Abstract
Insect metabolites are known for their preservative potential, but the time-consuming and unsustainable extraction process compromises their transferability. This study aimed to identify user-friendly solutions based on the use of insect meals that could improve microbiological safety as well as consumer acceptability. In this regard, the antimicrobial activity of Alphitobius diaperinus and Tenebrio molitor meals against surrogate strains of Gram-positive (Listeria monocytogenes) and Gram-negative (Escherichia coli) pathogenic bacteria and mycotoxin-producing fungi (Penicillium expansum) was evaluated. Minimum inhibitory concentration values of between 3.12 mg/mL vs. Listeria innocua and 12.50 mg/mL vs. Escherichia coli were found. Based on this finding, a model food was developed also considering consumer acceptance. Statistical analysis of food preferences showed that nutritional and sustainability claims were the independent variables of greatest interest. Therefore, waste or by-products from other food chains were selected as co-ingredients for sustainability, nutritional, and sensory claims. Analysis of the chemical composition showed that the insect bar-style snack qualifies as a “high-protein” food, as protein provides more than 20% of the energy value. Based on the moisture (30%) and water activity (0.77) values, the bar could be classified as an intermediate-moisture food. The challenge test showed that the insect meal prevented the proliferation of intentionally added undesirable microorganisms. Conclusively, the findings complement the knowledge on the antimicrobial activities of insect meals, offering new possibilities for their use as natural preservative ingredients with nutritionally relevant properties.
1. Introduction
Human evolution, progress, and the growth of civilizations are closely linked to the progress and transition of agri-food systems [1,2]. Today, more than any other time, with more than 8 billion people having the right to healthy food, profound innovation is needed. The availability of food divides the planet into two parts: on the one hand, rich geographical areas with a surplus of food and a worrying obesity rate, and on the other, geographical areas where the right to food is denied to more than a billion people [3]. The handling is paradoxical: almost 40 percent of food, which requires high pressure on the earth to be produced, is wasted before it reaches consumers’ tables [4,5]. Moreover, these two 21st-century paradoxes are accompanied by a rise in antimicrobial resistance of food-borne pathogenic and/or spoilage microorganisms [6,7,8,9]. The advances and knowledge gained in the last decade in the field of food biotechnology and food protection offer various insights into innovations in the food system [10,11,12]. The use of plant- or insect-based proteins or bioactive substances as an alternative to chemical ingredients or conventional additives are the pillars on which the food system transition rests [13,14,15,16,17]. To date, four edible insects are permitted as feed and food ingredients in Europe: the lesser insect worm Alphitobius diaperinus (A. diaperinus) [18], the house cricket Acheta domesticus [19,20], the locust migratory worm Locusta migratoria [21], and the yellow worm Tenebrio molitor (T. molitor) [22,23]. Besides the ability to convert and enhance by-products of different origins [24,25], insects are also high in protein, healthy fats, vitamins, and minerals, characteristics that make them a promising resource to meet the growing global demand for food [26,27]. Moreover, their use in the food chain goes beyond direct consumption: products derived from insects, such as extract or purified bioactive compounds, offer new opportunities to improve food quality and safety [28]. The study of the antimicrobial properties of insects is receiving increasing attention. The first evidence of antimicrobial properties in insects was reported in the 1970s [29]. Certainly chitin, a linear polymer composed of N-acetyl-glucosamine that is particularly abundant in the adult and larval forms of insects, is responsible for the antimicrobial interest. The antimicrobial activity of chitin has already been extensively characterized in recent decades [30], as has the enhanced efficacy of chitosan. Chitosan shows higher efficacy due to positively charged amine groups interacting with bacterial cell surface components and higher solubility [31]. To date, extracts and peptides derived from insects have demonstrated effective antimicrobial activity against a wide range of pathogenic and spoilage microorganisms [32]. This has led to proposals for their use as natural preservatives in food [33]. Recent studies highlighted the potential of these compounds in controlling several bacteria, including pathogenic species [34,35,36] that extracted chitin from larvae and adults of T. molitor by deproteinization and demineralization, with yields of around 6% in larvae and 12% in adults. The same authors observed that chitosan, obtained by deacetylation of chitin, produced inhibitory activity against sporogenesis bacteria such as Bacillus cereus vs. Gram-positive ones such as vs. Listeria monocytogenes and Staphylococcus aureus and Gram-negative ones such as Escherichia coli [37]. Anti-fungal activity expressed by chitosan from insects was also found. Until a few years ago, conventional methods of extraction and subsequent de-methylation of chitin, using considerable and impactful amounts of solvents, acids, and chemical bases, had several environmental drawbacks. Only recently, several green extraction methods have also been successfully developed to extract chitin and chitosan from various resources, including insect sources. Overall, techniques based on the use of chemicals have been supplemented with physical operations, ranging from thermal action to the application of high-pressure CO2 via high-voltage pulsed electric fields [38,39]. Although green approaches for the extraction of bioactive compounds have been developed [40], the extraction requires technologically complex processes that could limit their large-scale adoption. In addition, besides chitin and chitosan, other antimicrobial compounds are present, such as medium-chain saturated fatty acids, with lauric acid (LA) in particular, and antimicrobial peptides (AMPs) [34,41,42,43]. Based on these considerations, insect meals represent an attractive alternative to purified compounds or extracts, offering an integrated and less elaborate approach to harnessing the antimicrobial benefits of insects. Moreover, with the presence of bioactive compounds, insect meals retain all the nutritional properties of the whole organism, making it a versatile ingredient from both functional and nutritional perspectives. In addition, the use of insect meals simplifies the production process, reducing costs and complexity, and ensures a more sustainable approach, minimizing waste and exploiting the full potential of raw materials. However, the achievement of the plethora of benefits associated with the use of insect meals is related to their antimicrobial efficacy and the extent to which it is comparable to that of purified compounds or extracts. To date, although insect meals have been proposed as an ingredient for food or feed, emphasizing their antimicrobial potential [36,41,44,45], no exhaustive studies are available on the antimicrobial efficacy of insects used directly as meal in food preparation. Gaps or unresolved issues are also those related to meeting consumers’ demands or resolving their hesitations. The factors determining their acceptability or rejection have been investigated through specific research and analyzed in reviews [46,47,48,49]. However, further investigation that considers consumer expectations, the bioactive potential of edible insects, and their use in food preparation in an integrated manner is necessary and remains a priority. Based on these considerations, the antimicrobial activity expressed by two edible insect meals against various microorganisms, including Gram-negative bacteria (Escherichia coli), Gram-positive bacteria (Listeria innocua), and fungi (Penicilliium expansum), was evaluated and compared to that produced by purified compounds such as chitin and chitosan or insect extracts. The possibility of using insect meal was not only related to its antimicrobial efficacy but also to the expectations and requirements of a heterogeneous sample of consumers. Finally, for the validation of the use of edible insects, the integrated consideration of several factors was adopted, based on specific challenge tests and consumer feedback.
2. Materials and Methods
2.1. Pre-Treatment Edible Insect
A. diaperinus and T. molitor larvae were chosen as edible insects. Both larvae were purchased from an insect company (Fauna Topics Zoobedarf, breeding and trade LLC, Marbach am Neckar, Germany) and grown in a controlled rearing facility according to European food standards. They are fed exclusively with plant-based feeds, such as mixtures of cereal flour and bran, without the use of antibiotics, hormones, and pesticides, according to GMP+ FC (Good Manufacturing Practice Feed Certification system). Twenty-four hours prior to the technological scalding process, the larvae were not fed to allow the gut to empty, and the room temperature was lowered to promote hibernation. They were then freeze-dried, ground, and sieved to produce fine powder and stored at room temperature until use. The final weight of the larvae was 1/3 of the original live weight.
Antimicrobial Activity and Minimum Inhibitory Concentration
Meals from the two insects were evaluated for their antimicrobial activity against Escherichia coli ATCC 8739 (as a surrogate of enterohemorrhagic Escherichia coli) [50], Listeria innocua ATCC 33090 (as a surrogate of Listeria monocytogenes) [51], and Penicilliium expansum ATCC 36200. All strains were purchased from the ATCC—American Type Culture Collection. Strains of Escherichia coli (E. coli), Listeria innocua (L. innocua), and Penicilliu expansum (P. expansum) were revitalized at 37 °C in Luria–Bertani broth (LB—Oxoid, Milan, Italy), at 28 °C in brain–heart infusion (BHI—Oxoid, Milan, Italy), and at 28 °C in potato dextrose broth (PD—Oxoid, Milan, Italy), respectively. The insect meals were suspended in sterile distilled water at a ratio of 1:3 w/v, and their inhibitory action was assessed by the agar well diffusion assay as previously described [52,53]. Briefly, 1 mL (inoculum concentration of 4.0 log CFU/mL) of each bacterial suspension was inoculated in 19 mL of proper soft media (0.7% agar), gently mixed, and poured onto Petri plates (BHI for L. innocua, LB for E. coli, and PD for P. expansum). On each plate, 7 mm-diameter wells were set up, within which 70 µL of meal suspension were added. Tetracycline disk 30 µg (Sigma-Aldrich, Milan, Italy) and sterile distilled H2O (70 µL) were used as negative and positive control, respectively. Plates inoculated with P. expansum were incubated at 28 °C for 24 h, while plates containing L. innocua or E. coli were incubated at 37 °C for 24 h. Antimicrobial activity was assessed by measuring the diameter of the halo formed around the wells after 24 h. Based on the halo diameter, the inhibitory activity was described as low (halo ≤ 10 mm), moderate (10 < halo < 20 mm), or high (halo > 20 mm). Five independent replicates were performed. In addition, the minimum inhibitory concentration (MIC) was determined as described in the final EUCAST 3.1 document [54] with some modifications [14]. Specifically, each larval meal suspension was added to the different culture media to obtain a concentration of between 0.2 and 4% (w/v) and placed in Petri dishes. The surface of the plates was inoculated with the different microbial strains at a concentration of 4.0 log CFU/mL and incubated at the respective temperatures. At the end of the incubation period, the plates were evaluated for the presence or absence of growth. MIC values were recorded as the lowest concentration of natural extract that completely inhibited growth. Each test was performed in triplicate. Finally, the effect of meal suspensions on the kinetic parameters of the three strains was evaluated: E. coli, L. innocua, and P. expansum were inoculated (6 log CFU/mL) in phosphate-buffered saline (PBS) in the presence of T. molitor meal or A. diaperinus meal suspension at the MIC value, and the growth kinetics were assessed using the structured dynamic model of Baranyi and Roberts [55]. DMFit software (v2.0) was used for this purpose. Kinetic parameters were analyzed to determine the antimicrobial score, arbitrarily defined as follows:
where:
- N_end = number of cells at the end of the observation period;
- N_0 = number of cells at the beginning of the observation period.
2.2. Food Design
2.2.1. Consumer Survey—Assessment of Consumer Attitudes
Participants were recruited online by distributing a questionnaire to consumers from EU countries over a three-week period via the University of Molise’s e-mail networks, as well as among the research team’s social networks, and respondents were asked to share the survey link to increase coverage. To have a representative population of the different age groups, consumers aged 16 to 85 years were included. In addition, up to 10 consumers per year of age were accepted, with 700 interviewees collected (Table S1). To this end, an age-related question was asked upstream of the questionnaire, which allowed respondents to proceed only if they were within the age range of interest. All respondents provided informed consent (Document S1) and, in the case of respondents under the age of 18, also parental consent (Document S2). Data were collected and processed anonymously, without identification of participants, and analyzed in aggregate form. The survey consisted of three parts:
- -
- The first contained demographic questions regarding age, gender, level of education, and place of residence (city above or below 50,000 inhabitants or rural villages).
