Basic Amino Acids as Salt Substitutes in Low-Salt Gel-Based Meat Products: A Comprehensive Review of Mechanisms, Benefits, and Future Perspectives
Abstract
1. Introduction
2. The Role of NaCl in Traditional Meat Products
3. The Effect of BAAs on Protein Processing Properties
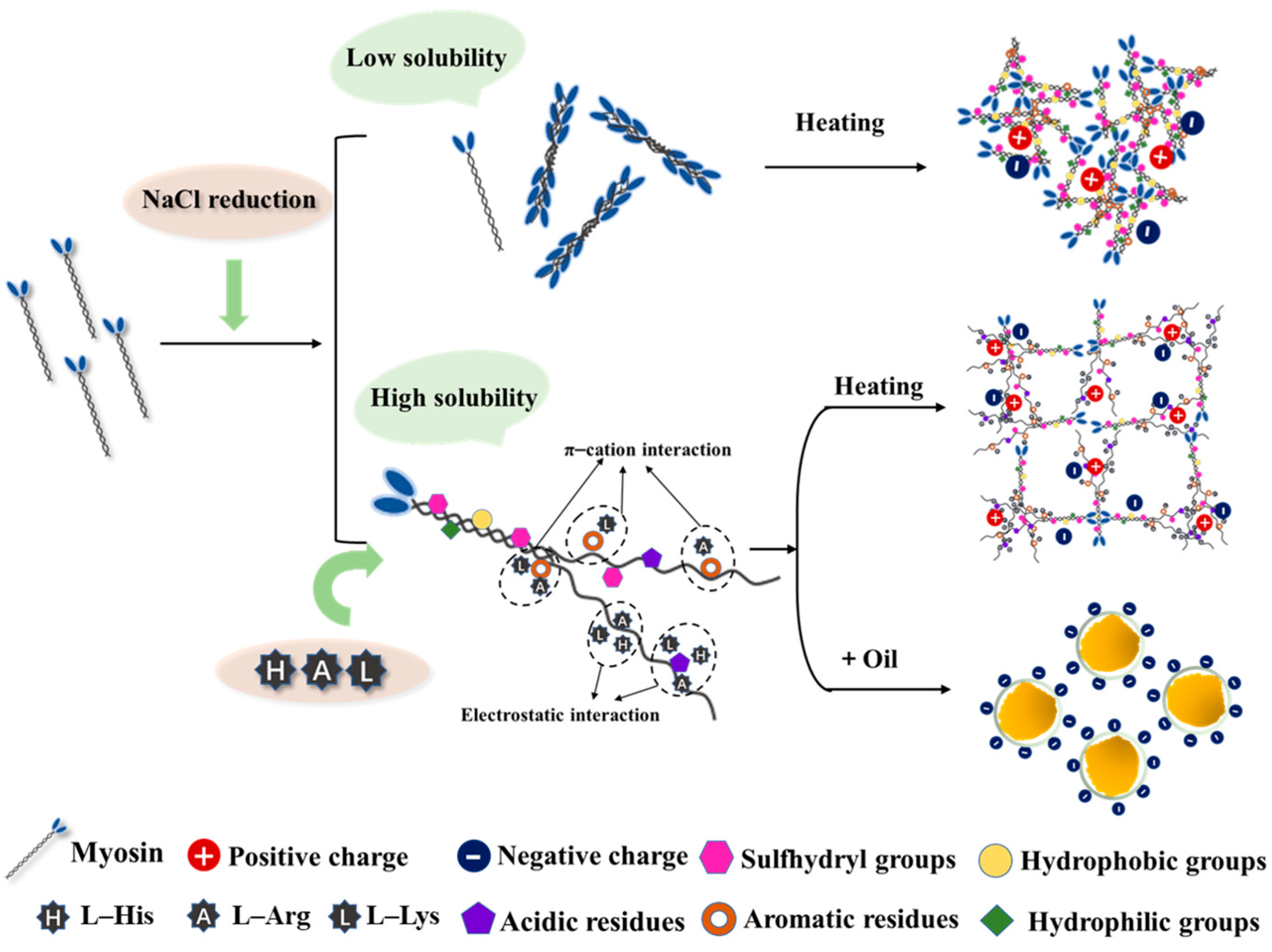
3.1. Solubility
3.2. Gel Properties
3.3. Emulsion Properties
4. The Effect of BAA on Low-Salt Gel-Based Meat Processing
4.1. Protein and Lipid Oxidation
4.2. Sensory Properties
4.3. Textural Properties
4.4. Influence of BAA on Harmful Substances in Low-Salt Meat
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Health Statistics 2024: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2024.
