The Concentrations of Fatty Acids, Cholesterol and Vitamin E in Cooked Longissimus, Semitendinosus, Psoas Major and Supraspinatus Muscles from Cattle Offered Grass Only, Concentrates Ad Libitum or Grass Silage Supplemented with Concentrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Sample Collection
2.2. Cooking Protocol
2.3. Chemical Analysis: Tissue
2.3.1. Moisture and Protein
2.3.2. Fatty Acids
2.3.3. Cholesterol and Vitamin E
2.3.4. Vitamin A and Beta-Carotene
2.4. Chemical Analysis: Feeds
2.5. Calculations and Statistical Analysis
3. Results
3.1. Feed Composition (Table 1)
3.2. Animal Production (Table 2)
3.3. General Chemical Composition (Table 3)
| Production System (PS) | |||||||
|---|---|---|---|---|---|---|---|
| Muscle 1 | CONC | GSS | GSL | Grass | Sed 2 | Significance | |
| Moisture (g/kg) | SUP | 638 a | 657 b | 644 a | 637 a | 6.0 | ** |
| ST | 619 a | 635 b | 634 b | 637 b | 7.2 | 0.06 | |
| LM | 631 | 628 | 623 | 614 | 8.8 | NS | |
| PM | 620 | 635 | 631 | 631 | 8.0 | NS | |
| Protein (g/kg) | SUP | 296 b | 282 a | 294 ab | 302 b | 6.6 | * |
| ST | 302 | 300 | 300 | 300 | 7.7 | NS | |
| LM | 290 | 298 | 303 | 291 | 8.9 | NS | |
| PM | 283 | 274 | 271 | 272 | 7.8 | NS | |
| Lipid (g/kg) | SUP | 45 ab | 48 b | 38 a | 41 a | 3.3 | * |
| ST | 54 b | 37 a | 40 a | 35 a | 5.4 | ** | |
| LM | 61 b | 49 a | 43 a | 44 a | 4.8 | ** | |
| PM | 85 b | 56 a | 57 a | 51 a | 5.8 | *** | |
| Energy (KJ/100 g) | SUP | 705 | 684 | 682 | 699 | 14.4 | NS |
| ST | 757 b | 696 a | 703 a | 687 a | 19.3 | ** | |
| LM | 750 | 732 | 727 | 745 | 18.7 | NS | |
| PM | 822 b | 734 a | 740 a | 730 a | 17.3 | *** | |
| Cholesterol (mg/g) | SUP | 0.29 a | 0.65 b | 0.73 bc | 0.78 c | 0.040 | *** |
| ST | 0.39 b | 0.31 a | 0.29 a | 0.30 a | 0.018 | *** | |
| LM | 0.66 bc | 0.58 a | 0.61 ab | 0.69 c | 0.038 | * | |
| PM | 0.57 a | 0.66 b | 0.74 c | 0.57 a | 0.035 | *** | |
| Vitamin E (mg/kg) | SUP | 2.44 a | 6.14 b | 5.88 b | 6.47 b | 0.640 | *** |
| ST | 1.17 a | 2.71 b | 3.18 bc | 3.50 c | 0.337 | *** | |
| LM | 1.35 a | 3.38 b | 3.25 b | 3.67 b | 0.301 | *** | |
| PM | 1.99 a | 5.83 bc | 5.17 b | 7.02 c | 0.800 | *** | |
3.4. Categories of Fatty Acids and Selected Nutritional Indices (Table 4)
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Muscle 3 | CONC | GSS | GSL | Grass | Sed 4 | Significance | |
| SFA | SUP | 1848 bc | 2092 c | 1483 a | 1583 ab | 151.2 | *** |
| ST | 2157 b | 1697 ab | 1769 ab | 1505 a | 243.0 | 0.07 | |
| LM | 2495 b | 2194 ab | 1808 a | 1823 a | 209.8 | ** | |
| PM | 3731 b | 2612 a | 2515 a | 2269 a | 272.6 | *** | |
| MUFA | SUP | 2218 b | 2149 b | 1773 a | 1912 ab | 166.2 | * |
| ST | 2858 b | 1759 a | 1901 a | 1695 a | 276.6 | *** | |