- -
- The second part of the survey included questions aimed at ascertaining knowledge of the possibility of eating insects and edible insect-based foods.
- -
- The third part included questions aimed at understanding the propensity to consume insect-based foods and the characteristics (nutritional, environmental, sustainability) that should accompany such foods, as well as a specific question aimed at understanding the type of insect-based food most preferred.
The interviewees answered each question with a numerical value according to a hedonistic scale from 1 to 10.
2.2.2. Analysis of Factors Influencing Consumer Attitudes
Data were collected using Google Forms and were analyzed to define a predictive model of the propensity to consume insects as a function of other variables, including respondents’ demographic characteristics (e.g., age, educational qualification, place of residence, etc.) as well as expected characteristics from insect foods (such as sustainability, nutritional claims). To this end, a multiple linear regression was applied using the R (v4.2.3) environment and RStudio software (v2022.07.0).
2.3. Insect-Based Model Food: Preparation and Characterization
A bar-type snack was made using insect larvae meal and other ingredients chosen for their workability, nutritional, and sustainability characteristics.
2.3.1. Ingredients and Bar-Snack Preparation
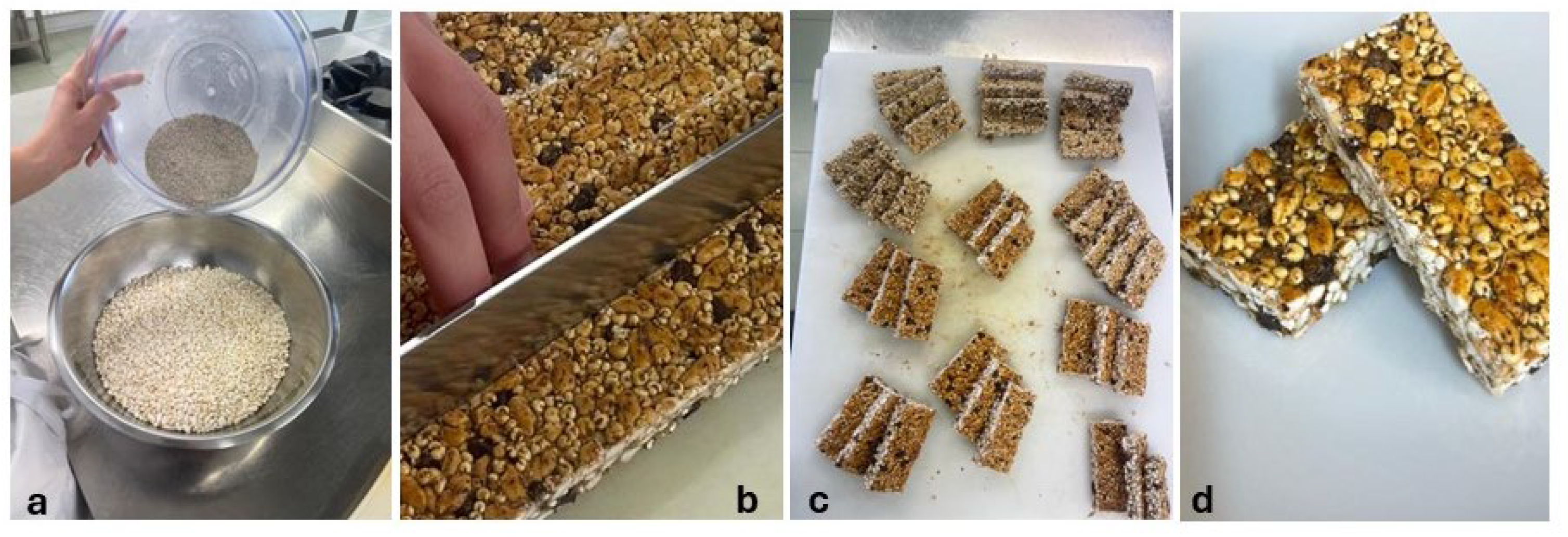
The ingredients used in the production of bars include edible insects, puffed grains and cereals, and waste and by-products of agro-food production. T. molitor larvae meal (8%) and A. diaperinus (8%) were used as edible insects. Marron glacé waste 8% (GMF Oliviero Ltd., Avellino, Italy), carob gum 6% (CioKarrua Ltd., Ragusa, Italy), and whey 26% (Mediterranea Biotecnologia Ltd., Termoli, Italy) were chosen for their sustainability potential as waste or by-products of other food processing. Puffed rice 4%, puffed quinoa 12%, flaxseed meal 8% (Prezzemolo & Vitale, Milan, Italy), chopped dark chocolate 6% (Barry Callebaut, Milan, Italy), and agave syrup 14% (Eridania, Bologna, Italy) were chosen for their sensory, nutritional, and technological properties. For the bar’s preparation (Figure 1), 80 g T. molitor larvae meal and 80 g A. diaperinus were combined with 80 g marron glacé waste, 40 g puffed rice, 120 g puffed quinoa, 80 g linseed meal, and 60 g chopped dark chocolate. The ingredients were thoroughly mixed and combined with 260 mL of whey in which 60 g of locust bean gum had previously been dissolved. Finally, 140 mL of agave syrup previously heated to 75 °C were added to the resulting mixture. The entire dough was shaped and cut to obtain 200 bars of approximately 50 g and a parallelepiped shape with a width of 2.5 cm, a height of 6 cm, and a depth of 0.6 cm.
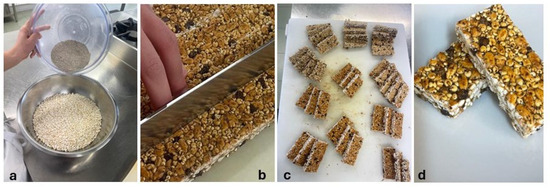
Figure 1.
Sequence of photos relating to snack-bar preparation: (a) insect meals (in upper bowl) and other ingredients (in lower bowl); (b) cutting of formed and shaped dough; (c,d) finished product.
2.3.2. Chemical Composition
Moisture, carbohydrates, protein, fat, ash, and fiber contents were determined in bars according to the official methods of AOAC 2023 [56], specified below. In detail, the moisture of the burgers was determined based on 5 g of sample placed in an oven for 16 h at 105 °C, calculated as the difference between the weight of the fresh sample and the weight of the sample after drying and expressed as percentage of dry matter (d.m.) [56]. Protein content was determined using the Kjeldhal method [56]. Crude fat content was measured in accordance with AOAC method 960.39 using a Soxhlet apparatus [56]. Ash content was determined by heating the samples for 3 h at 550 °C. Dietary fiber content was determined using AOAC method 985.29 [56]; carbohydrates (C) were calculated by the difference as follows:
where M = moisture (%), P = protein content (%), L = lipid content (%), A = ash content (%), and F = dietary fiber (%).
C% = 100 − (M + P + L + A + F)
2.3.3. Physical–Chemical and Microbiological Analyses
The pH was determined in three different points of two samples, using a pH meter with a Mettler Toledo spear probe (Novate Milanese, Italy). Water activity was measured in triplicate using a Water Activity Meter CR2 (AQUALAB Instrument, Washington, DC, USA) on two different samples of snack bars. Microbial groups were enumerated as follows: 10 g samples were decimal-diluted in a sterile solution of 0.1% peptone water, homogenized in a Stomacher 400 Lab Blender (Seward Medical, London, UK) for 3 min, and serially diluted in the same sterile solution. Total mesophilic bacteria (TMC), Enterobacteriaceae, Listeria spp., and fungi were detected after appropriate incubation in proper media and conditions. Briefly, TMC were enumerated on plate count agar (PCA) (Oxoid, Milan, Italy) after incubation at 30 °C for 72 h. Enterobacteriaceae were enumerated on violet red bile glucose agar (VRBGA) (Oxoid) after incubation at 37 °C for 24 h. Fungi were enumerated on rose bengal agar (RBA, Oxoid, Milan, Italy) after 48 h of incubation at 28 °C, and Listeria spp. were enumerated on Listeria selective Oxford agar base with modified Listeria selective supplement (Oxoid, Milan, Italy) after 24 and 48 h of incubation at 37 °C. Each experiment was carried out in duplicate, and results were expressed as the mean of measurements and standard error.
2.3.4. Sensorial Characterization
The sensory evaluation of snack bars was conducted by 20 judges, aged between 22 and 65 years, specifically trained in the sensory analysis of snacks with a focus on bars. From 40 candidates, after 10 training sessions, a panel of 20 judges was formed with optimal uniformity of response. All respondents provided informed consent (Document S3). The panel of judges, led by a panel leader, conducted a threefold sensory analysis on the bar using a 9-point hedonic scale to assess appearance, color, aroma, flavor, texture, stickiness, aftertaste, and overall liking. The panelists considered the specific evaluation criteria for each attribute (Table S2) as described below:
- -
- Appearance, an attribute describing the overall look of the bar, including shape, surface perception, presence of visible insect fragments, and uniformity, was evaluated according to the following criteria: Does the bar look attractive and appetizing? Is the shape consistent and well modeled? Are visible insect components present and do they affect acceptability?
- -
- Color, aimed at describing the appropriateness and attractiveness of the color in relation to consumer expectations, was expressed as a summative judgment with respect to the following criteria: Is the color uniform and natural? Does the color correspond to the expectations of a protein/energy bar? Are there any discolorations or unattractive shades?
- -
- Aroma was reported as a summative judgement with respect to the following questions: Is the aroma inviting or neutral? Are there strong or unpleasant aromas related to insects? Is the aroma consistent with that of conventional protein bars?
- -
- Flavor, which expresses the overall taste profile, including sweetness, bitterness, umami, and potential off flavors, was reported as the summative result of the following evaluation criteria: Is the taste balanced and pleasant? Are there any off flavors? Does the bar have a desirable level of taste?
- -
- Texture, understood as mouthfeel and structural integrity of the bar (hardness, chewability, and softness) is expressed as a summative judgement of the following criteria: Is the texture soft, firm, or too hard? Does it crumble, does it stick to the teeth, or is it grainy? Is chewability acceptable?
- -
- Stickiness, the degree of adherence of the bar to the fingers and to the oral cavity during consumption, is expressed as a summative judgement of the following criteria: Is it excessively sticky when handled? Does the bar leave a sticky residue in the mouth? Does the level of stickiness affect overall acceptability?
- -
- Aftertaste, which identifies the residual taste that remains in the mouth after swallowing, is expressed as a summative judgment of the following criteria: Is it pleasant, neutral or undesirable? Is it long lasting? Does the aftertaste encourage or discourage further consumption?
- -
- Overall liking, that is, the overall acceptability of the bar based on all sensory attributes combined, is expressed as a summative judgment of the following criteria: Would the consumer consume the bar again? Is the bar as enjoyable as or better than conventional protein bars?