- Adhikari, S.; Schop, M.; Boer, I.J.M.D.; Huppertz, T. Protein quality in perspective: A review of protein quality metrics and their applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef]
- Tan, K.; Huang, L.; Tan, K.; Lim, L.; Peng, Y.; Cheong, K. Effects of culinary treatments on the lipid nutritional quality of fish and shellfish. Food Chem. X 2023, 19, 100856. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Yu, B.; Amin, M.S.; Liu, R.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends Food Sci. Technol. 2022, 129, 657–666. [Google Scholar] [CrossRef]
- Hao, G.; Liu, K.; Halbert, J.D.; Chen, H.; Wu, J.; Jing, C. Dietary sodium and potassium and risk of diabetes: A prospective study using data from the China Health and Nutrition Survey. Diabetes Metab. 2020, 46, 377–383. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- The State Council. “Healthy China 2030” Plan Outline. 2016. Available online: https://www.gov.cn/zhengce/202407/content_6964985.htm (accessed on 23 December 2024).
- Zhou, C.; Ye, H.; Nishiumi, T.; Qin, H.; Chen, C. L-histidine enhances stability of hemoglobin concentrates by coordinating with free iron. Food Res. Int. 2014, 62, 637–643. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, J.; Tian, W.; Liu, Y.; Li, M. Contribution of histidine and lysine to the generation of volatile compounds in Jinhua Ham exposed to ripening conditions via Maillard reaction. J. Food Sci. 2017, 83, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tao, S.; Pan, J.; Lin, X.; Ji, C.; Liang, H.; Dong, X.; Li, S. Effects of L-Lysine on the physiochemical properties and sensory characteristics of salt-reduced reconstructed ham. Meat Sci. 2020, 166, 108133. [Google Scholar] [CrossRef]
- Fan, X.; Gao, X.; Zhou, C. L-arginine and L-lysine supplementation to NaCl tenderizes porcine meat by promoting myosin extraction and actomyosin dissociation. Food Chem. 2024, 446, 138809. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Chen, L.; Hu, Y.; Wang, Y.; Zhou, C. L-Arginine and L-lysine retard aggregation and polar residue modifications of myofibrillar proteins: Their roles in solubility of myofibrillar proteins in frozen porcine longissimus lumborum. Food Chem. 2022, 393, 133347. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Stein, L.J. Salt taste. In The Senses: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2008; Volume 4, pp. 401–408. [Google Scholar]
- Kuo, W.Y.; Lee, Y. Effect of food matrix on saltiness perception—Implications for sodium reduction. Compr. Rev. Food Sci. Food Saf. 2015, 13, 906–923. [Google Scholar] [CrossRef]
- Gan, X.; Li, H.; Wang, Z.; Emara, A.M.; Zhang, D.; He, Z. Does protein oxidation affect proteolysis in low sodium Chinese traditional bacon processing? Meat Sci. 2019, 150, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Wu, Q.; Wang, S.; Qiao, X.; Chen, W. Changes of protein oxidation, lipid oxidation and lipolysis in Chinese dry sausage with different sodium chloride curing salt content. Food Sci. Hum. Wellness 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Yu, C.; Chen, L.; Ouyang, K.; Chen, H.; Xu, M.; Lin, S.; Wang, W. Effect of partial substitution of NaCl by KCl on aggregation behavior and gel properties of beef myosin. Food Chem. 2024, 458, 140178. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xie, M.; Hu, Z.; Li, J.; Zheng, H.; Xie, N.; Zhen, Z. Optimizing preparation of low-NaCl protein gels from goose meat and understanding synergistic effects of pH/NaCl in improving gel characteristics. Food Chem. X 2024, 22, 101333. [Google Scholar] [CrossRef]
- Sow, L.C.; Yang, H. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Choi, J.S.; Chin, K.B. Influence of NaCl and phosphate on gelation properties of chicken breast myofibrillar protein gels and its application to in vitro digestion model. Food Chem. 2024, 460, 140638. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shen, H.; Wang, D.; Liu, S.; Ding, Y.; Zhou, X. Novel NaCl reduction technologies for dry-cured meat products and their mechanisms: A comprehensive review. Food Chem. 2024, 431, 137142. [Google Scholar] [CrossRef]