| LM | 2990 b | 2376 a | 2202 a | 2245 a | 244.4 | ** | |
| PM | 4064 b | 2464 a | 2653 a | 2308 a | 274.2 | *** | |
| PUFA | SUP | 284 a | 321 b | 282 a | 322 b | 17.1 | * |
| ST | 310 b | 214 a | 235 a | 240 a | 20.0 | *** | |
| LM | 327 b | 228 a | 217 a | 259 a | 21.5 | *** | |
| PM | 399 b | 324 a | 325 a | 330 a | 24.1 | ** | |
| n-3 PUFA | SUP | 21 a | 88 b | 90 b | 117 c | 6.5 | *** |
| ST | 47 a | 52 a | 70 b | 112 c | 7.0 | *** | |
| LM | 67 b | 45 a | 75 b | 107 c | 7.6 | *** | |
| PM | 60 a | 115 b | 106 b | 127 b | 11.0 | *** | |
| n-6 PUFA | SUP | 209 b | 151 a | 157 a | 152 a | 7.7 | *** |
| ST | 176 c | 123 b | 112 b | 97 a | 5.6 | *** | |
| LM | 176 c | 116 b | 107 ab | 100 a | 7.3 | *** | |
| PM | 287 c | 174 b | 178 b | 154 a | 9.7 | *** | |
| Trans FA | SUP | 70 a | 200 bc | 156 b | 235 c | 25.3 | *** |
| ST | 103 | 84 | 130 | 159 | 32.9 | NS | |
| LM | 152 | 123 | 113 | 168 | 24.6 | NS | |
| PM | 266 ab | 193 a | 203 ab | 272 b | 37.2 | 0.08 | |
| PUFA:SFA | SUP | 0.16 a | 0.16 a | 0.20 b | 0.21 b | 0.009 | *** |
| ST | 0.15 ab | 0.13 a | 0.14 a | 0.17 b | 0.012 | * | |
| LM | 0.14 b | 0.10 a | 0.13 b | 0.14 b | 0.009 | *** | |
| PM | 0.11 a | 0.13 b | 0.13 b | 0.15 c | 0.008 | *** | |
| n-6:n-3 PUFA | SUP | 11.5 a | 1.70 ab | 1.71 a | 1.33 a | 1.048 | *** |
| ST | 4.14 d | 2.45 c | 1.67 b | 0.89 a | 0.304 | *** | |
| LM | 2.71 c | 2.83 c | 1.47 b | 1.00 a | 0.187 | *** | |
| PM | 5.81 b | 1.53 a | 1.88 a | 1.26 a | 0.449 | *** | |
| AI | SUP | 0.62 b | 0.68 c | 0.52 a | 0.51 a | 0.020 | *** |
| ST | 0.65 a | 0.76 c | 0.71 b | 0.61 a | 0.024 | *** | |
| LM | 0.66 b | 0.75 c | 0.62 a | 0.60 a | 0.019 | *** | |
| PM | 0.72 b | 0.76 c | 0.65 a | 0.64 a | 0.019 | *** | |
| TI | SUP | 1.39 c | 1.33 c | 1.09 b | 1.03 a | 0.032 | *** |
| ST | 1.23 b | 1.41 c | 1.33 c | 1.06 a | 0.048 | *** | |
| LM | 1.30 c | 1.51 d | 1.20 b | 1.11 a | 0.030 | *** | |
| PM | 1.51 d | 1.43 c | 1.35 b | 1.27 a | 0.034 | *** | |
| HH | SUP | 1.83 b | 1.61 a | 2.02 c | 2.05 c | 0.057 | *** |
| ST | 1.68 c | 1.45 a | 1.56 b | 1.75 c | 0.049 | *** | |
| LM | 1.65 b | 1.45 a | 1.70 bc | 1.76 c | 0.044 | *** | |
| PM | 1.55 b | 1.46 a | 1.67 c | 1.70 c | 0.040 | *** | |
3.5. Individual Saturated Fatty Acids (Table 5)
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Muscle 1 | CONC | GSS | GSL | Grass | Sed 2 | Significance | |
| C14:0 | SUP | 113 b | 131 b | 68 a | 77 a | 10.6 | ** |
| ST | 159 b | 121 a | 111 a | 86 a | 18.5 | ** | |
| LM | 164 b | 143 b | 100 a | 103 a | 13.9 | *** | |
| PM | 262 c | 176 b | 141 ab | 129 a | 19.6 | *** | |
| C15:0 | SUP | 11 a | 22 b | 13 a | 17 a | 3.2 | ** |
| ST | 22 b | 7 a | 16 ab | 8 a | 4.9 | * | |
| LM | 21 b | 18 b | 5 a | 9 a | 3.7 | *** | |
