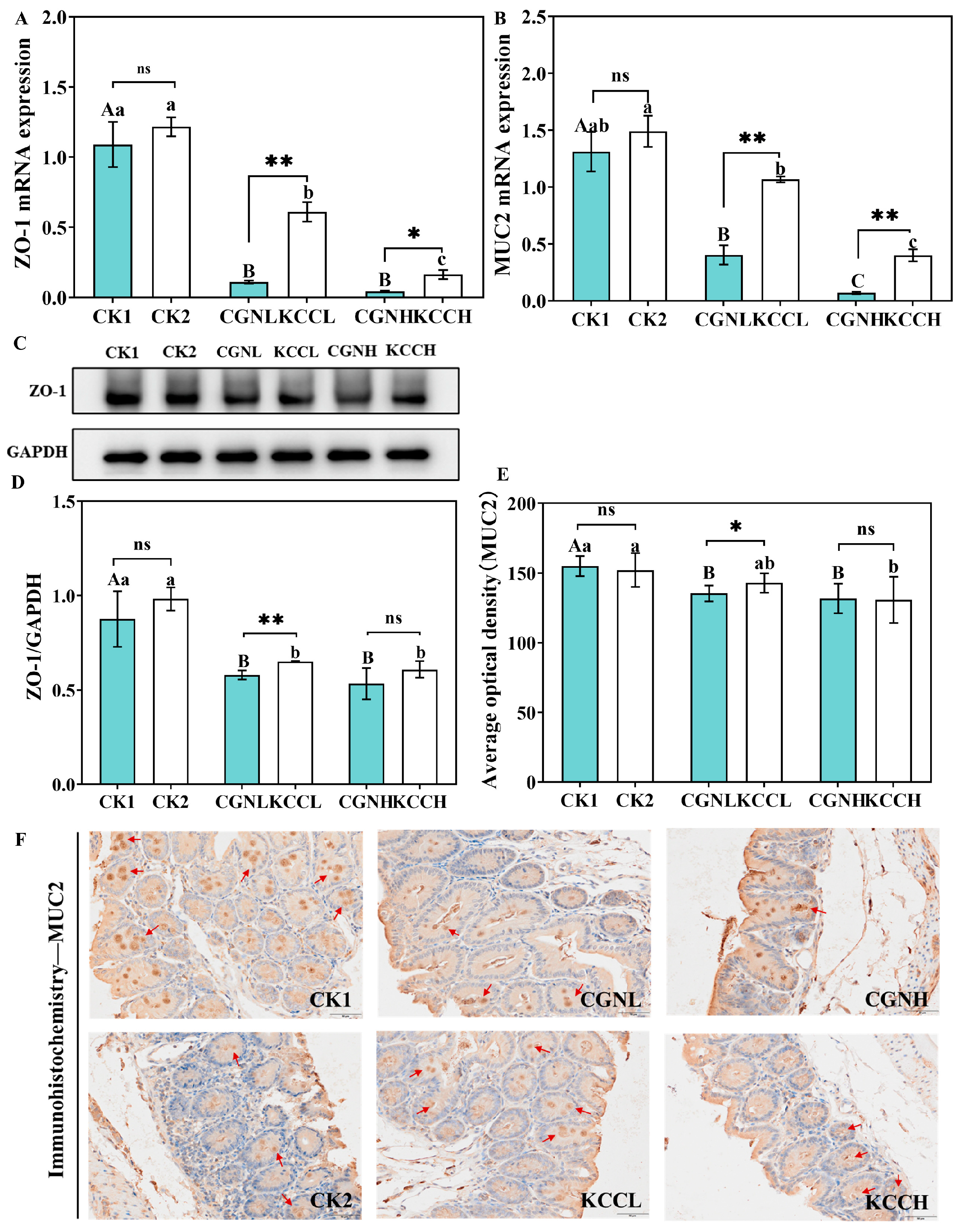
3.3. Effect of κ-CGN and κ-CC on Intestinal Barrier in Mice
3.4. Effect of κ-CGN and κ-CC on the Structure and Diversity of Gut Microbiota in Mice
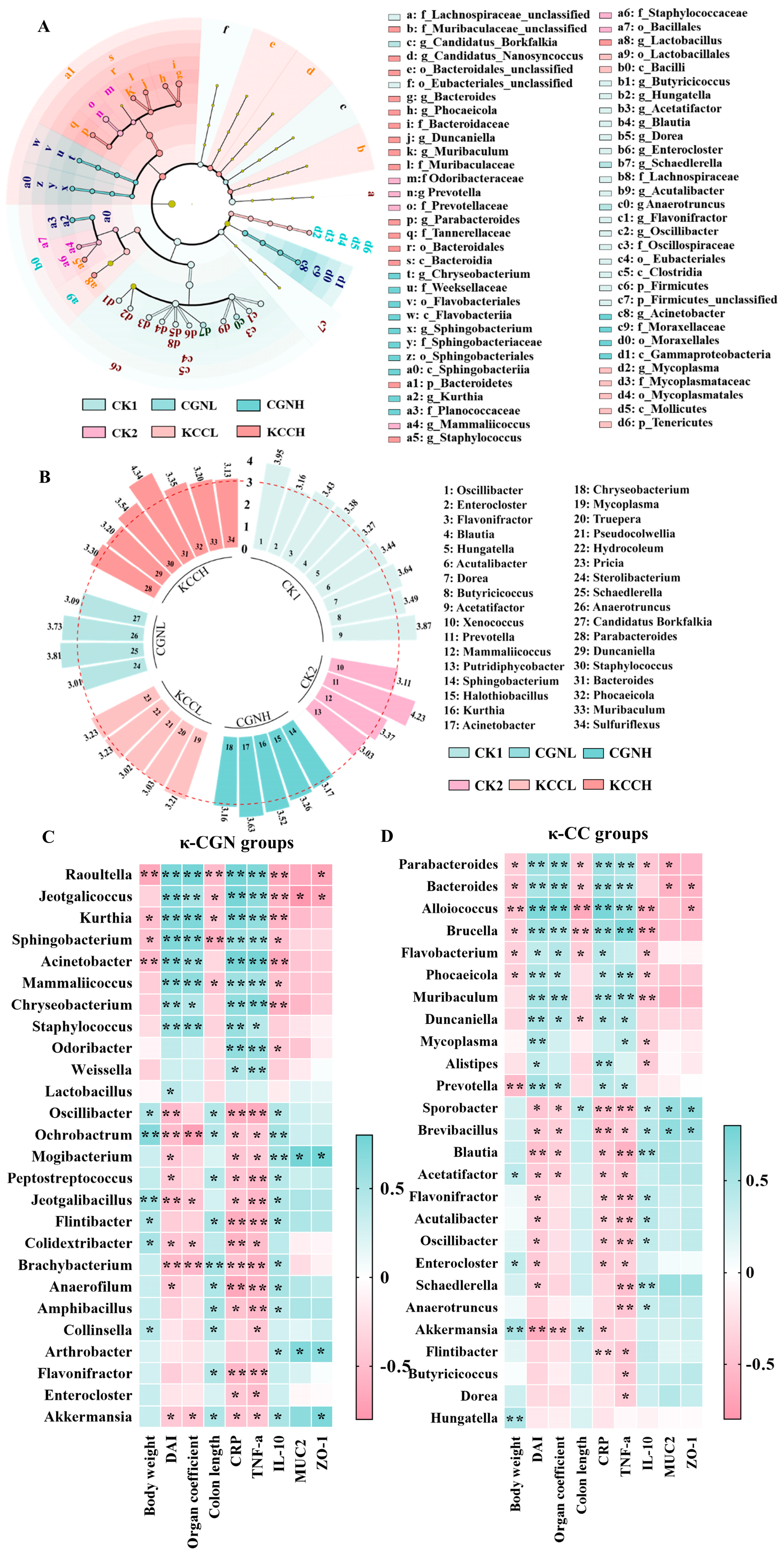
3.5. Effect of κ-CGN and κ-CC on the Composition and Abundance of the Gut Microbiota
3.6. The Correlation Among Biological Parameters and the Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Campo, V.L.; Kawano, D.F.; Silva, D.B.D.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis–A review. Carbohydr Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Liu, H.; Zhang, Z.; Jam, M.; Dudeja, P.K.; Michel, G.; Linhardt, R.J.; Tobacman, J.K. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nat. Biochem. 2010, 21, 906–913. [Google Scholar] [CrossRef]
- Błaszak, B.; Gozdecka, G.; Shyichuk, A. Carrageenan as a functional additive in the production of cheese and cheese-like products. Acta Sci. Pol. Technol. Aliment. 2018, 17, 107–116. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Xue, L.; Devkota, S.; Chang, E.; Morris, S.; Tobacman, J.K. Carrageenan-induced colonic inflammation is reduced in Bcl10 null mice and increased in IL-10-deficient mice. Mediat. Inflamm. 2013, 2013, 397642. [Google Scholar] [CrossRef]
- Borthakur, A.; Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G829–G838. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Wang, F.; Chen, H.M.; Yan, X.J. Kappa-carrageenan induces the disruption of intestinal epithelial Caco-2 monolayers by promoting the interaction between intestinal epithelial cells and immune cells. Mol. Med. Rep. 2013, 8, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M., Jr.; Wilga, P.C.; Pregenzer, J.F.; Blakemore, W.R. The common food additive carrageenan is not a ligand for Toll-Like-Receptor 4 (TLR4) in an HEK293-TLR4 reporter cell-line model. Food Chem. Toxicol. 2015, 78, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Weiner, M.L.; Ferguson, H.E.; Thorsrud, B.A.; Nelson, K.G.; Blakemore, W.R.; Zeigler, B.; Cameron, M.J.; Brant, A.; Cochrane, L.; Pellerin, M.; et al. An infant formula toxicity and toxicokinetic feeding study on carrageenan in preweaning piglets with special attention to the immune system and gastrointestinal tract. Food Chem. Toxicol. 2015, 77, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bixler, H.J. The carrageenan controversy. J. Appl. Phycol. 2017, 29, 2201–2207. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, D.; Xuan, R.; Zhou, J.; Liu, J.; Chen, J.; Han, H.; Niu, T.; Li, X.; Chen, H.; et al. Lambda-carrageenan exacerbates Citrobacter rodentium-induced infectious colitis in mice by targeting gut microbiota and intestinal barrier integrity. Pharmacol. Res. 2021, 174, 105940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; He, Y.; Zeng, L.; He, J.; Yang, Y.; Zhang, T. Effect of κ-carrageenan on glucolipid metabolism and gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2021, 86, 104707. [Google Scholar] [CrossRef]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin, C.; Xiaojun, Y. Enhanced effect of kappa-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study. Food Chem. Toxicol. 