- Roseiro, L.C.; Santos, C.; Gonçalves, H.; Serrano, C.; Aleixo, C.; Partidário, A.; Lourenço, A.R.; Dias, M.A.; Da Ponte, D.J.B. Susceptibility of dry-cured tuna to oxidative deterioration and biogenic amines generation: I. Effect of NaCl content, antioxidant type and ageing. Food Chem. 2017, 228, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Ding, Y.; Ke, Z.; Zhou, X.; Zhang, J. Diversity and succession of the microbial community and its correlation with lipid oxidation in dry-cured black carp (Mylopharyngodon piceus) during storage. Food Microbiol. 2021, 98, 103686. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Shang, S.; Zhu, K.; Miao, X.; Dong, X.; Jiang, P. Changes in bacterial flora and flavor of shrimp paste under different salt concentrations. LWT 2024, 205, 116534. [Google Scholar] [CrossRef]
- Michelakou, E.; Giaouris, E.; Doultsos, D.; Nasopoulou, C.; Skandamis, P. Evaluation of the microbial stability and shelf life of 50% NaCl-reduced traditional greek pork meat product “Syglino of Monemvasia” stored under vacuum at different temperatures. Heliyon 2021, 7, e8296. [Google Scholar] [CrossRef]
- Fougy, L.; Desmonts, M.-H.; Coeuret, G.; Fassel, C.; Hamon, E.; Hézard, B.; Champomier-Vergès, M.-C.; Chaillou, S. Reducing Salt in raw pork sausages increases spoilage and correlates with reduced bacterial diversity. Appl. Environ. Microbiol. 2016, 82, 3928–3939. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Barat, J.M.; Serra, J.A. Influence of sodium replacement and packaging on quality and shelf life of smoked sea bass (Dicentrarchus labrax L.). LWT Food Sci. Technol. 2011, 44, 917–923. [Google Scholar] [CrossRef]
- Armenteros, M.; Aristoy, M.C.; Barat, J.M.; Toldra, F. Biochemical and sensory changes in dry-cured ham salted with partial replacements of NaCl by other chloride salts. Meat Sci. 2012, 90, 361–367. [Google Scholar] [CrossRef]
- Zheng, J.; Han, Y.; Ge, G.; Zhao, M.; Sun, W. Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocoll. 2019, 96, 36–42. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Salt substitution in surimi seafood and its effects on instrumental quality attributes. LWT Food Sci. Technol. 2012, 48, 175–181. [Google Scholar] [CrossRef]
- Oliveira, D.; Andrade, A.; Rodrigues, J.; Natividade, M.; Bastos, S. Sodium reduction in butter using microparticulated salt. Br. Food J. 2019, 121, 874–881. [Google Scholar] [CrossRef]
- Wang, X.; Feng, T.; Xia, S. Saltiness perception related to salt release of surimi emulsified sausages: Modulation in texture and microstructure by polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 3893–3902. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, D.; Jiang, J.; Wu, X.; Ma, J.; Hu, X.; Sun, W.; Liu, J. Magnetic fields improve the gel properties of myofibrillar proteins in low-salt myofibrillar protein emulsion systems. Food Chem. 2025, 470, 142681. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A. The application of pulsed electric field as a sodium reducing strategy for meat products. Food Chem. 2020, 306, 125622. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef]
- Wang, X.; Luo, N.; Guo, C.; Wang, X.; Xia, S. Enhancing gel strength and saltiness perception of low-salt surimi gels: Synergistic effects of lysine assisted with water bath-microwave heating. Food Biosci. 2024, 61, 104827. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Mársico, E.T.; Cunha, L.C.M.; Rosenthal, A.; Deliza, R.; Conte-Junior, C.A. Application of emerging non-thermal technologies to sodium reduction in ready-to-eat fish products. Innov. Food Sci. Emerg. Technol. 2021, 71, 102710. [Google Scholar] [CrossRef]
- Man, H.; Sun, P.; Lin, J.; Ren, X.; Li, D. Based on hydrogen and disulfide-mediated bonds, L-lysine and L-arginine enhanced the gel properties of low-salt mixed shrimp surimi (Antarctic krill and Pacific white shrimp). Food Chem. 2024, 445, 138735. [Google Scholar] [CrossRef] [PubMed]