| PM | 40 b | 32 ab | 26 a | 27 a | 4.9 | * | |
| C16:0 | SUP | 1058 b | 1115 b | 773 a | 804 a | 77.5 | *** |
| ST | 1378 b | 1004 a | 1045 a | 828 a | 140.5 | ** | |
| LM | 1497 c | 1328 bc | 1088 ab | 1063 a | 127.6 | ** | |
| PM | 2115 b | 1413 a | 1338 a | 1143 a | 150.4 | *** | |
| C17:0 | SUP | 50 | 49 | 37 | 43 | 5.5 | NS |
| ST | 56 b | 46 b | 31 a | 39 a | 7.3 | * | |
| LM | 60 b | 49 ab | 40 a | 43 a | 5.8 | ** | |
| PM | 101 b | 69 a | 69 a | 67 a | 7.7 | *** | |
| C17:0 i+ C16:1 t9 | SUP | 9 b | 21 c | 3 a | 4 a | 2.3 | *** |
| ST | 16 b | 8 ab | 6 a | 16 b | 4.5 | * | |
| LM | 17 b | 18 b | 6 a | 13 ab | 3.5 | ** | |
| PM | 34 b | 35 b | 25 a | 28 ab | 4.4 | * | |
| C18:0 | SUP | 616 a | 736 b | 573 a | 626 ab | 57.7 | * |
| ST | 542 | 502 | 563 | 543 | 79.2 | NS | |
| LM | 726 | 654 | 575 | 604 | 64.9 | NS | |
| PM | 1197 b | 895 a | 940 a | 900 a | 94.9 | ** | |
3.6. Individual Monounnsaturated Fatty Acids (Table 6)
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Muscle 1 | CONC | GSS | GSL | Grass | Sed 2 | Significance | |
| C14:1 | SUP | 25 b | 27 b | 17 a | 17 a | 2.8 | *** |
| ST | 63 c | 39 b | 29 b | 9 a | 7.6 | *** | |
| LM | 46 c | 40 bc | 28 a | 29 ab | 5.8 | ** | |
| PM | 57 c | 34 b | 29 ab | 20 a | 5.7 | *** | |
| C16:1 t10 to 12 | SUP | 3 a | 13 b | 16 b | 21 c | 1.9 | *** |
| ST | 9 b | 1 a | 3 ab | 7 b | 3.4 | * | |
| LM | 12 b | 3 a | 1 a | 2 ab | 1.7 | *** | |
| PM | 24 b | 18 b | 1 a | 8 a | 3.7 | *** | |
| C16:1 c9+ C17:0 ai | SUP | 166 b | 160 b | 112 a | 121 a | 12.3 | *** |
| ST | 306 b | 169 a | 153 a | 126 a | 27.0 | *** | |
| LM | 250 b | 227 ab | 185 a | 192 a | 21.8 | * | |
| PM | 316 b | 199 a | 191 a | 158 a | 23.1 | *** | |
| C17:1 c9 | SUP | 31 b | 35 b | 13 a | 13 a | 2.0 | *** |
| ST | 25 b | 18 b | 11 a | 32 c | 3.5 | *** | |
| LM | 28 a | 27 a | 32 b | 34 b | 1.4 | *** | |
| PM | 32 a | 28 a | 44 b | 46 b | 2.8 | *** | |
| C18:1 t9 | SUP | 2 a | 10 c | 8 b | 9 bc | 1.1 | *** |
| ST | 5 | 3 | 6 | 1 | 2.5 | NS | |
| LM | 12 b | 1 a | 1 a | 1 a | 1.2 | *** | |
| PM | 19 c | 4 b | 1 a | 1 a | 1.8 | *** | |
| C18:1 t10 | SUP | 9 | 6 | 5 | 6 | 2.4 | NS |
| ST | 14 b | 1 a | 1 a | 1 a | 2.5 | *** | |
| LM | 18 c | 7 b | 1 a | 1 a | 2.3 | *** | |
| PM | 30 b | 3 a | 1 a | 1 a | 4.0 | *** | |
| C18:1 t11 | SUP | 39 a | 86 bc | 62 ab | 110 c | 12.8 | *** |
| ST | 41 a | 67 ab | 85 bc | 112 c | 16.6 | ** | |
| LM | 39 a | 78 b | 76 b | 126 c | 14.4 | *** | |
| PM | 102 a | 114 a | 128 a | 182 b | 19.5 | ** | |
| C18:1 c9 | SUP | 1837 b | 1670 ab | 1408 a | 1464 a | 131.0 | ** |
| ST | 2221 b | 1398 a | 1545 a | 1337 a | 218.4 | *** | |
| LM | 2387 b | 1905 a | 1774 a | 1779 a | 198.7 | * | |
| PM | 3263 b | 1959 a | 2145 a | 1805 a | 217.9 | *** | |