2.4. Antimicrobial Validation of Insect Larvae Meal
The antimicrobial effect of the two insects’ larvae meal was evaluated by means of in situ tests by setting up an appropriate challenge test to simulate multiple microbial contamination conditions. For this purpose, the insect-based bars were inoculated with 4 log CFU/g of a microbial cocktail consisting of a mixture of three microbial strains referring to L. innocua ATCC 33090, E. coli ATCC 8739, and P. expansum ATCC 36200. Prior to use, the microbial cultures were revitalized in nutrient broth (Oxoid, Milan, Italy) and, in the exponential phase, the strains were inoculated into the mixture for challenge testing. The development of Listeria spp., Escherichia spp., and Penicillium spp. in the bars was evaluated during the storage period (30 days).
The data were compared with those found in bars prepared with the same ingredients but without insects and inoculated equally with the same mixture of microorganisms.
2.5. Statistical Analyses
The data on the inhibition halo and antimicrobial score against the three target microorganisms were subjected to analysis of variance (ANOVA) followed by a Bonferroni’s post hoc test to identify differences and their levels of significance (p < 0.05, p < 0.01, or p < 0.001) between conditions. The influence of independent variables on the propensity to consume was assessed by means of multiple linear regression analysis. The data obtained from the chemical, sensory analysis, and challenge tests were subjected to a t-test to identify the differences and their significance levels (p < 0.05, p < 0.01, or p < 0.001) between the bars prepared without (control batch) and with insect meals (insect-based bar batch).
3. Results and Discussion
3.1. Meal Alphitobius diaperinus and Tenebrio molitor Larvae: Antimicrobial Activity
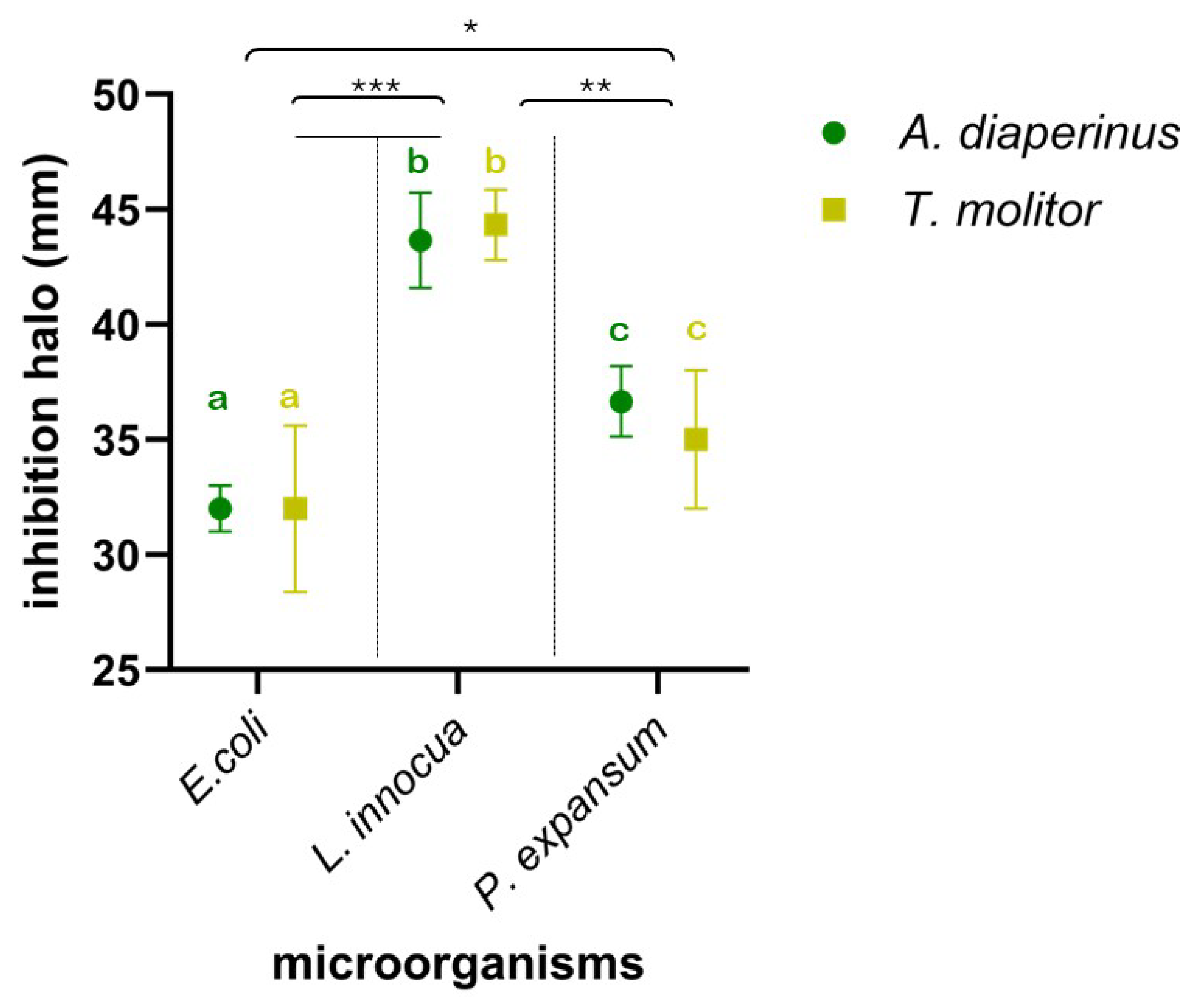
To assess the antimicrobial effectiveness expressed by edible insect larvae, used as nutritionally relevant ingredients in food preparation, their anti-Listeria, anti-Escherichia, and anti-Penicillium spectrum of action was evaluated. Specifically, the effect of meals from the two insects on the growth of E. coli ATCC 8739, L. innocua ATCC 33090, and P. expansum ATCC 36200 was investigated and is shown in Figure 2. Insect larvae meal in all tests significantly interfered with the growth of the three microorganisms, resulting in clear inhibition halos around the wells. The meal used, in suspension in DMSO at 10% (w/v), produced inhibition halos against the target microorganisms, ranging from 30 mm (vs. E. coli ATCC 8739) as the lowest value to the highest value of 45 mm against L. innocua ATCC 33090. The results obtained from the different meals by two insects did not show substantial or significant differences (p > 0.1) between them in terms of their effects on the target microorganisms. On the contrary, significant differences depended on the sensitivity of the different strains to the insect meals. L. innocua ATCC 33090 showed the highest sensitivity, resulting in significantly higher sensitivity to both P. expansum ATCC 36200 (p < 0.01) and E. coli ATCC 8739 (p < 0.001). In addition, the latter was the least sensitive strain, showing a significant difference (p < 0.05) even compared to P. expansum ATCC 36200.
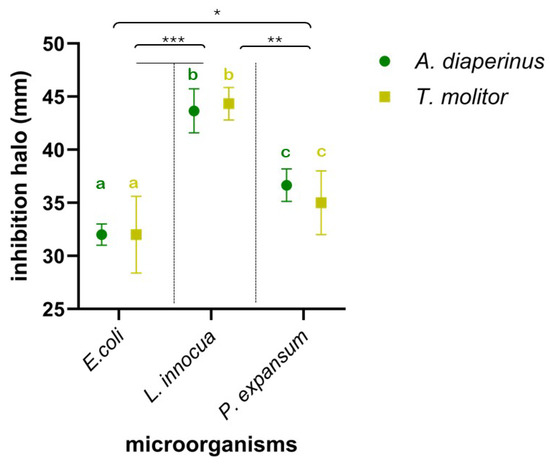
Figure 2.
Inhibition halo plot (mm) of Alphitobius diaperinus (green) and Tenebrio molitor (yellow) meals against Escherichia coli ATCC 8739, Listeria innocua ATCC 33090, and Penicilliium expansum ATCC 36200. Different letters indicate a significant difference between microorganisms based on the statistical ANOVA test. Asterisks indicate significant difference values (*, p < 0.05; **, p < 0.01; ***, p< 0.001) in attributes among the 3 microorganisms.
In all cases, the inhibition halos were significantly higher than those produced by antibiotic disks, namely, gentamycin disk (against E. coli and L. innocua) and cycloheximide disk (against P. expansum) used at a concentration of 10 mg/mL and 30 mg/mL, respectively. In fact, the diameter of the halos produced by both gentamicin and cycloheximide never exceeded 20 mm. These preliminary findings from the evaluation of inhibition halos are further confirmed by the relative MIC data (Table 1).

Table 1.
Minimum inhibitory concentration (MIC) values (mg/mL) of Alphitobius diaperinus and Tenebrio molitor meals against Escherichia coli ATCC 8739, Listeria innocua ATCC 33090, and Penicilliium expansum ATCC 36200.
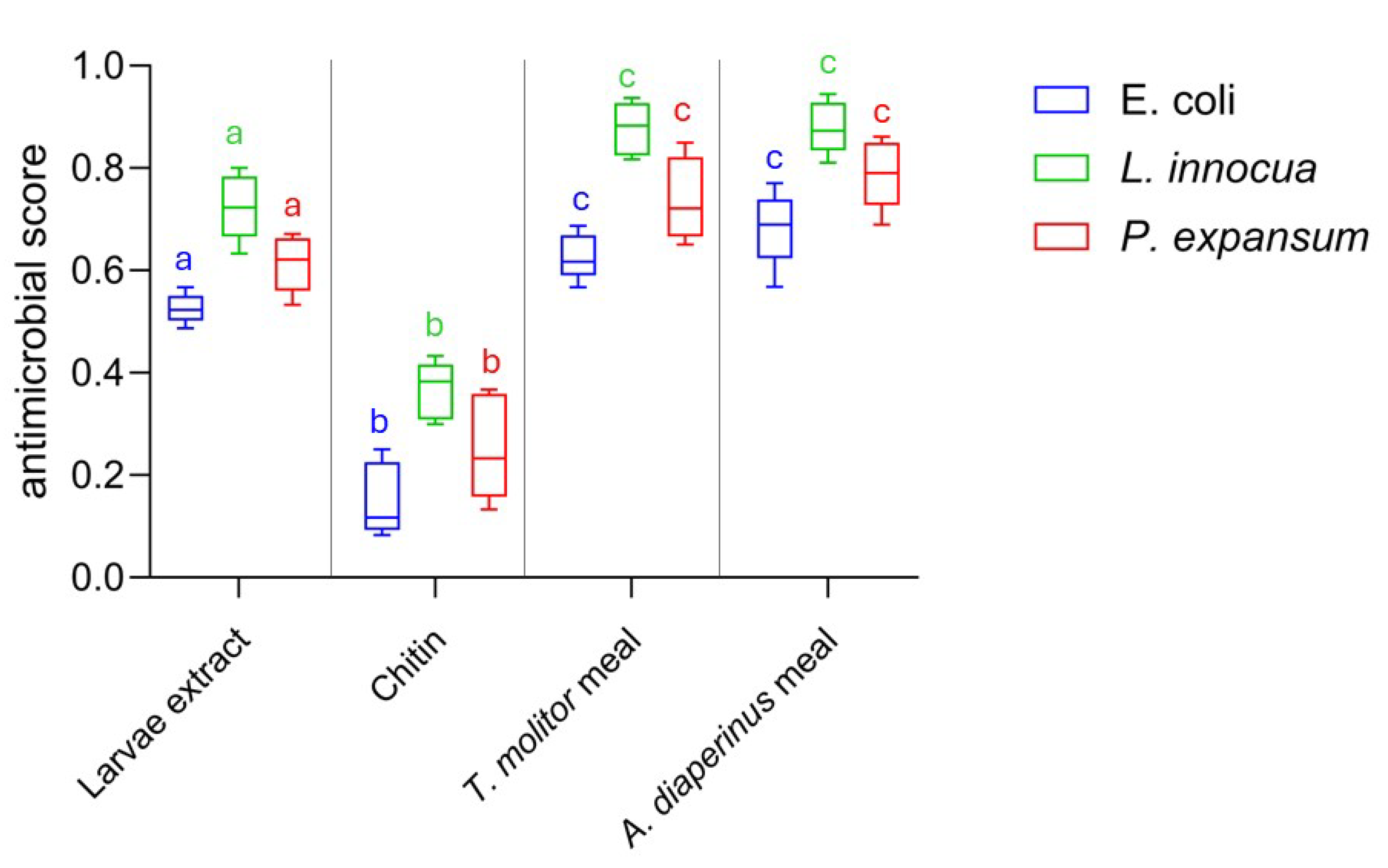
Both insect meals exhibited inhibiting activity, with MIC values of 3.12 mg/mL towards L. innocua ATCC 33090 and 6.25 mg/mL towards P. expansum ATCC 36200, while the highest MIC value of 12.5 mg/mL was observed towards E. coli ATCC 8739. Therefore, the meal showed an inhibitory capacity against target microorganisms when used at concentrations of between 0.6% and 1.5%. Considering that insect meals, as pointed out by several authors [57,58,59], can be used in food preparation at concentrations of 10% and even 50%, our results on antimicrobial efficacy are relevant for the biopreservation-conscious food industry. The remarkable antimicrobial activity of insect meals used as a raw material without any treatment is a novelty in scientific literature and in food industry research and development. To date, scientific literature includes numerous studies on the bioactive substances of edible insects and their antimicrobial potential [35,60]. However, the investigation of antimicrobial action has mainly focused on molecules extracted from edible insects, evaluating the efficacy of pure substances, their derivatives, or complex extracts [61,62,63]. Our results show that the antimicrobial action of insect meals is comparable or superior to that of insect complex extracts or pure insect compounds such as chitin. As can be seen from Figure 3, the antimicrobial effect expressed by the larvae meal of A. diaperinus and T. molitor in doses equal to MIC values is superior to both that produced by the protein extract of the larvae (p < 0.05) and that produced by pure chitin (p < 0.001) used in amounts compatible with MIC values [62,64,65].