2016, 87, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary kappa-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Guo, J.; Shang, X.; Chen, P.; Huang, X. How does carrageenan cause colitis? A review. Carbohydr. Polym. 2023, 302, 120374. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.E.; Khafipour, E. Carrageenan gum and adherent invasive Escherichia coli in a piglet model of inflammatory bowel disease: Impact on intestinal mucosa-associated microbiota. Front. Microbiol. 2016, 7, 00462. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native kappa-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2020, 58, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells 2020, 6, 698. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Macfarlane, S.; Macfarlane, G.T. Metabolic interactions involving sulphate-reducing and methanogenic bacteria in the human large intestine. FEMS Microbiol. Ecol. 1993, 12, 117–125. [Google Scholar] [CrossRef]
- Gibson, G.R. Physiology and ecology of the sulphate-reducing bacteria. J. Appl. Bacteriol. 1990, 69, 769–797. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Dwidar, M.; Gao, Y.; Xiang, L.; Wu, X.; Medema, M.H.; Xu, S.; Li, X.; Schäfer, H.; et al. Microbial assimilatory sulfate reduction-mediated H2S: An overlooked role in Crohn’s disease development. Microbiome 2024, 12, 2049–2618. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Lv, P.; Huang, X.; Pang, J. Physicochemical dynamic changes and differences of κ-carrageenan in different vehicles (aqueous and casein solution) during in vitro gastrointestinal digestion. Food Hydrocoll. 2022, 129, 107553. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, P.; Liu, Z.; Lin, L.; Zhang, J. Effect of physiological pH on the molecular characteristics, rheological behavior, and molecular dynamics of κ-carrageenan/casein. Front. Nutr. 2023, 10, 1174888. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, S.; Liu, B.; Zheng, M.; Chen, H.; Pang, J. Rheological behavior and molecular dynamics simulation of κ-carrageenan/casein under simulated gastrointestinal electrolyte conditions. Food Hydrocoll. 2023, 136, 108240. [Google Scholar] [CrossRef]
- Liu, F.; Hou, P.; Zhang, H.; Tang, Q.; Xue, C.; Li, R.W. Food-grade carrageenans and their implications in health and disease. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3918–3936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of milk protein-polysaccharide interactions on the stability of ice cream mix model systems. Food Hydrocoll. 2015, 45, 327–336. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Z. Changes in the nutrients of camels’ milk alter the functional features of the intestine microbiota. Food Funct. 2018, 9, 6484–6494. [Google Scholar] [CrossRef]