| C18:1 c11 | SUP | 84 b | 63 a | 52 a | 59 a | 5.3 | *** |
| ST | 113 b | 54 a | 53 a | 51 a | 7.5 | *** | |
| LM | 98 b | 68 a | 61 a | 66 a | 6.7 | *** | |
| PM | 128 b | 66 a | 69 a | 64 a | 6.7 | *** | |
| C18:1 c13 | SUP | 19 b | 16 ab | 14 a | 14 a | 2.0 | * |
| ST | 42 c | 6 ab | 10 b | 2 a | 4.0 | *** | |
| LM | 30 c | 17 b | 15 b | 1 a | 3.5 | *** | |
| PM | 35 c | 14 b | 12 ab | 5 a | 3.8 | *** | |
| C18:1 t16 | SUP | 1 a | 12 b | 11 b | 11 b | 1.2 | *** |
| ST | 1 | 1 | 3 | 2 | 2.1 | NS | |
| LM | 8 b | 1 a | 1 a | 1 a | 6.8 | *** | |
| PM | 12 | 9 | 12 | 8 | 3.6 | NS | |
3.7. Individual Polyunsaturated Fatty Acids (Table 7)
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Muscle 1 | CONC | GSS | GSL | Grass | Sed 2 | Significance | |
| C16:2 c9,c12 | SUP | 40 c | 28 b | 1 a | 1 a | 2.6 | *** |
| ST | 60 b | 1 a | 1 a | 1 a | 4.0 | *** | |
| LM | 46 c | 37 c | 10 a | 23 b | 6.1 | *** | |
| PM | 10 ab | 4 a | 20 b | 11 ab | 5.8 | 0.08 | |
| C18:2 c9,12 | SUP | 147 b | 109 a | 105 a | 102 a | 5.9 | *** |
| ST | 128 c | 89 b | 82 b | 68 a | 5.1 | *** | |
| LM | 133 c | 88 b | 80 ab | 72 a | 5.9 | *** | |
| PM | 219 c | 135 b | 136 b | 118 a | 8.2 | *** | |
| C18:3 c9,12,15 | SUP | 19 a | 41 b | 46 b | 60 c | 3.3 | *** |
| ST | 23 a | 34 b | 46 c | 43 c | 2.9 | *** | |
| LM | 22 a | 37 b | 40 b | 48 c | 2.6 | *** | |
| PM | 41 a | 54 b | 67 c | 74 c | 4.1 | *** | |
| C18:2 c9,t11 (CLA) | SUP | 13 a | 26 b | 22 b | 36 c | 4.2 | ** |
| ST | 21 | 15 | 23 | 30 | 6.1 | NS | |
| LM | 16 a | 28 bc | 34 c | 23 ab | 5.2 | * | |
| PM | 38 b | 27 a | 41 b | 49 b | 5.7 | ** | |
| C20:3 c8,c11, c14 | SUP | 16 b | 10 a | 11 a | 11 a | 1.4 | *** |
| ST | 10 b | 1 a | 1 a | 1 a | 1.3 | *** | |
| LM | 14 b | 1 a | 1 a | 1 a | 0.4 | *** | |
| PM | 19 b | 1 a | 1 a | 1 a | 0.9 | *** | |
| C20:4 c5,8,11, 14 | SUP | 47 c | 31 a | 40 b | 37 b | 1.7 | *** |
| ST | 37 c | 34 b | 30 a | 29 a | 1.4 | *** | |
| LM | 28 | 28 | 27 | 28 | 2.6 | NS | |
| PM | 49 c | 40 ab | 41 b | 36 a | 2.0 | *** | |
| C20:5 c5,8,11, 14,17 | SUP | 2 a | 15 b | 22 c | 27 d | 1.2 | *** |
| ST | 14 a | 17 a | 19 a | 25 b | 2.8 | ** | |
| LM | 11 bc | 3 a | 5 ab | 16 c | 3.1 | *** | |
| PM | 2 a | 13 b | 21 c | 29 d | 3.1 | *** | |
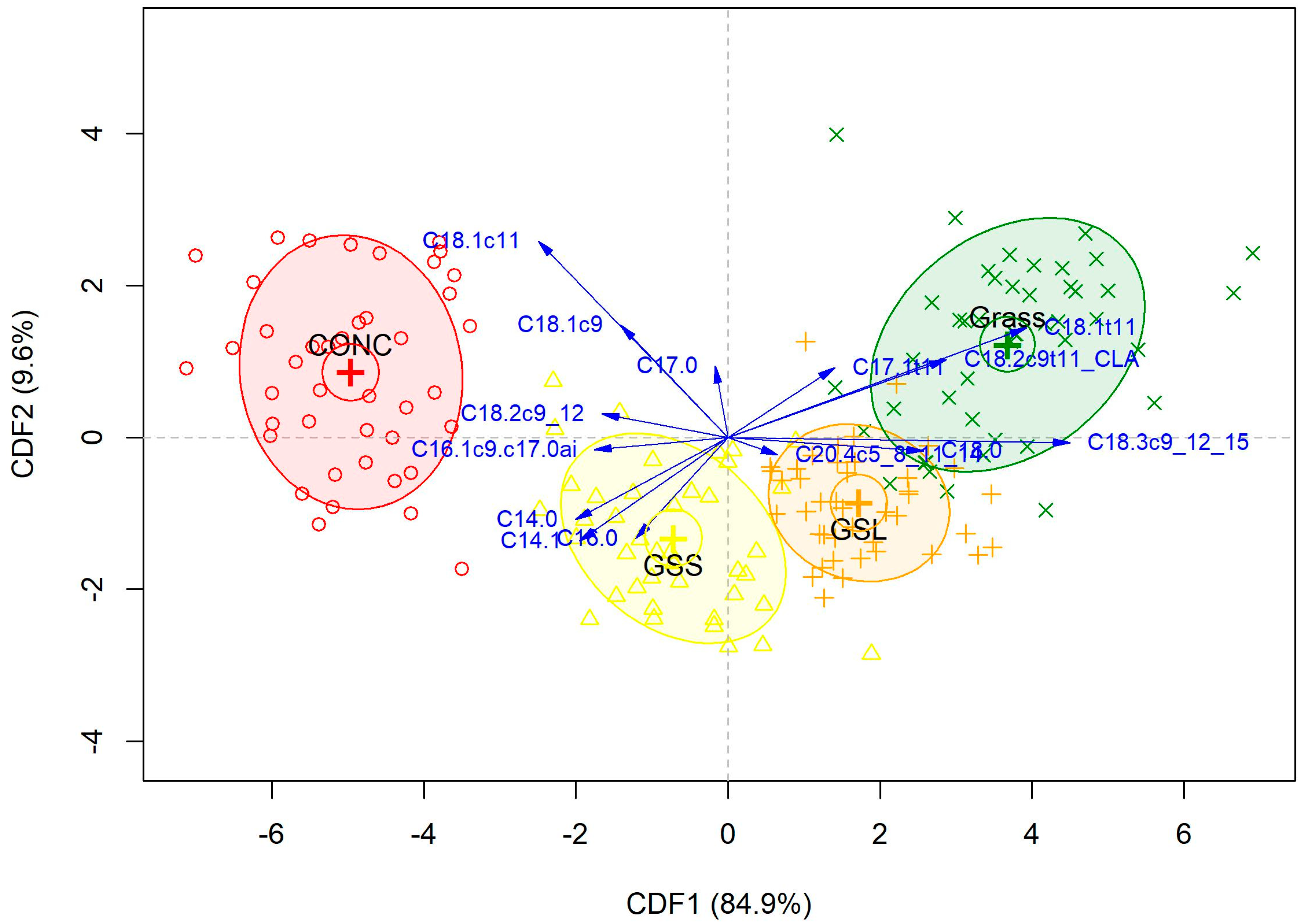
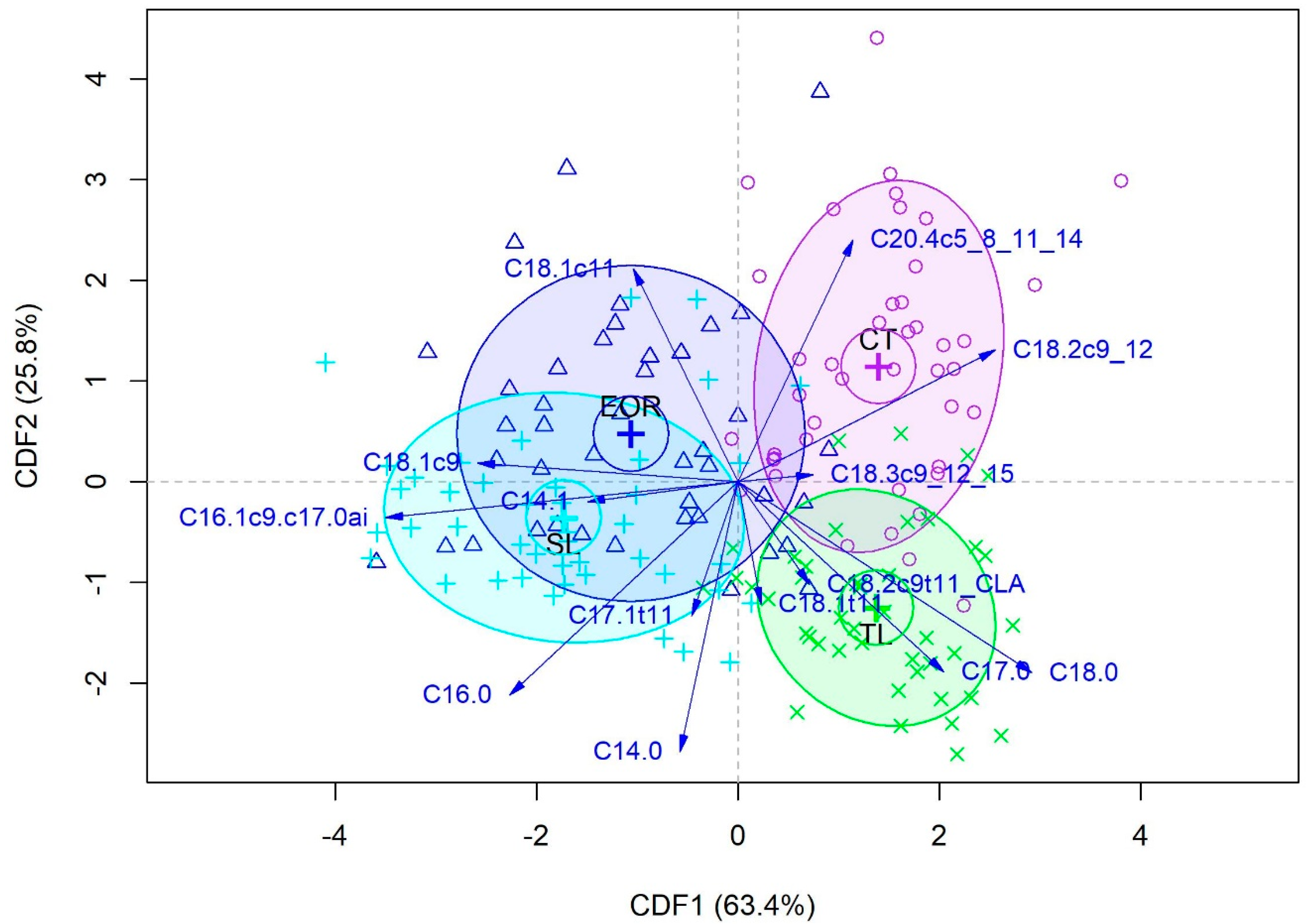
3.8. Discrimination According to Dietary Treatment
3.9. Discrimination According to Muscle Type
4. Discussion
4.1. Context
4.2. General Chemical Composition
4.3. Individual Fatty Acids and Nutritional Indices
4.4. Discrimination According to Dietary Treatment or Muscle Type