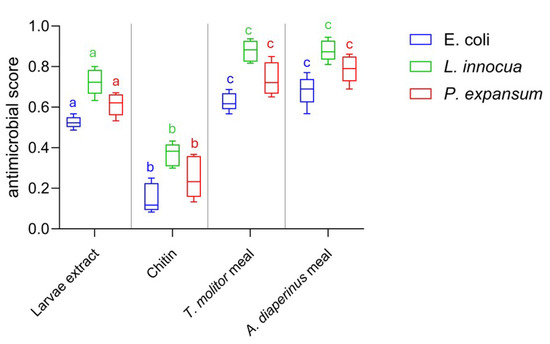
Figure 3.
Box-and-whisker plots showing the antimicrobial score produced by A. diaperinus meal, T. molitor meal, chitin, and larvae extract against Escherichia coli ATCC 8739 (blue), L. innocua ATCC 33090 (green), and P. expansum ATCC 36200 (red). Different letters with the same color indicate significance difference between microorganisms depending on different treatments (larvae extract, chitin, insect meals) based on the statistical ANOVA test.
Thus, the antimicrobial activity of insect larvae meals can be attributed to various bioactive molecules. Further studies will be necessary to identify and quantify the contribution of each bioactive substance. However, it can now be assumed that meals, without further treatment, can easily be used as an ingredient to counteract the development of specific microbial species, such as L. innocua, E. coli, or P. expansum, which are feared not only in high-moisture but also in low-moisture foods [66,67,68].
3.2. Use of Edible Insects as Antimicrobial Ingredients: Food Design
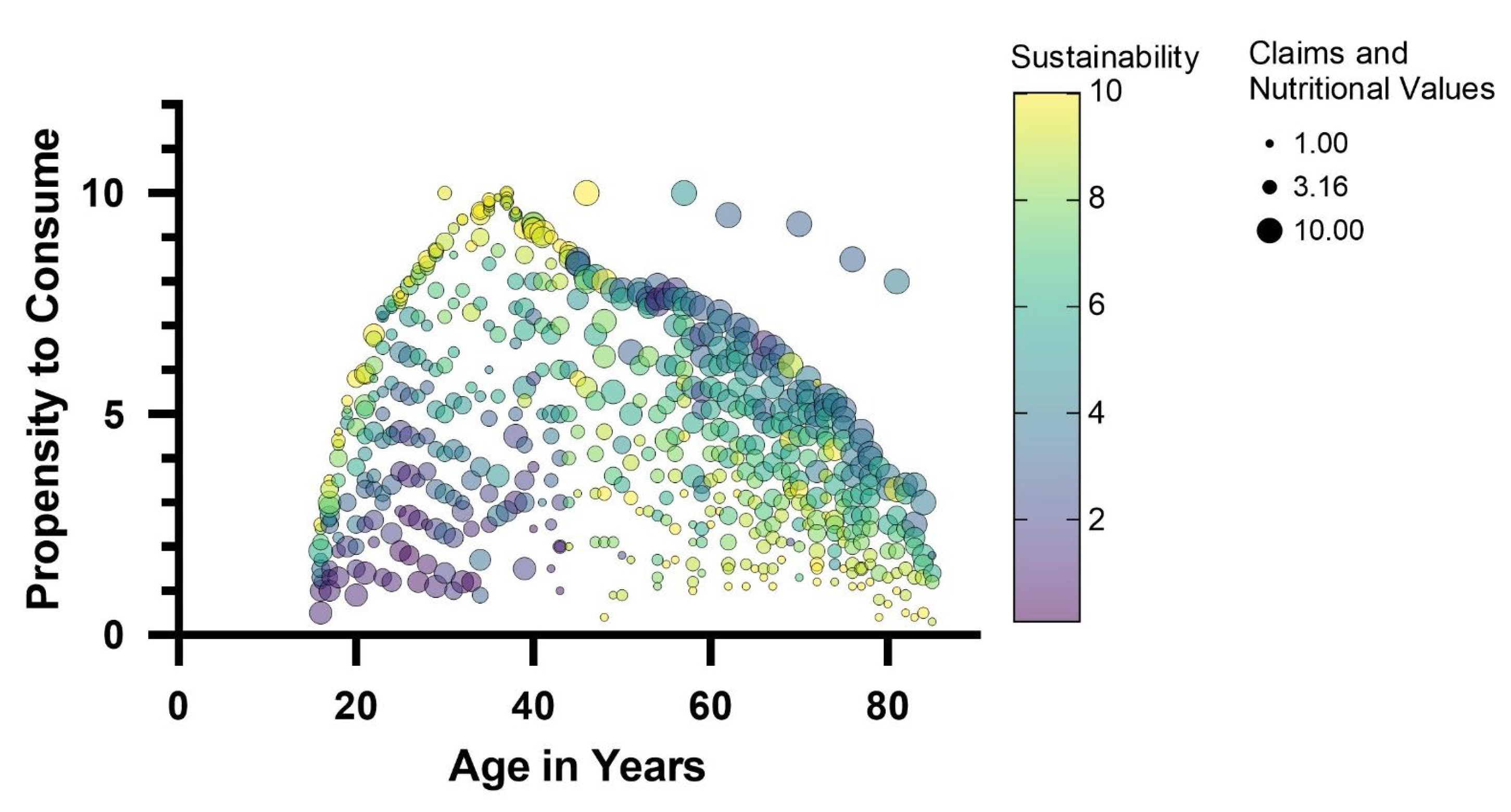
It is widely recognized that consumer opinion plays a crucial role in the design of novel foods [69,70]. Understanding consumer attitudes cannot possibly be neglected if the innovation intends to introduce much-discussed ingredients, such as edible insects [71]. For this reason, responses from a large sample of consumers (700 consumers evenly distributed over an age range of 16 to 85 years) were collected via a questionnaire designed to investigate familiarity with edible insects, propensity to purchase or consume insect products, and factors that might influence the acceptability of any insect-based food product. The population of respondents is representative of different age groups, diverse levels of education, and different places of residence (Table S1). Specifically, the interviewees are equally distributed between male and female genders and equally distributed according to the size of their cities of residence. On the contrary, the majority stated that they had a university degree in terms of qualification. The survey showed that most consumers interviewed were aware of the possibility of using insects in food preparations. In fact, out of the total number of respondents, only 7% did not know that insects, whole or as meal, could be used as a food ingredient. Different attitudes emerge regarding the propensity to consume insects. The analysis of the answers shows that place of residence and level of education are not factors influencing propensity to purchase. On the contrary, a relationship was found between age and propensity to purchase. The predictive model of the propensity to consume (Figure 4), based on multiple regression analysis, highlighted a relationship between age and propensity to consume.
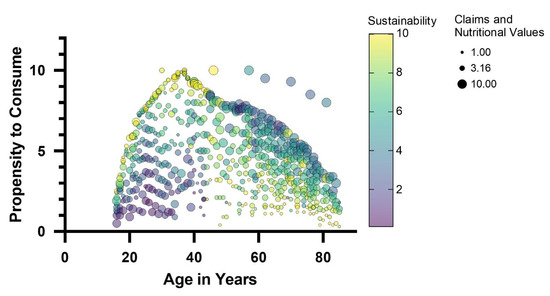
Figure 4.
Predictive model of the propensity to consume insect-based foods by multiple linear regression of several variables, including consumers age as well as sustainability and nutritional claims. The size of the bubbles is directly related to the expectation of nutritional claims; the color of the bubbles (purple, yellow) is related to the expectation of nutritional claims on a scale of 1 to 10.
A bell-shaped (Gaussian) curve with a smaller slope on the right side than on the left describes the relationship between the propensity to consume insect-based foods and the age of the interviewees. Thus, the youngest age groups, 16–18 years, and adults over 70 years declared a lower propensity to consume insect-based foods. The 35–40 age group contained the consumers who declared the highest propensity to consume these foods. These findings are in line with evidence found in other European regions, according to which older individuals show greater resistance to incorporating insects into their diet than younger ones [71,72]. The predictive model of the propensity to consume insect foods also revealed which factors most influence consumer attitudes: sustainability characteristics and nutritional claims are the factors that showed a relationship with propensity to consume, also as a function of age. Specifically, a positive regression between sustainability traits and propensity to purchase was found in the younger consumer groups (age < 45 years). In the age groups of over 45 years, sustainability traits were found to be indifferent without influencing the propensity to consume. On the contrary, in these age groups (over 45 years), nutritional claims regarding insect-based foods positively influenced the choice. Based on consumer attitudes and the predictive model of consumer propensity, an insect-based food model was defined for the validation of the antimicrobial action and acceptability of A. diaperinus and T. molitor. Considering that the bar-type snack is the preferred staple food with insects (Figure 5), a bar-type snack was developed to meet specific nutritional and sustainability claims.