- Si, X.; Song, Z.; Liu, N.; Jia, H.; Liu, H.; Wu, Z. α-ketoglutarate restores intestinal barrier function through promoting intestinal stem cells-mediated epithelial regeneration in colitis. J. Agric. Food Chem. 2022, 70, 13882–13892. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Martínková, K.; Vítězová, M.; Rittmann, S.K.M. Intestinal microbiota and perspectives of the use of meta-analysis for comparison of ulcerative colitis studies. J. Clin. Med. 2021, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Hazem, S.H.; Shaker, M.E.; Ashamallah, S.A.; Ibrahim, T.M. The novel Janus kinase inhibitor ruxolitinib confers protection against carbon tetrachloride-induced hepatotoxicity via multiple mechanisms. Chem-Biol. Interact. 2014, 220, 116–127. [Google Scholar] [CrossRef]
- Osman, N.; Adawi, D.; Ahrné, S.; Jeppsson, B.; Molin, G. Endotoxin- and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig. Liver Dis. 2007, 39, 849–856. [Google Scholar] [CrossRef]
- Liu, H.; Liu, W.; Huang, X.; Feng, Y.; Lu, J.; Gao, F. Intestinal flora differences between patients with ulcerative colitis of different ethnic groups in China. Medicine 2021, 100, e26932. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Agrawal, A. Evolution of C-reactive protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Slaats, J.; Ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1beta/IL-6/CRP and IL-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef] [PubMed]
- Morio, L.A.; Chiu, H.; Sprowles, K.A.; Zhou, P.; Heck, D.E.; Gordon, M.K.; Laskin, D.L. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol. Appl. Pharmacol. 2001, 172, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Karamese, M.; Aydin, H.; Gelen, V.; Sengul, E.; Karamese, S.A. The anti-inflammatory, anti-oxidant and protective effects of a probiotic mixture on organ toxicity in a rat model. Future Microbiol. 2020, 15, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.G.; Haribhai, D.; Williams, J.B.; Aggarwal, P.; Jia, S.; Charbonnier, L.M.; Yan, K.; Lorier, R.; Turner, A.; Ziegelbauer, J.; et al. IL-10 produced by induced regulatory T cells (iTregs) controls colitis and pathogenic ex-iTregs during immunotherapy. J. Immunol. 2012, 189, 5638–5648. [Google Scholar] [CrossRef]
- Tu, A.; Wang, X.C.; Chen, H.; Jia, X.; Wang, T.; Yi, Y.; Liu, B.; Xin, W.; Lü, X.; Shan, Y. Ovomucin ameliorates intestinal barrier and intestinal bacteria to attenuate DSS-induced colitis in mice. J. Agric. Food Chem. 2021, 69, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, L.Y.; Dou, C.Z.; Guan, X.; Wu, H.G.; Liu, H.R. Research advance in intestinal mucosal barrier and pathogenesis of Crohn’s disease. Gastroenterol. Res. Pract. 2016, 2016, 9686238. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Allaire, J.M.; Morampudi, V.; Crowley, S.M.; Stahl, M.; Yu, H.; Bhullar, K.; Knodler, L.A.; Bressler, B.; Jacobson, K.; Vallance, B.A. Frontline defenders: Goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G360–G377. [Google Scholar] [CrossRef] [PubMed]