- Ban, Q.; Lin, Y.; Li, J.; Cheng, J.; Jiang, Y.; An, J. Effect of non-covalently bound alkaline amino acids on the structural characterization, microstructure, and rheological properties of whey protein emulsion gel. LWT 2024, 209, 116809. [Google Scholar] [CrossRef]
- Dong, M.; Xu, Y.; Zhang, Y.; Han, M.; Wang, P.; Xu, X.; Zhou, G. Physicochemical and structural properties of myofibrillar proteins isolated from pale, soft, exudative (PSE)-like chicken breast meat: Effects of pulsed electric field (PEF). Innov. Food Sci. Emerg. Technol. 2020, 59, 102277. [Google Scholar] [CrossRef]
- Guo, X.Y.; Peng, Z.Q.; Zhang, Y.W.; Liu, B.; Cui, Y.Q. The solubility and conformational characteristics of porcine myosin as affected by the presence of L-lysine and l-histidine. Food Chem. 2015, 170, 212–217. [Google Scholar] [CrossRef]
- Craig, R.; Woodhead, J.L. Structure and function of myosin filaments. Curr. Opin. Struct. Biol. 2006, 16, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Ito, T.; Wakamatsu, J.; Nishimura, T.; Hattori, A. Myosin filament depolymerizes in a low ionic strength solution containing L-histidine. Meat Sci. 2010, 84, 742–746. [Google Scholar] [CrossRef]
- Chen, X.; Zou, Y.; Han, M.; Pan, L.; Xing, T.; Xu, X.; Zhou, G. Solubilization of myosin in a solution of low ionic strength l-histidine: Significance of the imidazole ring. Food Chem. 2016, 196, 42–49. [Google Scholar] [CrossRef]
- Baynes, B.M.; Trout, B.L. Rational design of solution additives for the prevention of protein aggregation. Biophys. J. 2004, 87, 1631–1639. [Google Scholar] [CrossRef]
- Arakawa, T.; Tsumoto, K. The effects of arginine on refolding of aggregated proteins: Not facilitate refolding, but suppress aggregation. Biochem. Biophys. Res. Commun. 2003, 304, 148–152. [Google Scholar] [CrossRef]
- Shi, T.; Xiong, Z.; Jin, W.; Yuan, L.; Sun, Q.; Zhang, Y.; Li, X.; Gao, R. Suppression mechanism of L-arginine in the heat-induced aggregation of bighead carp (Aristichthys nobilis) myosin: The significance of ionic linkage effects and hydrogen bond effects. Food Hydrocoll. 2020, 102, 105596. [Google Scholar] [CrossRef]
- Lu, S.; Wang, H.; Kang, R.; Feng, Z.; Liu, D.; Pei, Z.; Liu, J.; Xue, C.; Shen, X.; Cao, J.; et al. Impact of pH and NaCl on the molecular conformation of myosin and myosin microgel and underlying mechanism: A comprehensive study using spectroscopy and molecular dynamics simulation. LWT 2023, 189, 115517. [Google Scholar] [CrossRef]
- Fu, Y.; Zheng, Y.; Lei, Z.; Xu, P.; Zhou, C. Gelling properties of myosin as affected by L-lysine and L-arginine by changing the main molecular forces and microstructure. Int. J. Food Prop. 2017, 20, S884–S898. [Google Scholar] [CrossRef]
- Lei, Z.; Fu, Y.; Xu, P.; Zheng, Y.; Zhou, C. Effects of L-arginine on the physicochemical and gel properties of chicken actomyosin. Int. J. Biol. Macromol. 2016, 92, 1258–1265. [Google Scholar] [CrossRef]
- Qin, H.; Xu, P.; Zhou, C.; Wang, Y. Effects of L-arginine on water holding capacity and texture of heat-induced gel of salt-soluble proteins from breast muscle. LWT Food Sci. Technol. 2015, 63, 912–918. [Google Scholar] [CrossRef]
- Khemakhem, M.; Attia, H.; Ayadi, M.A. The effect of pH, sucrose, salt and hydrocolloid gums on the gelling properties and water holding capacity of egg white gel. Food Hydrocoll. 2019, 87, 11–19. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Tan, S. Effect of L-lysine on the physicochemical properties of pork sausage. Food Sci. Biotechnol. 2014, 23, 775–780. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Mu, J.; Shi, T.; Yuan, L. Effect of L-histidine on the heat-induced aggregation of bighead carp (Aristichthys nobilis) myosin in low/high ionic strength solution. Food Chem. 2018, 75, 174–181. [Google Scholar] [CrossRef]