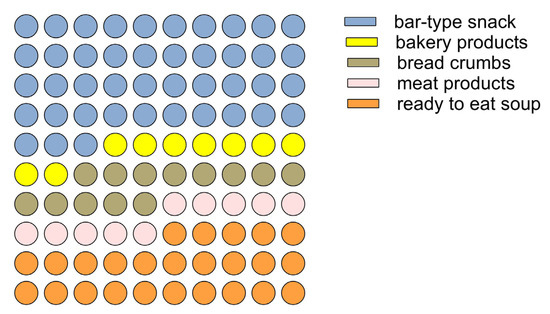
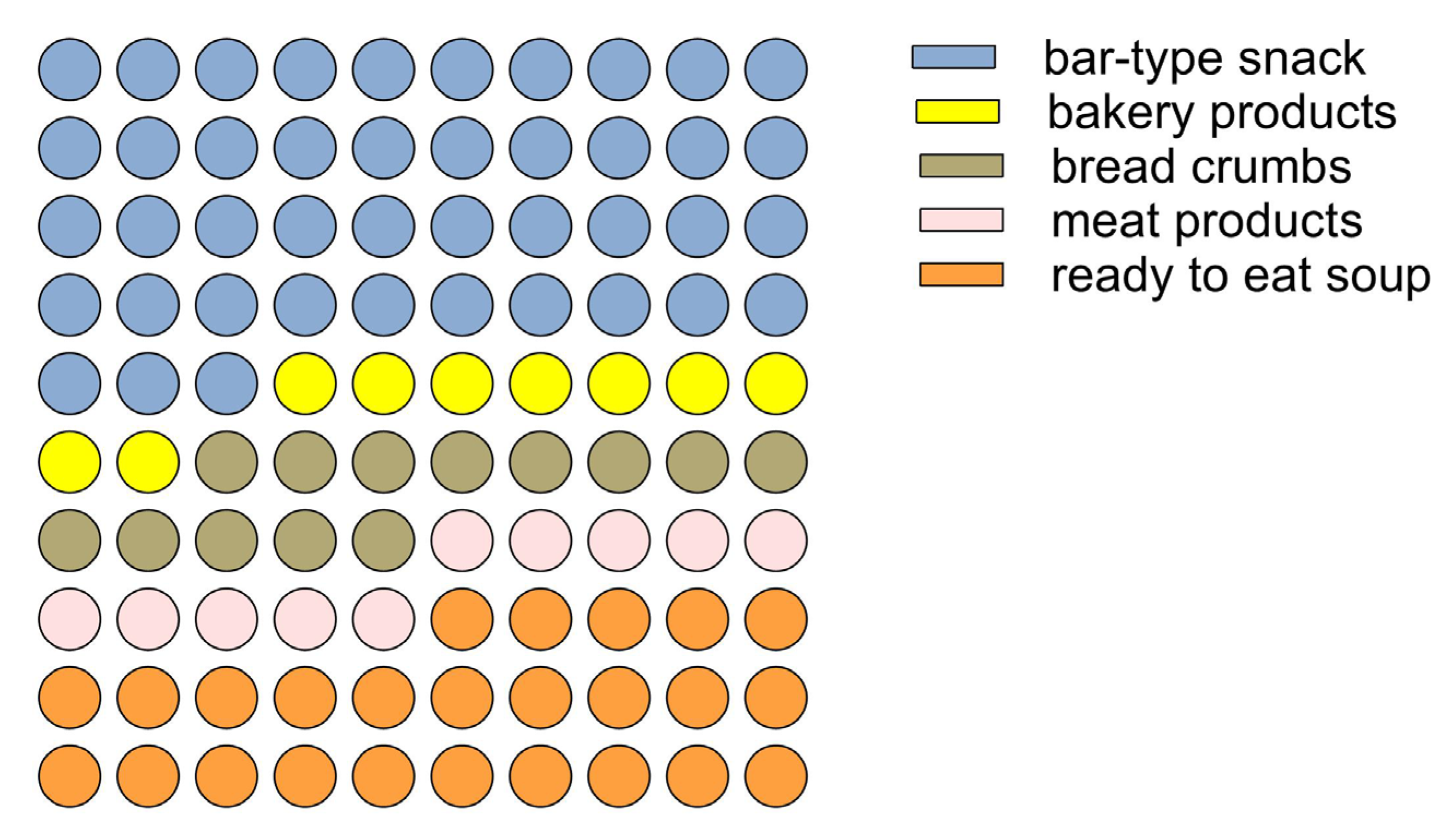
Figure 5.
Part of the whole graph shows the preference for insect-based food types.
To this end, ingredients were selected that not only satisfy the intake of specific nutrients but also meet the aspects of the circular economy by reusing by-products and food waste from different food supply chains. The environmental pressure of food industry waste and by-products and their possible reuse has been widely studied and is still a topic of cutting-edge research [73,74,75,76]. Based on these acquisitions, among the ingredients to be used in the formulation of bar-type snacks, waste from confectionery processing (marron glacé waste); milk-processing waste or by-products, such as whey [77]; as well as waste from carob processing, such as carob gum [14], were selected.
3.3. Insect-Based Bar-Type Snack: Chemical, Nutritional, Microbiological, and Sensorial Features
The choice of ingredients and their use have resulted in a product for which 50% of the raw materials used are by-products, waste, or co-products of other food processes. If the use of insects is added to this percentage, about 60% of the product is obtained from sustainable or low-impact sources. Sustainability claims, as also highlighted by a recent review [78], could remove some consumer distrust and increase the propensity to consume atypical foods, such as insect-based foods. Considering the data acquired through our survey, the sustainability claim would increase the propensity to consume, especially among younger age groups. Noteworthy are the benefits related to macronutrient composition provided by the special choice of raw materials combined with the use of insect meals (Table 2). The use of ingredients such as carob bean gum, shifted linseed meal, and agave syrup, together with insect larvae meal, allows the bar to meet the requirements of Regulation (EC) [79] to qualify as a “protein source” food. In addition, the use of insects provides a significant protein contribution that allows the bar to qualify as a “high-protein” food [79], as more than 20% of the energy value of the food is provided by protein.

Table 2.
Chemical composition of the conventional snack bar (control) and the snack bar with the insect meal (insect-based bar).
Achieving these characteristics in composition and, consequently, in nutritional claims makes it possible to meet the expectations of specific consumer groups, increasing their propensity to consume insect-based food products. The combination and concentration of the different ingredients also influenced the moisture content and the chemical–physical characteristics of the bars. The samples of the batch of bars prepared with the use of insects and those of the control batch without insects were characterized by the same moisture and water activity (aw) values: 30% and 0.776, respectively. These values are characteristic of intermediate-moisture food (IMF), a product category that is gaining more and more attention, as it has very similar characteristics to fresh food products, but with a longer shelf life [80]. However, the scientific literature in recent years has highlighted evidence and concerns regarding the microbiological safety of low-to-intermediate-moisture food products [81,82,83]. In addition, the pH of our samples is not a limiting factor for microbial survival or proliferation, being around 6.3. Samples from both batches immediately after packaging showed microbial-load levels of no concern: total mesophilic bacteria never exceeded 1000 CFU/g, yeast load levels were around 1000 CFU/g, and both Listeria spp. and E. coli were undetectable. No significant differences between the samples of the two batches (with or without insect meal) were found. Although microbiological-count levels are not of concern, several authors have pointed out that diverse food-borne illnesses are associated with low-moisture foods [81]. In the development of a new food, it is widely recognized that the assessment of sensory quality is crucial feedback to validate innovative solutions [84,85,86]. Sensory acceptance gains crucial importance in the case of the use of insects, which, despite being part of the diets of more than 100 countries in Asia, Africa, and South America, are still poorly accepted in the usual diet [57]. Novel foods based on the use of edible insects, if adapted to Western sensory preferences and food aesthetics, could be more appealing to consumers. Therefore, incorporation into bar-type snacks could bode well for better acceptance. As is shown in Figure 6, consumer acceptance was medium to high, with no significant differences compared to the control. Other authors [87] have pointed out that the practice of incorporating insects into familiar products without them being easily recognizable as insects can help overcome neophobia and increase acceptability. In terms of color, the use of insects also produced no significant differences (p > 0.05). This result can be attributed to the use of other ingredients, which, having a similar color to insect meal, helped camouflage the presence of insects. Parameters ranging from texture to flavor and aroma through stickiness could be influenced by using insects.
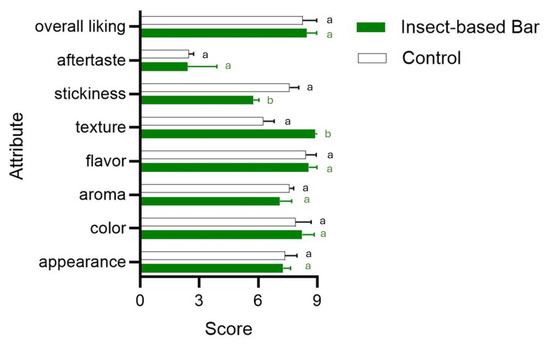
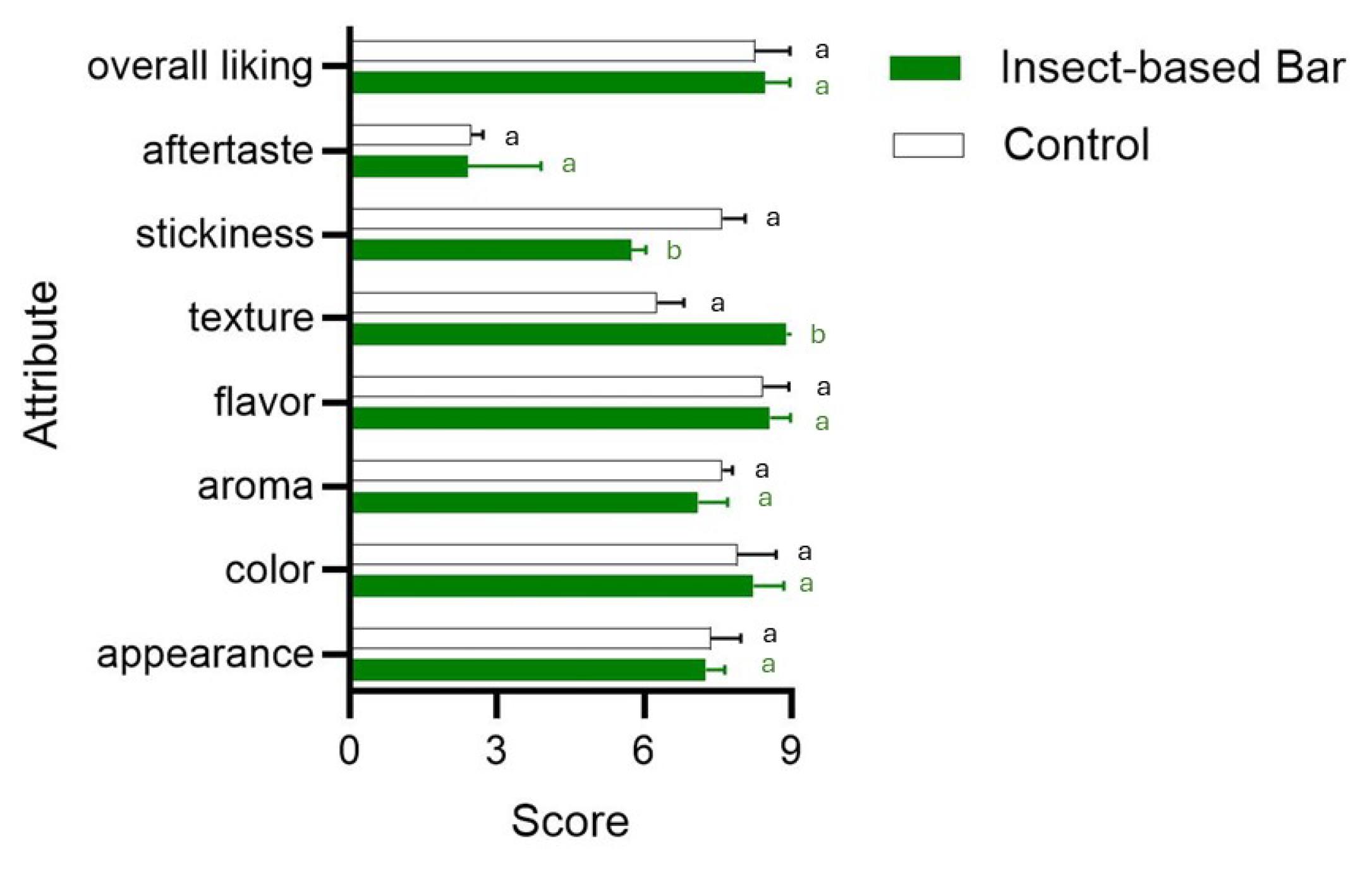
Figure 6.