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flowers, S.; McFadden, B.R.; Carr, C.C.; Mateescu, R.G. Consumer preferences for beef with improved nutrient profile. J. Anim. Sci. 2019, 97, 4699–4709. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Stampa, E.; Schipmann, C.; Ham, U. Consumer perceptions, preferences, and behaviour regarding pasture-raised livestock products: A review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- Jones, S.J.; Guru, A.; Singh, V.; Carpenter, B.; Calkins, C.R.; Johnson, D. Bovine Myology and Muscle Profiling. 2004. Available online: https://bovine.unl.edu (accessed on 1 September 2024).
- Alfaia, C.P.M.; Alves, S.P.; Martins, S.I.V.; Costa, A.S.H.; Fontes, C.M.G.A.; Lemos, J.P.C.; Bessa, R.J.B.; Prates, J.A.M. Effect of the feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- Cama-Moncunill, R.; Moloney, A.P.; Röhrle, F.T.; Luciano, G.; Monahan, F.J. Canonical discriminant analysis of the fatty acid profile of muscle to authenticate beef from grass-fed and other beef production systems: Model development and validation. Food Control 2021, 122, 107820. [Google Scholar] [CrossRef]
- Hannah, H.; Cama-Moncunill, R.; Dunne, P.G.; McElhinney, C.; Monahan, F.J.; Schmidt, O.; Moloney, A.P. The potential of the mineral composition to discriminate between beef from different cattle diets and between individual muscles. Food Control 2024, 163, 110539. [Google Scholar]
- AOAC. Official Methods of Analysis, Method 992.15; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Brunton, N.P.; Mason, C.; Collins, M.J. Rapid microwave assisted preparation of fatty acid methyl esters for the analysis of fatty acid profiles in foods. J. Anal. Chem. 2015, 70, 1218–1224. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Ahvenjärvi, S.; Toivonen, V.; Ärölä, A.; Nurmela, K.V.V.; Huhtanen, P.; Griinari, J.M. Effect of dietary fish oil on biohydrogenation of fatty acids and milk fatty acid content in cows. Anim. Sci. 2003, 77, 165–169. [Google Scholar] [CrossRef]
- Grasso, S.; Brunton, N.P.; Monahan, F.J.; Harrison, S. Development of a method for the analysis of sterols in sterol-enriched deli-style turkey with GC-FID. Food Anal. Methods 2016, 9, 724–728. [Google Scholar] [CrossRef]
- Dunne, P.G.; Monahan, F.J.; O’Mara, F.P.; Moloney, A.P. Colour stability, under simulated retail display conditions, of M. longissimus dorsi and M. semimembranosus from steers given long-term daily exercise and supplemented with vitamin E. Meat Sci. 2005, 71, 480–489. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and faeces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- AFRC. Energy and Protein Requirements of Ruminants. An Advisory Manual Prepared by the AFRC Technical Committee on Responses to Nutrients; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 1 November 2024).