- Gao, R.; Shi, T.; Sun, Q.; Li, X.; Mcclements, D.J.; Yuan, L. Effects of L-arginine and L-histidine on heat-induced aggregation of fish myosin: Bighead carp (Aristichthys nobilis). Food Chem. 2019, 295, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, L.; Xu, M.; Ouyang, K.; Chen, H.; Lin, S.; Wang, W. The effect of pH and heating on the aggregation behavior and gel properties of beef myosin. LWT 2024, 191, 115615. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Ning, C.; Bao, P.; Fang, H.; Zhou, C. L-arginine and L-lysine improve the physical stability of soybean oil-myosin emulsions by changing penetration and unfolding behaviors of interfacial myosin. Food Hydrocoll. 2020, 98, 105261–105265. [Google Scholar] [CrossRef]
- Li, L.; Cai, R.; Wang, P.; Xu, X.; Zhou, G.; Sun, J. Manipulating interfacial behavior and emulsifying properties of myosin through alkali-heat treatment. Food Hydrocoll. 2018, 85, 69–74. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Li, S.; Ning, C.; Zhou, C. L-arginine/L-lysine improves emulsion stability of chicken sausage by increasing electrostatic repulsion of emulsion droplet and decreasing the interfacial tension of soybean oil-water. Food Hydrocoll. 2019, 89, 492–502. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, J.H.; Teng, S.; Peng, Z.Q.; Jamali, M.A. Quality improvement of prerigor salted ground chicken breast with BAAs at low NaCl level. Poult. Sci. 2023, 102, 102871. [Google Scholar] [CrossRef]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food Sci. 2010, 70, 54–66. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Jamali, M.A.; Peng, Z. Manipulating interfacial behaviour and emulsifying properties of myofibrillar proteins by L-arginine at low and high salt concentration. Int. J. Food Sci. Technol. 2020, 56, 999–1012. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhu, X.; Ning, C.; Cai, K.; Zhou, C. Conformational and charge changes induced by L-arginine and L-lysine increase the solubility of chicken myosin. Food Hydrocoll. 2019, 89, 330–336. [Google Scholar] [CrossRef]
- Guo, X.; Gao, F.; Zhang, Y.; Peng, Z.; Jamali, M.A. Effect of L-histidine and L-lysine on the properties of oil-in-water emulsions stabilized by porcine myofibrillar proteins at low/high ionic strength. LWT 2021, 141, 110883. [Google Scholar] [CrossRef]
- Han, Z.; Li, X.; Liu, Y.; Yue, X.; Wu, Z.; Shao, J. The evolution of pork myosin aggregates and the relationship between aggregation modes and microstructures of O/W emulsions. Food Hydrocoll. 2021, 119, 106825. [Google Scholar] [CrossRef]
- Lobo, F.; Ventanas, S.; Morcuende, D.; Estévez, M. Underlying chemical mechanisms of the contradictory effects of NaCl reduction on the redox-state of meat proteins in fermented sausages. LWT 2016, 69, 110–116. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; Guo, J.; Feng, X.; Yin, X.; Wang, L.; Wu, W.; Li, Z.; Sun, W.; Ma, J. Effects of oxidative modification on textural properties and gel structure of pork myofibrillar proteins. Food Res. Int. 2019, 121, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibrillar proteins. Int. J. Food Sci. Technol. 2018, 53, 2687–2696. [Google Scholar] [CrossRef]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- Amici, A.; Levine, R.L.; Tsai, L.; Stadtman, E.R. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J. Biol. Chem. 1989, 264, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Kawakishi, S. 2-Oxo-histidine as a novel biological marker for oxidatively modified proteins. FEBS Lett. 1993, 332, 208–210. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, Y.; Zhu, X.; Li, S.; Zhou, C. L-lysine and L-arginine inhibit the oxidation of lipids and proteins of emulsion sausage by chelating iron ion and scavenging radical. Asian-Australas. J. Anim. Sci. 2018, 31, 905–913. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, B.; Zhang, Y.; Huang, Y.; Chen, L.; Bao, P.; Fang, H.; Zhou, C. L-arginine/L-lysine alleviated the deterioration of emulsion sausages with partial replacement of porcine backfat by soybean oil by hindering hydroxyl radical stressed oxidation of meat proteins. ACS Food Sci. Technol. 2021, 1, 967–974. [Google Scholar] [CrossRef]
- Mei, L.; Mcclements, D.J.; Wu, J.; Decker, E.A. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl. Food Chem. 1998, 61, 307–312. [Google Scholar] [CrossRef]