- Firdayanti, L.; Yanti, R.; Rahayu, E.S.; Hidayat, C. Carrageenan extraction from red seaweed (Kappaphycopsis cottonii) using the bead mill method. Algal Res. 2023, 69, 102906. [Google Scholar] [CrossRef]
- Sari, D.K.; Barleany, D.; Lestari, R.S.D.; Mustikawati, L. Extraction refined carrageenan using ultrasonic irradiation in from Kappaphycus Alvarezii originated from Lontar. IOP Conf. Ser. Mater. Sci. Eng. 2019, 673, 012015. [Google Scholar] [CrossRef]
- Setijawati, D.; Nursyam, H.; Salis, H. Carrageenan: The difference between PNG and KCL gel precipitation method as Lactobacillus acidophilus encapsulation material. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 012073. [Google Scholar] [CrossRef]
- Brownlee, I.A. The physiological roles of dietary fibre. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Southgate, D.A. The role of dietary fibre in the diet. J. R. Soc. Health 1990, 110, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Interactions between gut microbes and host cells control gut barrier and metabolism. Int. J. Obes. Suppl. 2016, 6, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Li, Q.-M.; Zha, X.-Q.; Luo, J.-P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef]
- Yu, W.; Su, X.; Chen, W.; Tian, X.; Zhang, K.; Guo, G.; Zhou, L.; Zeng, T.; Han, B. Three types of gut bacteria collaborating to improve Kui Jie’an enema treat DSS-induced colitis in mice. Biomed. Pharmacother. 2019, 113, 108751. [Google Scholar] [CrossRef]
- Hsiao, Y.-P.; Chen, H.-L.; Tsai, J.-N.; Lin, M.-Y.; Liao, J.-W.; Wei, M.-S.; Ko, J.-L.; Ou, C.-C. Administration of Lactobacillus reuteri combined with clostridium butyricum attenuates cisplatin-induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients 2021, 13, 2792. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Xia, B.; Tang, S.; Xie, J.; Zhang, H. Modulation of pectin on mucosal innate immune function in pigs mediated by gut microbiota. Microorganisms 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Ogita, T.; Yamamoto, Y.; Mikami, A.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii strongly suppresses Th2 immune responses in mice. Front. Immunol. 2020, 11, 00379. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, P.; Zhang, C.; Jiang, G.; Zheng, W.; Song, S.; Ai, C. Sulfated polysaccharides from pacific abalone attenuated DSS-induced acute and chronic ulcerative colitis in miceviaregulating intestinal micro-ecology and the NF-κB pathway. Food Funct. 2021, 12, 11351–11365. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Y.; Itagaki, K.; Zhu, B.; Lin, Y.; Song, H.; Wang, L.; Xiong, L.; Weng, Z.; Shen, X. Wheat germ-derived peptide alleviates dextran sulfate sodium-induced colitis in mice. J. Agric. Food Chem. 2023, 71, 15593–15603. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium pseudocatenulatum ameliorates DSS-induced colitis by maintaining intestinal mechanical barrier, blocking proinflammatory cytokines, inhibiting TLR4/NF-κB signaling, and altering gut microbiota. J. Agric. Food Chem. 2021, 69, 1496–1512. [Google Scholar] [CrossRef]