- U.S. Environmental Protection Agency (EPA). Office of Pesticide Programs. Assigning Values to Non-Detected/Non-Quantified Pesticide Residues in Human Health Food Exposure Assessments; Office of Pesticide Programs, U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Cui, Y. Discriminant Analyisis. In Encyclopedia of Research Design; Salkind, N.J., Ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010. [Google Scholar] [CrossRef]
- Moloney, A.P.; O’ Riordan, E.G.; Monahan, F.J.; Richardson, R.I. The colour and sensory characteristics of longissimus muscle from beef cattle that grazed grass or consumed concentrates prior to slaughter. J. Sci. Food Agric. 2022, 102, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Teagasc. Beef Production System Guidelines; Teagasc: Carlow, Ireland, 2015; 36p. [Google Scholar]
- Aldai, N.; Dugan, M.; Kramer, J.; Martínez, A.; López-Campos, O.; Mantecón, A.R.; Osoro, K. Length of concentrate finishing affects the fatty acid composition of grass-fed and genetically lean beef: An emphasis on trans-18: 1 and conjugated linoleic acid profiles. Animal 2011, 5, 1643–1652. [Google Scholar] [CrossRef]
- Moloney, A.P.; McGettrick, S.; Dunne, P.G.; Shingfield, K.J.; Richardson, R.I.; Monahan, F.J.; Mulligan, F.J.; Ryan, M.; Sweeney, T. Supplementation with Sunflower/Fish Oil-Containing Concentrates in a Grass-Based Beef Production System: Influence on Fatty Acid Composition, Gene Expression, Lipid and Colour Stability and Sensory Characteristics of Longissimus Muscle. Foods 2022, 11, 4061. [Google Scholar] [CrossRef] [PubMed]
- Gravador, R.S.; Valentini, V.; Harrison, S.M.; Fahey, A.; Moloney, A.P.; Diskin, M.G.; Monahan, F.J. Impact of cooking on the content of nutritionally important fatty acids in muscle from lambs raised to have divergent intramuscular fatty acid compositions. Small Rumin. Res. 2022, 216, 106802. [Google Scholar] [CrossRef]
- EU Regulation No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2007, 12, 3–18.
- EU Regulation No 116/2010 of 9 February 2010 amending Regulation No 1924/2006 of the European Parliament and of the Council with regard to the list of nutrition claims. Off. J. Eur. Union 2010, 37, 16–18.
- Descalzo, A.M.; Insani, E.M.; Biolatto, A.; Sancho, A.M.; García, P.T.; Pensel, N.A.; Josifovich, J.A. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Sci. 2005, 70, 35–44. [Google Scholar] [CrossRef]
- Doyle, P.; O’Riordan, E.G.; McGee, M.; Crosson, P.; Kelly, A.K.; Moloney, A.P. Temperate pasture- or concentrate beef production systems: Steer performance, meat nutritional value, land-use, food–feed competition, economic and environmental sustainability. J. Agric. Sci. 2023, 161, 704–719. [Google Scholar] [CrossRef]
- Alfaia, C.M.M.; Ribeiro, V.V.V.; Lourenco, M.R.A.; Quaresma, M.A.G.; Martins, S.I.V.; Portugal, A.P.V.; Fontes, C.M.G.A.; Bessa, R.J.B.; Castro, M.L.F.; Prates, J.A.M. Fatty acid composition, conjugated linoleic acid isomers and cholesterol in beef from crosssbred bullocks intensively produced and from the Alentejana purebred bullocks reared according to Carnalentejana-PDO specifications. Meat Sci. 2006, 72, 425–436. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion: Labeling reference intake values for n-3 and n-6 polyunsaturated fatty acids. EFSA J. 2009, 1176, 1–11. [Google Scholar]
- Warren, H.; Scollan, N.; Enser, M.; Hughes, S.; Richardson, R.; Wood, J. Effects of breed and a concentrate or grass silage diet on beef quality in cattle of 3 ages. I: Animal performance, carcass quality and muscle fatty acid composition. Meat Sci. 2008, 78, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Noci, F.; Monahan, F.J.; French, P.; Moloney, A.P. The fatty acid composition of muscle fat and subcutaneous adipose tissue of pasture-fed beef heifers: Influence of the duration of grazing. J. Anim. Sci. 2005, 83, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Pavan, E.; Duckett, S.K. Fatty acid composition and interrelationships among eight retail cuts of grass-fed beef. Meat Sci. 2013, 93, 371–377. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, S.B.; Richardson, I.; Kim, E.J.; Dannenberger, D.; Coilmier, D.; Scollan, N.D. Addition of an extract of Lucerne (Medicago sativa L.) to cattle diets—Effects on fatty acid profile, meat quality and eating quality of the M. longissimus muscle. Meat Sci. 2017, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; Report of the joint WHO/FAO Expert Consultation; WHO: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Dal Bosco, A.; Mancinelli, A.C.