- Cui, L.; Shen, P.; Gao, Z.; Yi, J.; Chen, B. New insights into the impact of sodium chloride on the lipid oxidation of oil-in-water emulsions. J. Agric. Food Chem. 2019, 67, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Cui, L. Impact of NaCl substitution with KCl on the lipid oxidation in oil-in-water emulsions. Food Chem. 2025, 466, 142154. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Jaffe, R. Lipid peroxidation of muscle food as affected by sodium chloride. J. Agric. Food Chem. 1991, 39, 1017–1021. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, J.; Mao, J.; Dong, X.; Chen, Y. Lipidomics reveals the relationship between lipid oxidation and flavor formation of basic amnio acids participated Low-Sodium cured large yellow croaker. Food Chem. 2023, 429, 136888. [Google Scholar] [CrossRef]
- Liu, X.; Piao, C.; Ju, M.; Zhang, J.; Zhang, W.; Cui, F.; Li, G.; Cui, M. Effects of low salt on lipid oxidation and hydrolysis, fatty acids composition and volatiles flavor compounds of dry-cured ham during ripening. LWT 2023, 187, 115347. [Google Scholar] [CrossRef]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Teng, S.; Zhou, Y.; Liu, Y.; Liao, B.; Ren, X.; Zhang, Y. Effects of BAAs on heterocyclic amines and quality characteristics of fried beef patties at low NaCl level. Meat Sci. 2024, 215, 109541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Peng, Z.; Jamali, M.A. A review of recent progress in reducing NaCl content in meat and fish products using BAAs. Trends Food Sci. Technol. 2022, 119, 215–226. [Google Scholar] [CrossRef]
- Qiu, H.M. Effect of L-Arginine in Combination with Table Salt on Saltiness Perception and Application in Low-Salt Sausages. Master’s Thesis, Huaiyin Institute of Technology, Huai’an, China, 2022. (In Chinese). [Google Scholar]
- Silva, S.L.; Lorenzo, J.M.; Machado, J.M.; Manfio, M.; Cichoski, A.J.; Fries, L.L.M.; Morgano, M.A.; Campagnol, P.C.B. Application of arginine and histidine to improve the technological and sensory properties of low-fat and low-sodium bologna-type sausages produced with high levels of KCl. Meat Sci. 2020, 159, 107939. [Google Scholar] [CrossRef]
- Campagnol, P.C.B.; Santos, B.A.D.; Morgano, M.A.; Terra, N.N.; Pollonio, M.A.R. Application of lysine, taurine, disodium inosinate and disodium guanylate in fermented cooked sausages with 50% replacement of NaCl by KCl. Meat Sci. 2011, 87, 239–243. [Google Scholar] [CrossRef]
- Vidal, V.A.S.; Santana, J.B.; Paglarini, C.S.; Silva, M.A.A.P.; Pollonio, M.A.R. Adding lysine and yeast extract improves sensory properties of low sodium salted meat. Meat Sci. 2019, 159, 107911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, N.; Gao, P.; Yu, D.; Yang, F.; Xu, Y.; Xia, W. Influence of L-arginine addition on the gel properties of reduced-salt white leg shrimp (Litopenaeus vannamei) surimi gel treated with microbial transglutaminase. LWT Food Sci. Technol. 2023, 173, 114310. [Google Scholar] [CrossRef]
- Guo, X.; Wang, R.; Han, B.; Shao, W.; Chen, L.; Feng, X. A novel EGCG-histidine complex improves gelling and physicochemical properties of porcine myofibrillar proteins: Insight into underlying mechanisms. Food Chem. 2024, 448, 139070. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, Y.; Zhou, T.; Wang, Y.; Yan, J.; Lai, B.; Wang, C.; Wu, H. Gelation improvement of low-salt Chinese shrimp (Fenneropenaeus chinensis) surimi gel by L-arginine. Food Chem. 2025, 465, 142020. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Chen, Q.; He, Z.; Wang, Z.; Qin, F.; Yang, T.; Chen, J.; Zeng, M. Non-precursors amino acids can inhibit β-carbolines through free radical scavenging pathways and competitive inhibition in roast beef patties and model food systems. Meat Sci. 2020, 169, 108203. [Google Scholar] [CrossRef] [PubMed]
- Linghu, Z.; Karim, F.; Smith, S. Amino acids inhibitory effects and mechanism on 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) formation in the Maillard reaction model systems. J. Food Sci. 2017, 82, 3037–3045. [Google Scholar] [CrossRef]