- Xie, P.; Chen, B.; Ran, J.; Zhan, M.; Du, H.; Hong, F.; Lu, M.; Cao, Y.; Xiao, H.; Song, M. Dietary 5-demethylnobiletin attenuated dextran sulfate sodium-induced colitis in mice by inhibiting immune response and regulating gut microbiota. Food Funct. 2023, 14, 6248–6261. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-inflammatory properties and gut microbiota modulation of Porphyra tenera extracts in dextran sodium sulfate-induced colitis in mice. Antioxidants 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Jean Wilson, E.; Sirpu Natesh, N.; Ghadermazi, P.; Pothuraju, R.; Shanmugam, M.; Prajapati, D.R.; Pandey, S.; Kaifi, J.T.; Dodam, J.R.; Bryan, J.; et al. Red cabbage juice-mediated gut microbiota modulation improves intestinal epithelial homeostasis and ameliorates colitis. Int. J. Mol. Sci. 2023, 25, 539. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, Y.; Li, J.; Bai, G.; Liu, L.; Zhong, R.; Ma, T.; Pan, H.; Zhang, H. Supplementation of multi-enzymes alone or combined with inactivated Lactobacillus benefits growth performance and gut microbiota in broilers fed wheat diets. Front. Microbiol. 2022, 13, 927932. [Google Scholar] [CrossRef]
- Chen, P.; Xu, H.; Tang, H.; Zhao, F.; Yang, C.; Kwok, L.Y.; Cong, C.; Wu, Y.; Zhang, W.; Zhou, X.; et al. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb. Biotechnol. 2020, 13, 2032–2043. [Google Scholar] [CrossRef]
- Huang, L.; Cui, K.; Mao, W.; Du, Y.; Yao, N.; Li, Z.; Zhao, H.; Ma, W. Weissella cibaria attenuated LPS-induced dysfunction of intestinal epithelial barrier in a Caco-2 cell monolayer model. Front. Microbiol. 2020, 11, 02039. [Google Scholar] [CrossRef]
- Sandes, S.; Figueiredo, N.; Pedroso, S.; Sant’Anna, F.; Acurcio, L.; Abatemarco, M.; Barros, P.; Oliveira, F.; Cardoso, V.; Generoso, S.; et al. Weissella paramesenteroides WpK4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res. Int. 2020, 137, 109741. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, N.; Wang, X.; Yan, H.; Guan, L.; Kong, L.; Chen, J.; Zhang, H.; Ma, H. Weissella cibaria relieves gut inflammation caused by Escherichia coli through inflammation modulation and gut microbiota regulation. Foods 2024, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Singh, S.; Singh, V.; Roberts, K.D.; Zaidi, A.; Rodriguez-Palacios, A. The Weissella genus: Clinically treatable bacteria with antimicrobial/probiotic effects on inflammation and cancer. Microorganisms 2022, 10, 2427. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Hawai, S. Effect of minocycline, methyl prednisolone, or combination treatment on the colonic bacterial population in a state of colonic inflammation using the murine dextran sulfate sodium model. Microb. Cell Factories 2023, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, M.; Ye, C.; Sun, X.; Jiang, N.; Zou, X.; Yang, H.; Liu, H. BuZangTongLuo decoction improved hindlimb ischemia by activating angiogenesis and regulating gut microbiota in diabetic mice. J. Ethnopharmacol. 2020, 248, 112330. [Google Scholar] [CrossRef]