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of fatty acids in poultry meat for its characterisation in healthy human nutrition: A comprehensive application of the scientific literature and new proposals. Nutrients 2022, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Jefferis, B.J.; Lennon, L.; Papacosta, O.; Whincup, P.H.; Hingorani, A. Serum conjugated linoleic acid and risk of incident heart failure in older men: The British regional heart study. J. Am. Heart Assoc. 2018, 7, e006653. [Google Scholar] [CrossRef] [PubMed]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Siurana, A.; Calsamiglia, S. A meta-analysis of feeding strategies to increase the content of conjugated linoleic acid (CLA) in dairy cattle milk and the impact on human consumption. Anim. Feed Sci. Technol. 2016, 217, 13–26. [Google Scholar] [CrossRef]
- Scollan, N.D.; Choi, N.J.; Kurt, E.; Fisher, A.V.; Enser, M.; Wood, J.D. Manipulating of fatty acid composition of muscle and adipose tissue in beef cattle. Br. J. Nutr. 2001, 85, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Kraft, J.; Moeckel, P.; Jahreis, G. Trans 18:12 is effectively delta-9 desaturated compared with trans-12-18:1 in humans. Br. J. Nutr. 2006, 95, 752–761. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The effects of trans fatty acids on human health: Regulation and consumption patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- Verneque, B.J.F.; Machado, A.M.; Silva, L.d.A.; Lopes, A.C.S.; Duarte, C.K. Ruminant and industrial trans-fatty acids consumption and cardiometabolic risk markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.T.; Pensel, N.A.; Sancho, A.M.; Latimori, N.J.; Kloster, A.M.; Amigone, M.A.; Casal, J.J. Beef lipids in relation to animal breed and nutrition in Argentina. Meat Sci. 2008, 79, 500–508. [Google Scholar] [CrossRef] [PubMed]
| Standard Concentrate | Linseed Concentrate | Grass | Medium Silage | High Silage | Finishing Silage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Ingredient (g/kg) | ||||||||||||
| Rolled barley | 862 | 813 | ||||||||||
| Linseeds | 46 | |||||||||||
| Soyabean meal | 60 | 57 | ||||||||||
| Linseed oil | 14 | |||||||||||
| Cane molasses | 50 | 47 | ||||||||||
| Mineral/Vitamin Mix | 28 | 27 | ||||||||||
| Chemical composition | ||||||||||||
| Dry matter (DM, g/kg) | 793 | (7.3) | 827 | (11.4) | 208 | (20.3) | 211 | (31.2) | 289 | (69.8) | 251 | (15.2) |
| Ash 1 | 58 | (5.8) | 77 | (10.5) | 86 | (9.1) | 77 | (7.0) | 101 | (2.8) | 80 | (6.0) |
| Crude protein 1 | 125 | (8.0) | 126 | (12.5) | 203 | (29.4) | 162 | (6.1) | 136 | (1.8) | 166 | (5.3) |
| Oil B 1,2 | 24 | (2.0) | 59 | (16.6) | 40 | (8.9) | 36 | (2.0) | 42 | (8.4) | 48 | (2.5) |
| DM digestibility (g/kg) | - | 800 | (27.1) | 699 | (24.2) | 767 | (19.0) | 778 | (16.2) | |||
| NCGD (g/kg) 3 | 839 | (20.6) | 791 | (20.5) | - | - | - | - | - | - | - | - |
| NDF 1,4 | 193 | (22.4) | 203 | (11.7) | 428 | (21.4) | 515 | (29.2) | 426 | (8.4) | 425 | (45.3) |
| β-carotene (mg/kg) 1 | ND | ND | 26 | (25.8) | 7 | (2.5) | 8 | (2.0) | 35 | (9.5) | ||
| Vitamin E (mg/kg) 1 | 13 | (13.8) | 14 | (4.7) | 12 | (10.9) | 27 | (14.8) | 15 | (2.1) | 39 | (5.0) |
| Fatty acids (g/kg fatty acids) | ||||||||||||
| C12:0 | 6.6 | (6.22) | 1.7 | (1.60) | 1.7 | (0.36) | 2.6 | (0.43) | 2.4 | (0.28) | 1.8 | (0.18) |
| C14:0 | 6.7 | (2.09) | 1.9 | (0.96) | 4.4 | (0.93) | 9.0 | (2.61) | 6.2 | (0.18) | 5.7 | (0.37) |
| C16:0 | 265.8 | (20.65) | 106.3 | (14.61) | 134.9 | (18.23) | 144.7 | (11.25) | 138.7 | (5.15) | 126.0 | (3.50) |
| C16:1 | 2.7 | (0.33) | 1.1 | (0.52) | 1.9 | (1.00) | 4.1 | (1.51) | 3.8 | (0.62) | 2.4 | (0.19) |
| C18:0 | 18.1 | (2.98) | 33.4 | (1.39) | 15.1 | (2.13) | 15.7 | (1.37) | 13.8 | (0.66) | 12.2 | (0.61) |
| C18:1 | 148.8 | (13.93) | 182.1 | (8.14) | 15.3 | (3.38) | 20.5 | (3.24) | 19.0 | (2.48) | 14.0 | (0.69) |
| C18:2 | 430.0 | (43.79) | 241.8 | (17.11) | 89.0 | (7.06) | 111.6 | (4.84) | 109.6 | (3.60) | 110.8 | (0.95) |
| C18:3 | 31.0 | (5.70) | 374.6 | (38.68) | 457.1 | (50.63) | 348.1 | (44.29) | 413.9 | (16.65) | 442.7 | (19.87) |
| C20:0 | 2.5 | (0.55) | 1.9 | (0.16) | 4.6 | (1.07) | 5.8 | (0.58) | 4.6 | (0.29) | 3.8 | (0.09) |
| C20:1 | 6.9 | (0.70) | 0.6 | (1.11) | 0.6 | (0.10) | 1.0 | (0.23) | 0.8 | (0.33) | 0.5 | (0.07) |