Bar plot showing the acceptability level (9-point hedonic scale) of sensory attributes in samples from conventional (control) and insect-based snack bars. Different letters indicate significant differences in attributes between the batches. Statistical tests were carried out using a t-test.
Some authors [88,89] have shown that the use of up to 15 percent insect powder does not significantly affect the sensory quality of the products. In the present study, the insect larvae meal of the two insects, used to the extent of 16%, produced a positive effect on texture and stickiness. No significant differences from the control were found in aroma, flavor, or the sensory attribute “aftertaste”.
3.4. Antimicrobial Validation
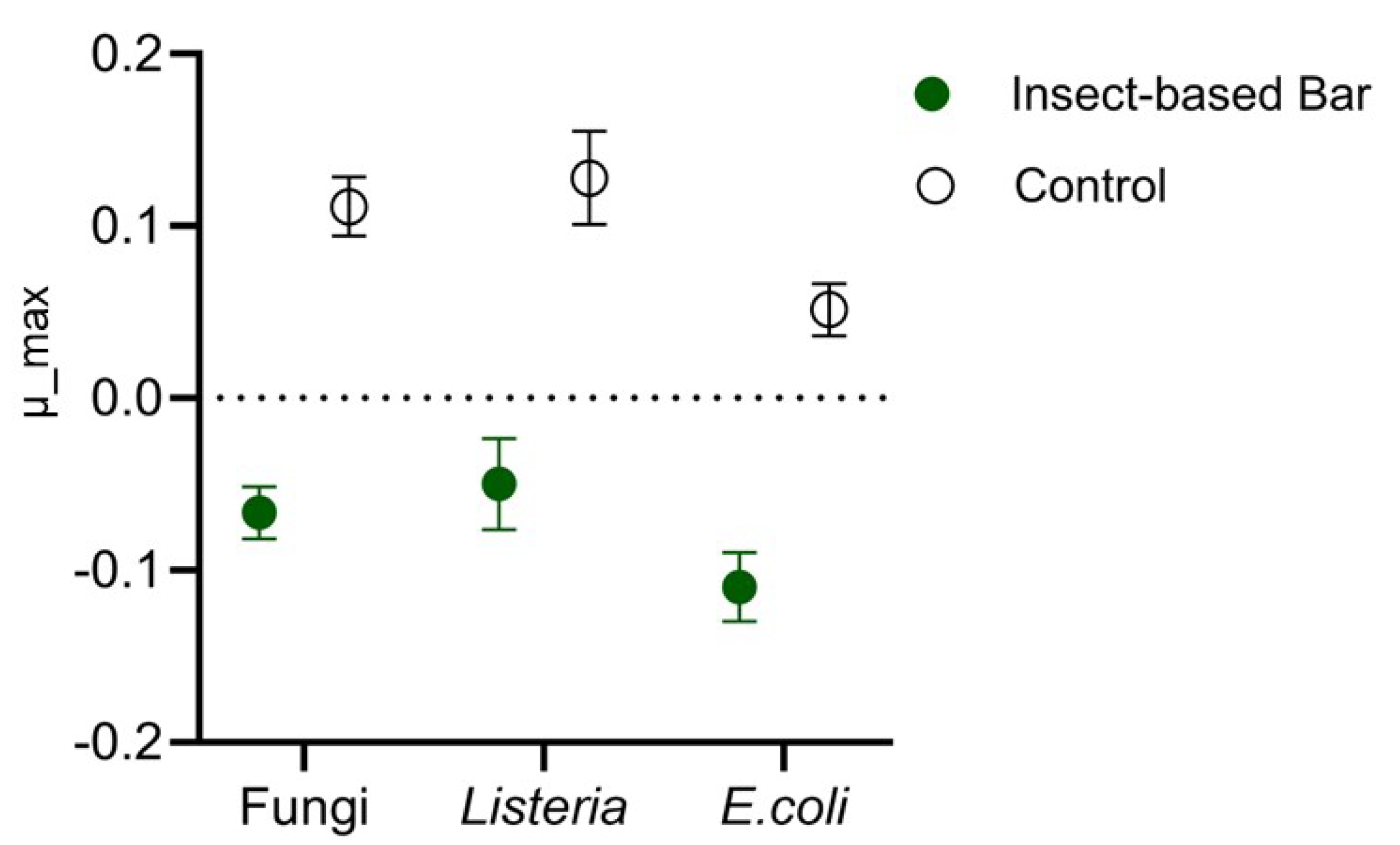
The effectiveness of anti-Listeria, anti-E. coli, and anti-Penicillium action was evaluated by performing a specific challenge test. The load trends of the three microorganisms in the insect-based bar intentionally inoculated with a multi-strain cocktail referring to the three species were compared with those found in control samples inculcated with the strain cocktail but without having been fortified with insect meal. Significant differences (p < 0.05) as a function of meal use were found by studying the kinetic parameters of the microbial populations. Indeed, as previously reported by other authors [90], the study and comparison of growth rate is a useful approach to evaluate the effect of specific treatments. In our study, the growth rate (Figure 7) for all microorganisms analyzed in the control samples was positive and showed an increase in loading over time. In contrast, negative growth-rate values were found for the microorganisms analyzed in the insect samples, indicating a decrease during the storage time.
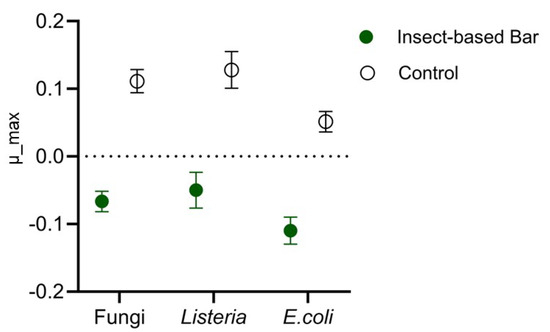
Figure 7.
Maximum growth rate (m_max) of fungi (presumably Penicillium), Listeria, and Escherichia in insect-based or conventional (control) snack bars intentionally inoculated with a pathogen surrogate microorganism cocktail.
Furthermore, significant differences (p < 0.05) were found between the growth or growth rates of the different microorganisms in both the conventional and the insect-based bars. In contrast to what was observed in vitro, Escherichia spp. was the most sensitive (p < 0.05) compared to both Listeria spp. and the presumed Penicillium spp. These findings could be due to the higher resistance of Listeria and Penicillium to environmental factors. Indeed, it is recognized that Listeria and fungi are the microorganisms most feared in products with low or intermediate moisture [81,91]. In contrast, in the model food, Escherichia, although less sensitive to insect meals in vitro than Listeria, was affected not only by the inhibiting effect of the meal but also by environmental factors, particularly moisture and aw. Overall, two fundamental aspects emerge from the results obtained: in products with intermediate moisture such as snack bars, possible pathogenic contaminants such as Listeria, Escherichia, and Penicillium can develop, and insect meals allow for an effective reduction in microorganisms. Based on these results, insect meals not only represent an important ingredient for nutritional claims but could also serve as a bio-preservative ingredient. Furthermore, these results fill critical knowledge gaps on the antimicrobial properties of insect meals and complement previous research, which has largely focused on compounds derived or extracted from insects. Although purified extracts such as chitin, chitosan, and antimicrobial peptides (AMPs) are recognized for their antimicrobial activity and proposed as potential food additives, extraction processes are often resource-intensive and have environmental impacts [35,36]. Insect meal, on the other hand, offers a more practical, sustainable, and effective alternative, as it maintains the nutritional properties of the whole organism and simplifies the production process. Therefore, insect meals as bio-preservatives represent an interesting innovation in the food industry. At the same time, however, they raise safety concerns and the need to establish a new regulatory framework, as they may pose a risk to allergy sufferers [92]. Since insect products are considered “novel food”, they require a preliminary scientific assessment by the EFSA. In this regard, it should be noted that the use of A. diaperinus and T. molitor meals in food preparations is properly authorized and regulated [19,20,23].
4. Conclusions
The findings of this study provide a valuable foundation for the use of insect meals as a multifunctional ingredient with both nutritional and bio-preservative properties. Notably, sensory analysis and consumer surveys highlighted the broad acceptance of insect meals, demonstrating their potential for incorporation into food products. In addition, Insect meals have demonstrated their antimicrobial efficacy against surrogate strains of L. monocytogenes, P. expansum and E. coli in intermediate moisture food models, contributing new evidence to the growing body of research on the control of microorganisms in low-moisture foods, a difficult area where conventional antimicrobial strategies often fail. In conclusion, this study improves the current knowledge on the antimicrobial properties of insect meals, demonstrating their viability as a natural and sustainable antimicrobial ingredient for food protection. By addressing both microbial safety and consumer expectations, the results align with ongoing efforts to develop innovative and sustainable solutions to agribusiness and food safety challenges. These findings further underscore the potential of insect meals as a practical alternative to conventional additives, offering the food industry new opportunities for innovation in line with nutritional, safety, and sustainability priorities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14040702/s1, Table S1. Interviewees of different age groups and distinguished by gender, educational qualification and place of residence; Table S2. Sensory analysis form; Document S1. Online Anonymous Survey Consent; Document S2. Online Anonymous Survey Consent—PARENTAL PERMISSION—Minor Assent (ages 16–<18 years); Document S3. Consent Sensory Evaluations.