- Ma, F.; Li, Y.; Zhang, Y.; Zhang, Q.; Li, X.; Cao, Q.; Ma, H.; Xie, D.; Zhang, B.; Yu, J.; et al. Effects of umami substances as taste enhancers on salt reduction in meat products: A review. Food Res. Int. 2024, 185, 114248. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhang, Q.; Hayat, K.; Ho, C.T. Proline-glucose amadori compounds: Aqueous preparation, characterization and saltiness enhancement. Food Res. Int. 2021, 144, 110319. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J. The Enhancement of Saltiness Perception in Fish Products by Microwave Treatment and the Processing Adaptability of Reduced-Salt Surimi. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2021. (In Chinese). [Google Scholar]
- Alio, M.; Fuentes, A.; Fernández-Segovia, I.; Barat, J.M. Development of a low-sodium ready-to-eat desalted cod. J. Food Eng. 2011, 107, 304–310. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Xu, Y.; Mi, H.; Yi, S.; Gao, R.; Li, X.; Li, J. Effects of chickpea protein-stabilized Pickering emulsion on the structure and gelling properties of hairtail fish myosin gel. Food Chem. 2023, 417, 135821. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; Yan, H.; Lu, Y.; Deng, X.; Hu, Y.; Xiong, S.; Yang, H. The influence of pH and monovalent/divalent cations on the structural and physicochemical properties of myofibrillar protein from silver carp. Food Chem. 2023, 404, 134519. [Google Scholar] [CrossRef] [PubMed]
- Horita, C.N.; Messias, V.C.; Morgano, M.A.; Hayakawa, F.M.; Pollonio, M.A.R. Textural, microstructural and sensory properties of reduced sodium frankfurter sausages containing mechanically deboned poultry meat and blends of chloride salts. Food Res. Int. 2014, 66, 29–35. [Google Scholar] [CrossRef]
- Horita, C.N.; Morgano, M.A.; Celeghini, R.M.S.; Pollonio, M.A.R. Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci. 2011, 89, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Inguglia, E.S.; Burgess, C.M.; Kerry, J.P.; Tiwari, B.K. Ultrasound-assisted marination: Role of frequencies and treatment time on the quality of sodium-reduced poultry meat. Foods 2019, 8, 473. [Google Scholar] [CrossRef] [PubMed]



| Type | Equations | |
| Protein oxidation | (1) | |
| (2) | ||
| Lipid oxidation | (3) | |
| (4) | ||
| (5) |
| Product | Salt Mixture | Mechanism | Result | Reference |
|---|---|---|---|---|
| Harbin dry sausage | 70% NaCl, 20% KCl, 4% L-Lys, 1% L-Arg, 0.5% citric acid, 1% Ca-lactate, and 3.5% maltodextrin | Enhancing the formation of volatile compounds from carbohydrate and amino acid metabolism, β-lipid oxidation, and esterification. | Improving the flavor and reducing NaCl by 30%. | [69] |
| Pork emulsion sausages | 2.2 g of low-sodium salt + 0.6 g L-Arg and L-Lys | L-Arg and L-Lys could retard the total SH content reduction. | L-Arg and L-Lys could retard meat proteins form oxidation under ·OH stress. | [73] |
| Low-fat bologna sausages | 1% NaCl + 1.5% KCl + 1% L-Arg + 0.2% L-His | Increasing the pH values and deviating from the isoelectric point of MP via the formation of hydrogen bonds and ion–dipole interactions between the side chains of these amino acids and water. | Producing products with a 40% sodium reduction while ensuring adequate processing and sensory properties. | [84] |
| Fermented cooked sausages | 0.313% L-Lys and a mixture of taurine (750 mg/kg) with disodium inosinate (300 mg/kg) and disodium guanylate (300 mg/kg) | The pH decreased significantly, while Aw ranged from 0.905 to 0.916, showing no significant difference between the modified products and the control. | Improving the flavor issues due to KCl. | [85] |
| Salted meat | 50% NaCl, 25% KCl, 25% CaCl2 + 3% L-Lys | Decreasing moisture content. | Minimizing the negative sensory impact of KCl and CaCl2, decreasing the salty taste and aftertaste in mixed-salt meat products, without affecting physicochemical quality parameters. | [86] |
| Ground chicken breast meat | 1% NaCl + 0.06% L-Arg, L-Lys, and L-His (w/w) | Increasing pH, WHC, and solubility. | Compared to the 1% NaCl (w/w) treatment, adding 0.06% BAAs (w/w) significantly increased MP solubility, emulsion activity, storage modulus change rate, gel WHC, and hardness. | [60] |