| C20:2 | 1.1 | (0.33) | 0.4 | (0.20) | 3.8 | (1.92) | 1.2 | (0.57) | 1.0 | (0.31) | 0.7 | (0.11) |
| C22:0 | 2.7 | (0.48) | 2.1 | (0.55) | 9.4 | (1.92) | 11.0 | (0.89) | 8.5 | (0.77) | 8.9 | (0.32) |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| CONC | GSS | GSL | Grass | Sed 2 | Significance | |
| Weight (kg) | ||||||
| Initial | 296 | 297 | 297 | 296 | 2.3 | NS |
| Final | 479 a | 516 b | 530 b | 510 b | 12.7 | ** |
| Duration (days) | 188 | 293 | 294 | 336 | - | - |
| Age at slaughter (days) | 465 a | 567 b | 581 b | 630 c | 11.9 | *** |
| Overall growth (g/day) | 978 a | 747 b | 790 b | 636 c | 47.6 | *** |
| Carcass weight (kg) | 258 | 265 | 269 | 267 | 6.4 | NS |
| Conformation 3 | 7.1 | 6.5 | 7.2 | 6.9 | 0.45 | NS |
| Fat classification 4 | 10.3 | 9.7 | 10.0 | 10.6 | 0.512 | NS |
| Diet 2 Discrimination | Muscle 3 Discrimination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictions | Predictions | |||||||||
| CONC | GSS | GSL | Grass | SUP | ST | LM | PM | |||
| Training | CONC (n = 42) | 42.0 | 0.0 | 0.0 | 0.0 | SUP (n = 43) | 37.0 | 1.7 | 1.0 | 3.3 |
| GSS (n = 41) | 0.0 | 39.3 | 1.7 | 0.0 | ST (n = 42) | 2.3 | 31.7 | 6.3 | 1.7 | |
| GSL (n = 42) | 0.0 | 0.0 | 42.0 | 0.3 | LM (n = 43) | 3.3 | 2.7 | 34.7 | 2.3 | |
| Grass (n = 45) | 0.0 | 0.3 | 3.3 | 41.3 | PM (n = 43) | 2.7 | 0.7 | 0.7 | 39.0 | |
| Sensitivity (%) | 100.0 | 95.9 | 100.0 | 91.9 | Sensitivity (%) | 86.0 | 75.4 | 80.6 | 90.7 | |
| Specificity (%) | 100.0 | 99.7 | 96.1 | 99.7 | Specificity (%) | 93.5 | 96.1 | 93.8 | 94.3 | |
| Accuracy (%) | 96.9 | Accuracy (%) | 83.2 | |||||||
| CV-LOO | CONC (n = 42) | 42.0 | 0.0 | 0 | 0 | SUP (n = 43) | 34.0 | 2.0 | 2.0 | 5.0 |
| GSS (n = 41) | 0.0 | 36.3 | 3.3 | 1.3 | ST (n = 42) | 4.0 | 26.7 | 9.0 | 2.3 | |
| GSL (n = 42) | 0 | 1.3 | 38.0 | 2.7 | LM (n = 43) | 4.7 | 4.7 | 31.0 | 2.7 | |
| Grass (n = 45) | 0 | 0.7 | 5.0 | 39.3 | PM (n = 43) | 3.0 | 1.0 | 1.7 | 37.3 | |
| Sensitivity (%) | 100.0 | 88.6 | 90.5 | 87.4 | Sensitivity (%) | 79.1 | 63.5 | 72.1 | 86.8 | |
| Specificity (%) | 100.0 | 98.4 | 93.5 | 96.8 | Specificity (%) | 90.9 | 94.1 | 90.1 | 92.2 | |
| Accuracy (%) | 91.6 | Accuracy (%) | 75.4 | |||||||
| Test | CONC (n = 14) | 14.0 | 0.0 | 0.0 | 0.0 | SUP (n = 14) | 11.0 | 0.3 | 0.3 | 2.3 |
| GSS (n = 14) | 0.0 | 11.7 | 2.0 | 0.3 | ST (n = 14) | 0.7 | 9.0 | 2.7 | 1.7 | |
| GSL (n = 14) | 0.0 | 0.7 | 12.0 | 1.3 | LM (n = 14) | 0.3 | 1.7 | 11.7 | 0.3 | |
| Grass (n = 15) | 0.0 | 0.3 | 2.7 | 12.0 | PM (n = 14) | 1.7 | 0.3 | 0.3 | 11.7 | |
| Sensitivity (%) | 100.0 | 83.3 | 85.7 | 80.0 | Sensitivity (%) | 78.6 | 64.3 | 83.3 | 79.8 | |
| Specificity (%) | 100.0 | 97.7 | 89.1 | 96.0 | Specificity (%) | 93.7 | 94.4 | 92.1 | 89.7 | |
| Accuracy (%) | 87.1 | Accuracy (%) | 77.4 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloney, A.P.; McElhinney, C.; Cama-Moncunill, R.; O′Riordan, E.G.; Monahan, F.J. The Concentrations of Fatty Acids, Cholesterol and Vitamin E in Cooked Longissimus, Semitendinosus, Psoas Major and Supraspinatus Muscles from Cattle Offered Grass Only, Concentrates Ad Libitum or Grass Silage Supplemented with Concentrates. Foods 2025, 14, 747. https://doi.org/10.3390/foods14050747
Moloney AP, McElhinney C, Cama-Moncunill R, O′Riordan EG, Monahan FJ. The Concentrations of Fatty Acids, Cholesterol and Vitamin E in Cooked Longissimus, Semitendinosus, Psoas Major and Supraspinatus Muscles from Cattle Offered Grass Only, Concentrates Ad Libitum or Grass Silage Supplemented with Concentrates. Foods. 2025; 14(5):747. https://doi.org/10.3390/foods14050747
Chicago/Turabian StyleMoloney, Aidan P., Cormac McElhinney, Raquel Cama-Moncunill, Edward G. O′Riordan, and Frank J. Monahan. 2025. "The Concentrations of Fatty Acids, Cholesterol and Vitamin E in Cooked Longissimus, Semitendinosus, Psoas Major and Supraspinatus Muscles from Cattle Offered Grass Only, Concentrates Ad Libitum or Grass Silage Supplemented with Concentrates" Foods 14, no. 5: 747. https://doi.org/10.3390/foods14050747
APA StyleMoloney, A. P., McElhinney, C., Cama-Moncunill, R., O′Riordan, E. G., & Monahan, F. J. (2025). The Concentrations of Fatty Acids, Cholesterol and Vitamin E in Cooked Longissimus, Semitendinosus, Psoas Major and Supraspinatus Muscles from Cattle Offered Grass Only, Concentrates Ad Libitum or Grass Silage Supplemented with Concentrates. Foods, 14(5), 747. https://doi.org/10.3390/foods14050747