Author Contributions
F.C.: formal analysis, data curation. S.J.L.: methodology, data curation, validation, original draft preparation. P.T.: conceptualization, writing—original draft preparation, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Agriculture, Environmental and Food Sciences at the University of Molise; Project funds “Development of bioprotective strategies for food quality and safety assurance”, CUP H34I19001230005.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the BioEthics Committee of the University of Molise (0005912-2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the Higher Education Institute Lombardo-Radice (Bojano—Vinchiaturo) for the practical support in the snack-bar preparation from the cooking teacher Luana Bernardette Marino.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bruckmeier, K.; d’Andria, D.; Wiemers, J. Universal, Targeted or Both: Effects of Different Child Support Policies on Labour Supply and Poverty: A Simulation Study. J. Context. Econ. 2022, 142, 159–206. [Google Scholar] [CrossRef]
- Reisman, E.; Fairbairn, M. Agri-food systems and the Anthropocene. In The Anthropocene; Routledge: Abingdon, UK, 2021; pp. 57–67. [Google Scholar]
- Shannon, D. Food Insecurity. In Inequality Around the World: Understanding the Rich-Poor Divide from America to Zimbabwe [2 volumes]; Bloomsbury Publishing: London, UK, 2025; p. 180. [Google Scholar]
- Sattler, M. Rethinking peripheral geographies of innovation: Towards an ordinary periphery approach. Eurasian Geogr. Econ. 2024, 1–27. [Google Scholar] [CrossRef]
- Iori, E.; Masotti, M.; Falasconi, L.; Risso, E.; Segrè, A.; Vittuari, M. Tell me what you waste and I’ll tell you who you are: An eight-country comparison of consumers’ food waste habits. Sustainability 2022, 15, 430. [Google Scholar] [CrossRef]
- Tremonte, P.; Succi, M.; Coppola, R.; Sorrentino, E.; Tipaldi, L.; Picariello, G.; Pannella, G.; Fraternali, F. Homology-based modeling of universal stress protein from Listeria innocua up-regulated under acid stress conditions. Front. Microbiol. 2016, 7, 1998. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Tremonte, P.; Mercurio, I.; Vergalito, F.; Caturano, C.; Maiuro, L.; Iorizzo, M.; Succi, M.; Sorrentino, E.; et al. Fungi occurrence in ready-to-eat hazelnuts (Corylus avellana) from different boreal hemisphere areas. Front. Microbiol. 2022, 13, 900876. [Google Scholar] [CrossRef]
- Colautti, A.; Orecchia, E.; Coppola, F.; Iacumin, L.; Comi, G. Cyberlindnera fabianii, an Uncommon Yeast Responsible for Gluten Bread Spoilage. Foods 2024, 13, 2381. [Google Scholar] [CrossRef]
- Comi, G.; Colautti, A.; Bernardi, C.E.M.; Stella, S.; Orecchia, E.; Coppola, F.; Iacumin, L. Leuconostoc gelidum Is the Major Species Responsible for the Spoilage of Cooked Sausage Packaged in a Modified Atmosphere, and Hop Extract Is the Best Inhibitor Tested. Microorganisms 2024, 12, 1175. [Google Scholar] [CrossRef]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Ibanez, E.; Mendiola, J.A.; Di Renzo, T.; Reale, A.; Coppola, R. Antimicrobial effect of Malpighia punicifolia and extension of water buffalo steak shelf-life. J. Food Sci. 2016, 81, M97–M105. [Google Scholar] [CrossRef]
- Ombra, M.N.; Cozzolino, A.; Nazzaro, F.; d’Acierno, A.; Tremonte, P.; Coppola, R.; Fratianni, F. Biochemical and biological characterization of two Brassicaceae after their commercial expiry date. Food Chem. 2017, 218, 335–340. [Google Scholar] [CrossRef]
- Tremonte, P.; Pannella, G.; Lombardi, S.J.; Iorizzo, M.; Vergalito, F.; Cozzolino, A.; Maiuro, L.; Succi, M.; Sorrentino, E.; Coppola, R. Low-fat and high-quality fermented sausages. Microorganisms 2020, 8, 1025. [Google Scholar] [CrossRef]
- Picariello, L.; Errichiello, F.; Coppola, F.; Rinaldi, A.; Moio, L.; Gambuti, A. Effect of chitosan addition on acetaldehyde and polymeric pigments production after oxidation of red wines with different tannin/anthocyanins ratio. Eur. Food Res. Technol. 2023, 249, 2447–2455. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Coppola, F.; Vergalito, F.; Maiuro, L.; Succi, M.; Tremonte, P.; Coppola, R. Plant-Based Ingredients Utilized as Fat Replacers and Natural Antimicrobial Agents in Beef Burgers. Foods 2024, 13, 3229. [Google Scholar] [CrossRef]
- Zengin, G.; Uba, A.I.; Abul, N.; Gulcin, I.; Koyuncu, I.; Yuksekdag, O.; Sathish Kumar, M.; Ponnaiya, S.K.M.; Tessappan, S.; Nazzaro Fratianni, F.; et al. A multifunctional natural treasure based on a “one stone, many birds” strategy for designing health-promoting applications: Tordylium apulum. Food Biosci. 2024, 62, 105088. [Google Scholar] [CrossRef]
- Brai, A.; Trivisani, C.I.; Vagaggini, C.; Stella, R.; Angeletti, R.; Iovenitti, G.; Francardi, V.; Dreassi, E. Proteins from Tenebrio molitor: An interesting functional ingredient and a source of ACE inhibitory peptides. Food Chem. 2022, 393, 133409. [Google Scholar] [CrossRef]
- Bresciani, A.; Cardone, G.; Jucker, C.; Savoldelli, S.; Marti, A. Technological performance of cricket powder (Acheta domesticus L.) in wheat-based formulations. Insects 2022, 13, 546. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU). 1975 of 12 November 2021 authorising the placing on the market of frozen, dried and powder forms of Locusta migratoria as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA relevance), C/2021/7987. Orkesterjournalen L 2021, 402, 10–16. [Google Scholar]
- Commission Implementing Regulation (EU). 882 of 1 June 2021 authorising the placing on the market of dried Tenebrio molitor larva as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA relevance), C/2021/3744. Orkesterjournalen L 2021, 194, 16–20. [Google Scholar]
- Commission Implementing Regulation (EU). 169 of 8 February 2022 authorising the placing on the market of frozen, dried and powder forms of yellow mealworm (Tenebrio molitor larva) as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA relevance), C/2022/658. Orkesterjournalen L 2022, 28, 10–16. [Google Scholar]
- Commission Implementing Regulation (EU). 188 of 10 February 2022 authorising the placing on the market of frozen, dried and powder forms of Acheta domesticus as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA relevance), C/2022/695. Orkesterjournalen L 2022, 30, 108–113. [Google Scholar]
- Commission Implementing Regulation (EU). 5 of 3 January 2023 authorising the placing on the market of Acheta domesticus (house cricket) partially defatted powder as a novel food and amending Implementing Regulation (EU) 2017/2470 (Text with EEA relevance), C/2023/6. Orkesterjournalen L 2023, 2, 9–14. [Google Scholar]
- Commission Implementing Regulation (EU). 58 of 5 January 2023 authorising the placing on the market of the frozen, paste, dried and powder forms of Alphitobius diaperinus larvae (lesser mealworm) as a novel food and amending Implementing Regulation (EU) 2017/2470 (Text with EEA relevance), C/2023/20. Orkesterjournalen L 2023, 5, 10–15. [Google Scholar]
- Jucker, C.; Belluco, S.; Oddon, S.B.; Ricci, A.; Bonizzi, L.; Lupi, D.; Savoldelli, S.; Biasato, I.; Caimi, C.; Mascaretti, A.; et al. Impact of some local organic by-products on Acheta domesticus growth and meal production. J. Insects Food Feed 2022, 8, 631–640. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic agricultural waste valorization to obtain valuable products: An overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Guiné, R.P.; Florença, S.G.; Costa, C.A.; Correia, P.M.; Cruz-Lopes, L.; Esteves, B.; Ferreira, M.; Fragata, A.; Cardoso, A.P.; Campos, S.; et al. Edible Insects: Consumption, Perceptions, Culture and Tradition Among Adult Citizens from 14 Countries. Foods 2024, 13, 3408. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Lopresto, C.G.; Casella, P.; Errico, S. Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems. Nutraceuticals 2023, 3, 451–480. [Google Scholar] [CrossRef]
- Boman, H.G.; Nilsson-Faye, I.; Paul, K.; Rasmuson, T., Jr. Insect immunity I. Characteristics of an inducible cell-free antibacterial reaction in hemolymph of Samia cynthia pupae. Infect. Immun. 1974, 10, 136–145. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.P.; Jia, S.R.; Chu, L.Q.; Xie, Y.Y.; Li, Z.X.; Zhong, C. Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: Current state and perspectives. Appl. Microbiol. Bbiot. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Razo, A.N.D.; Herrera, R.R.; Lalanne, G.M.; Campos, M.R.S. Insect-derived antimicrobial peptides and their potential use as antibiotic and foodborne preservative. In Improving Health and Nutrition Through Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2025; pp. 253–265. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Leon, M.J.; Montserrat-de la Paz, S. Potential applications of antimicrobial peptides from edible insects in the food supply chain: Uses in agriculture, packaging, and human nutrition. Food Biosci. 2024, 62, 105396. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]
- Saadoun, J.H.; Sogari, G.; Bernini, V.; Camorali, C.; Rossi, F.; Neviani, E.; Lazzi, C. A critical review of intrinsic and extrinsic antimicrobial properties of insects. Trends Food Sci. Technol. 2022, 122, 40–48. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, G.; Zhu, L.; Ma, L.; Chen, K. Insect Antimicrobial Peptides as Guardians of Immunity and Beyond: A Review. Int. J. Mol. Sci. 2024, 25, 3835. [Google Scholar] [CrossRef]
- Sheng, Z.; Guo, A.; Wang, J.; Chen, X. Preparation, physicochemical properties and antimicrobial activity of chitosan from fly pupae. Heliyon 2022, 8, e11168. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef]
- Zarzosa, G.I.G.; Kobayashi, T. Insect Resources for Chitin Biomass. In Building a Low-Carbon Society Through Applied Environmental Materials Science; IGI Global: Hershey, PA, USA, 2025; pp. 241–268. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]
- Patyra, E.; Kwiatek, K. Insect meals and insect antimicrobial peptides as an alternative for antibiotics and growth promoters in livestock production. Pathogens 2023, 12, 854. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Pucciarelli, V.; Borrelli, L.; Addeo, N.F.; Bovera, F.; Laginestra, A.; Schmitt, E.; Falabella, P. Antimicrobial activity of lipids extracted from Hermetia illucens reared on different substrates. Appl. Microbiol. Biotechnol. 2024, 108, 167. [Google Scholar] [CrossRef]
- Muñoz-Seijas, N.; Fernandes, H.; Fernández, B.; Domínguez, J.M.; Salgado, J.M. Eco-friendly technologies for obtaining antioxidant compounds and protein hydrolysates from edible insect Tenebrio molitor beetles. Food Chem. 2025, 464, 141726. [Google Scholar] [CrossRef]