| Reduced-salt whiteleg shrimp surimi | 0.5% NaCl, 0.75% MTGase, and different contents (0.5%, 1%, 1.5%, 2.0%, and 2.5%) of L-Arg | Adding L-Arg and MTGase together significantly increased disulfide bonds and the protein β-sheet structure of SSG, while enhancing moisture distribution and rheological properties. | Combining L-Arg and MTGase improved the gel characteristics of SSG while reducing NaCl content. | [87] |
| Pork meat | EGCG–His complex at a molar ratio of 1:5 | Forming an EGCG–Histidine complex through covalent binding of histidine to EGCG via Michael addition or Schiff base reaction significantly increases the antioxidant activity of the complex compared to EGCG or histidine alone. | Decreasing cooking loss (40.3 ± 2.02%), enhancing rheological properties, and enhancing gel strength (0.22 ± 0.03 N) of MP. | [88] |
| Chinese shrimp surimi | 0.75% L-Arg (w/w) | Enhancing protein solubility, hydrogen bonds, and disulfide bonds in SSG, L-Arg addition resulted in a denser network structure, as observed by Cryo-SEM. Molecular docking revealed L-Arg interaction with myosin through hydrogen bonds, significantly increasing protein solubility to 74.89%. | Increasing protein solubility, hydrogen bonds, and disulfide bonds with 0.75% L-Arg, forming a denser gel network structure for low-salt SSG, thereby improving gel properties. | [89] |
| Roast beef patties | 0.1%, 0.5%, 1.0% L-His (w/w) | Attributing the inhibitory mechanism of L-His to free radical scavenging and competitive inhibition. | Demonstrating excellent alkyl radical scavenging ability, with a maximum of 82.59%, L-His effectively reduced radical activity. | [90] |
| A model system consisting of reducing sugars, creatinine, and phenylalanine to investigate PhIP formation | Reactivity of each AA to the total PheAce in PheAce-containing model system, wherein 0.04 mmol PheAce used as precursor reacted with AA solution (0.4 M) in final molar ratio of PheAce:AA at 1:0, 1:0.125, 1:0.25, 1:0.5, 1:1, and 1:2. | Scavenging HAs directly by L-Lys and forming adducts with L-Lys, which may contribute to reducing HA content. | Inhibiting the aldol condensation between creatinine and phenylacetaldehyde to form PhIP, phenylacetaldehyde-Lys adducts in the Maillard reaction reduced PhIP content in the final product in a dose-dependent manner. | [91] |
| Emulsion sausage | 0.4% L-Arg and L-Lys (w/w) | Sharing a similar molecular structure with L-Lys and L-Arg exhibits comparable capacity to scavenge free radicals. | Effectively inhibiting lipid and protein oxidation in emulsion sausage, both L-Lys and L-Arg scavenged free radicals and chelated ferrous ions. | [72] |
| Fried beef patties | 1% NaCl + L-Arg, L-Lys, and L-His (0.1%, 0.5%, 1%, w/w) | - | The addition of BAAs at 1% NaCl significantly enhanced the quality characteristics compared to 3% NaCl. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Hu, W.; Chen, L.; Ouyang, K.; Chen, H.; Lin, S.; Wang, W. Basic Amino Acids as Salt Substitutes in Low-Salt Gel-Based Meat Products: A Comprehensive Review of Mechanisms, Benefits, and Future Perspectives. Foods 2025, 14, 637. https://doi.org/10.3390/foods14040637
Yu C, Hu W, Chen L, Ouyang K, Chen H, Lin S, Wang W. Basic Amino Acids as Salt Substitutes in Low-Salt Gel-Based Meat Products: A Comprehensive Review of Mechanisms, Benefits, and Future Perspectives. Foods. 2025; 14(4):637. https://doi.org/10.3390/foods14040637
Chicago/Turabian StyleYu, Chuanlong, Wenbing Hu, Lingli Chen, Kehui Ouyang, Hui Chen, Suyun Lin, and Wenjun Wang. 2025. "Basic Amino Acids as Salt Substitutes in Low-Salt Gel-Based Meat Products: A Comprehensive Review of Mechanisms, Benefits, and Future Perspectives" Foods 14, no. 4: 637. https://doi.org/10.3390/foods14040637
APA StyleYu, C., Hu, W., Chen, L., Ouyang, K., Chen, H., Lin, S., & Wang, W. (2025). Basic Amino Acids as Salt Substitutes in Low-Salt Gel-Based Meat Products: A Comprehensive Review of Mechanisms, Benefits, and Future Perspectives. Foods, 14(4), 637. https://doi.org/10.3390/foods14040637