- Hu, T.-G.; Wen, P.; Shen, W.-Z.; Liu, F.; Li, Q.; Li, E.-N.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Effect of 1-deoxynojirimycin isolated from mulberry leaves on glucose metabolism and gut microbiota in a streptozotocin-induced diabetic mouse model. J. Nat. Prod. 2019, 82, 2189–2200. [Google Scholar] [CrossRef]
- Du, J.; Zayed, A.A.; Kigerl, K.A.; Zane, K.; Sullivan, M.B.; Popovich, P.G.; Arumugam, M. Spinal cord injury changes the structure and functional potential of gut bacterial and viral communities. mSystems 2021, 6, e01356-20. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yang, S.M.; Kim, H.Y. Weissella and the two Janus faces of the genus. Appl. Microbiol. Biotechnol. 2023, 107, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.C.; Cummings, J.H. Hydrogen sulphide: A bacterial toxin in ulcerative colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen sulfide as a toxic product in the small–large intestine axis and its role in IBD development. J. Clin. Med. 2019, 8, 1054. [Google Scholar] [CrossRef]


| Score | 0 | 1 | 2 |
|---|---|---|---|
| Changes of body weight | 1–5% | 5–10% | 10–15% |
| Hair condition | Neat gloss | Cluttered and shiny | Cluttered, sparse, dull |
| Activity | Normal | Inactive | Motionless |
| Stool color | Black | Yellow | White |
| Stool shape | Normal | Soft | Watery |
| Bloody stool | — | Few | obvious |
| Score | Epithelial Damage | Infiltration of Inflammatory Cells |
|---|---|---|
| 0 | Normal | None |
| 1 | A small loss of goblet cells | Infiltrate around the base of the crypt |
| 2 | Massive loss of goblet cells | Infiltration of the basal mucosa |
| 3 | A small loss of crypts and massive loss of goblet cells | extensively infiltrated and edematous of mucosa |
| 4 | Massive loss of crypts | Submucosal infiltration |
| Target Gene | Forward Primer (5′→3′) | Revere Primer (5′→3′) |
|---|---|---|
| ZO-1 | AAATCATCCGACTCCTCGTCG | GACAGAAACACAGTTGGCTCC |
| MUC2 | GGCCAGGAGTTTACCAACGA | CAGGGCAAGGCAGGTCTTTA |
| GAPDH | CCCAGCTTAGGTTCATCAGG | CCAAATCCGTTCACACCGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Guo, J.; Chen, P. Effect of Food Matrix on Regulation of Intestinal Barrier and Microbiota Homeostasis by Polysaccharides Sulfated Carrageenan. Foods 2025, 14, 635. https://doi.org/10.3390/foods14040635
Shang X, Guo J, Chen P. Effect of Food Matrix on Regulation of Intestinal Barrier and Microbiota Homeostasis by Polysaccharides Sulfated Carrageenan. Foods. 2025; 14(4):635. https://doi.org/10.3390/foods14040635
Chicago/Turabian StyleShang, Xuke, Juanjuan Guo, and Peilin Chen. 2025. "Effect of Food Matrix on Regulation of Intestinal Barrier and Microbiota Homeostasis by Polysaccharides Sulfated Carrageenan" Foods 14, no. 4: 635. https://doi.org/10.3390/foods14040635
APA StyleShang, X., Guo, J., & Chen, P. (2025). Effect of Food Matrix on Regulation of Intestinal Barrier and Microbiota Homeostasis by Polysaccharides Sulfated Carrageenan. Foods, 14(4), 635. https://doi.org/10.3390/foods14040635