- Elahi, U.; Xu, C.C.; Wang, J.; Lin, J.; Wu, S.G.; Zhang, H.J.; Qi, G.H. Insect meal as a feed ingredient for poultry. Anim. Biosci. 2022, 35, 332. [Google Scholar] [CrossRef]
- Willora, F.P.; Farris, N.W.; Ghebre, E.; Zatti, K.; Bisa, S.; Kiron, V.; Verlhac-Trichet, V.; Danielsen, M.; Dalsgaard, T.K.; Sørensen, M. Full-fat black soldier fly larvae meal and yellow mealworm meal: Impact on feed protein quality, growth and nutrient utilization of Atlantic salmon (Salmo salar) post smolts. Aquaculture 2025, 595, 741648. [Google Scholar] [CrossRef]
- Rovai, D.; Michniuk, E.; Roseman, E.; Amin, S.; Lesniauskas, R.; Wilke, K.; Garza, J.; Lammert, A. Insects as a sustainable food ingredient: Identifying and classifying early adopters of edible insects based on eating behavior, familiarity, and hesitation. J. Sens. Stud. 2021, 36, e12681. [Google Scholar] [CrossRef]
- Hopkins, I.; Farahnaky, A.; Gill, H.; Newman, L.P.; Danaher, J. Australians’ experience, barriers and willingness towards consuming edible insects as an emerging protein source. Appetite 2022, 169, 105832. [Google Scholar] [CrossRef] [PubMed]
- Dagevos, H.; Taufik, D. Eating full circle: Exploring consumers’ sympathy for circularity in entomophagy acceptance. Food Qual. Prefer. 2023, 105, 104760. [Google Scholar] [CrossRef]
- Ranga, L.; Noci, F.; Dermiki, M. Insect-Based Foods: A Preliminary Qualitative Study Exploring Factors Affecting Acceptance and New Product Development Ideas through Focus Groups. Challenges 2024, 15, 40. [Google Scholar] [CrossRef]
- Gędas, A.; Schmidt, H.; Weiss, A. Identification and evaluation of Escherichia coli strain ATCC 8739 as a surrogate for thermal inactivation of enterohemorrhagic Escherichia coli in fruit nectars: Impact of applied techniques on the decimal reduction time. Food Microbiol. 2024, 122, 104544. [Google Scholar] [CrossRef]
- Sorrentino, E.; Tremonte, P.; Succi, M.; Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Sturchio, M.; Coppola, R. Detection of antilisterial activity of 3-phenyllactic acid using Listeria innocua as a model. Front. Microbiol. 2018, 9, 1373. [Google Scholar] [CrossRef]
- Tremonte, P.; Succi, M.; Reale, A.; Di Renzo, T.; Sorrentino, E.; Coppola, R. Interactions between strains of Staphylococcus xylosus and Kocuria varians isolated from fermented meats. J. Appl. Microbiol. 2007, 103, 743–751. [Google Scholar] [CrossRef]
- Tremonte, P.; Pannella, G.; Succi, M.; Tipaldi, L.; Sturchio, M.; Coppola, R.; Sorrentino, E. Antimicrobial activity of Lactobacillus plantarum strains isolated from different environments: A preliminary study. Int. Food Res. J. 2017, 24, 852–859. [Google Scholar]
- Eucast, D. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 22nd ed.; Online; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- Mishyna, M.; Chen, J.; Benjamin, O. Sensory attributes of edible insects and insect-based foods–Future outlooks for enhancing consumer appeal. Trends Food Sci. Technol. 2020, 95, 141–148. [Google Scholar] [CrossRef]
- Carpentieri, S.; Orkusz, A.; Ferrari, G.; Harasym, J. Effect of replacing durum wheat semolina with Tenebrio molitor larvae powder on the techno-functional properties of the binary blends. Curr. Res. Food Sci. 2024, 8, 100672. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Wu, Y.S.; Vijeepallam, K.; Batumalaie, K.; Hatta, M.M.; Lutuf, H.; Castro-Munoz, R.; Ibrahim, S.A. Alphitobius diaperinus larvae (lesser mealworm) as human food–An approval of the European Commission–A critical review. J. Insects Food Feed. 2024, 1, 1–40. [Google Scholar] [CrossRef]
- De la Luz Sánchez-Estrada, M.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A. Bioactive compounds and biological activity in edible insects: A review. Heliyon 2024, 10, e24045. [Google Scholar] [CrossRef]
- De Sousa Araujo, A.C.; Pereira, A.C.; Gomes, R.M.M.; Ramirez, J.R.B.; da Silva Noda, K.; Santos, L.G.; Latorres, J.M.; Ramos, D.F.; Monserrat, J.M.; Martins, V.G. Protein hydrolysates derived from superworm (Zophobas morio): Composition, bioactivity, and techno-functional properties. Int. J. Biol. Macromol. 2025, 295, 139668. [Google Scholar] [CrossRef]
- Tafi, E. Chitosan and Chitin Sources and Their Isolation. In Chitin and Chitosan; Jenny Stanford Publishing: Singapore, 2025; pp. 33–62. [Google Scholar]
- Yang, S.; Li, X.; Li, Q. Deep Eutectic Solvent extraction and biological activity of polysaccharides from Tenebrio molitor. Heliyon 2025, 11, e41790. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, M.W.; Moon, C.; Seo, D.J.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.K.; Kim, S.A.; Kim, Y.W.; et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Navarro, P.; Ribeiro, J.C.; Luís, A.; Domingues, F.; Anjos, O.; Cunha, L.M. Insect-based chitin and chitosan from whole body sources and rearing by-products: Extraction, physicochemical, structural and bioactivity characterisation. J. Insects Food Feed. 2025, 1, 1–19. [Google Scholar] [CrossRef]
- Taylor, M.H.; Zhu, M.J. Control of Listeria monocytogenes in low-moisture foods. Trends Food Sci. Technol. 2021, 116, 802–814. [Google Scholar] [CrossRef]
- Bento de Carvalho, T.; Silva, B.N.; Tomé, E.; Teixeira, P. Preventing fungal spoilage from raw materials to final product: Innovative preservation techniques for fruit fillings. Foods 2024, 13, 2669. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, S.J.; Jeong, H.J.; Lee, J.H. Development of a novel serogrouping method for the rapid detection of 21 Escherichia coli O-serotypes using multiplex real-time PCR. Food Contr. 2024, 157, 110171. [Google Scholar] [CrossRef]
- Siegrist, M.; Hartmann, C. Consumer acceptance of novel food technologies. Nat. Food 2020, 1, 343–350. [Google Scholar] [CrossRef]
- Galanakis, C.M. The future of food. Foods 2024, 13, 506. [Google Scholar] [CrossRef]
- Molina-Castillo, S.; Espinoza-Ortega, A.; Sánchez-Vega, L. Perception of non-conventional food consumption: The case of insects. Br. Food J. 2025, 127, 1013–1028. [Google Scholar] [CrossRef]
- Castro-Alija, M.J.; Zolfaghari, G.; Fernandez, C.G.; Álvarez, C.; Ramón-Carreira, L.C.; Jiménez, J.M.; Albertos, I. Elderly Resistance vs. Youthful Acceptance: A Study on Insect Consumption across Age Groups. Foods 2024, 13, 2641. [Google Scholar] [CrossRef]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob seeds: Food waste or source of bioactive compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef]
- Poništ, J.; Dubšíková, V.; Schwarz, M.; Samešová, D. Methods of processing whey waste from dairies. A review. Environ. Prot. Eng. 2021, 47, 67–84. [Google Scholar] [CrossRef]
- Di Giorgio, L.; Marcantonio, M.A.; Salgado, P.R.; Mauri, A.N. Isolation, characterization, and industrial processing of soybean proteins. In Handbook of Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 557–575. [Google Scholar] [CrossRef]
- Ospina-Maldonado, S.; Martin-Gómez, H.; Cardoso-Ugarte, G.A. From waste to wellness: A review on the harness of food industry by-products for sustainable functional food production. Int. J. Food Sci. Technol. 2024, 59, 8680–8692. [Google Scholar] [CrossRef]
- Iosca, G.; Turetta, M.; De Vero, L.; Bang-Berthelsen, C.H.; Gullo, M.; Pulvirenti, A. Valorization of wheat bread waste and cheese whey through cultivation of lactic acid bacteria for bio-preservation of bakery products. Lwt 2023, 176, 114524. [Google Scholar] [CrossRef]
- Laureati, M.; De Boni, A.; Saba, A.; Lamy, E.; Minervini, F.; Delgado, A.M.; Sinesio, F. Determinants of Consumers’ Acceptance and Adoption of Novel Food in View of More Resilient and Sustainable Food Systems in the EU: A Systematic Literature Review. Foods 2024, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- Regulation, E.C. No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2007, 12, 3–18. [Google Scholar]
- Qiu, L.; Zhang, M.; Tang, J.; Adhikari, B.; Cao, P. Innovative technologies for producing and preserving intermediate moisture foods: A review. Food Res. Int. 2019, 116, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.J.; Quinn, A.R.; Kataoka, A. Listeria monocytogenes in low-moisture foods and ingredients. Food Control 2019, 103, 153–160. [Google Scholar] [CrossRef]
- Coppola, F.; Picariello, L.; Forino, M.; Moio, L.; Gambuti, A. Comparison of three accelerated oxidation tests applied to red wines with different chemical composition. Molecules 2021, 26, 815. [Google Scholar] [CrossRef]
- Aydın, A.; Yüceer, M.; Ulugergerli, E.U.; Caner, C. Improving food security as disaster relief using intermediate moisture foods and active packaging technologies. Appl. Food Res. 2024, 4, 100378. [Google Scholar] [CrossRef]
- Karuppuchamy, V.; Heldman, D.R.; Snyder, A.B. A review of food safety in low-moisture foods with current and potential dry-cleaning methods. J. Food Sci. 2024, 89, 793–810. [Google Scholar] [CrossRef]
- Huey, S.L.; Bhargava, A.; Friesen, V.M.; Konieczynski, E.M.; Krisher, J.T.; Mbuya, M.N.; Mehta, N.H.; Monterrosa, E.; Nyangaresi, A.M.; Mehta, S. Sensory acceptability of biofortified foods and food products: A systematic review. Nutr. Rev. 2024, 82, 892–912. [Google Scholar] [CrossRef]
- Awaeloh, N.; Limsuwan, S.; Na-Phatthalung, P.; Kaewmanee, T.; Chusri, S. Novel Development and Sensory Evaluation of Extruded Snacks from Unripe Banana (Musa ABB cv. Kluai ‘Namwa’) and Rice Flour Enriched with Antioxidant-Rich Curcuma longa Microcapsules. Foods 2025, 14, 205. [Google Scholar] [CrossRef]
- Wilkinson, K.; Muhlhausler, B.; Motley, C.; Crump, A.; Bray, H.; Ankeny, R. Australian consumers’ awareness and acceptance of insects as food. Insects 2018, 9, 44. [Google Scholar] [CrossRef]
- Niaba, K.P.V.; Gildas, G.K.; Avit, B.; Thierry, A.; Anglade, M.K.; Dago, G. Nutritional and sensory qualities of wheat biscuits fortified with deffatted Macrotermes subhyalinus. Int. J. Chem. Sci. Technol. 2013, 3, 25–32. [Google Scholar]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foglini, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. A focus on the death kinetics in predictive microbiology: Benefits and limits of the most important models and some tools dealing with their application in foods. Foods 2015, 4, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Bigi, F.; Maurizzi, E.; Quartieri, A.; De Leo, R.; Gullo, M.; Pulvirenti, A. Non-thermal techniques and the “hurdle” approach: How is food technology evolving? Trends Food Sci. Technol. 2023, 132, 11–39. [Google Scholar] [CrossRef]
- Yue, S.R.; Verhoeckx, K.C.; Houben, G.F.; Bøgh, K.L. Analysis of trends and allergenicity risk assessments in novel food approvals within the European Union between 2018 and 2023. Food Chem. Toxicol. 2025, 197, 115249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).