Ursolic Acid Induces Multifaceted Defense Responses Against Postharvest Blue Mold Rot in Apple Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit and Microbial Material
2.2. Antifungal Activity Measurement
2.3. UA Concentration on the Induced Resistance
2.4. Induction Time Interval for the Induced Resistance
2.5. UA-Induced Resistance Against Blue Mold Rot in Harvested Apple Fruit
2.6. Total Phenolics, Flavonoid, and Lignin Contents
2.7. Hydrogen Peroxide Content
2.8. Measurement of Enzymatic Activities
2.9. Data Analyses
3. Results and Discussion
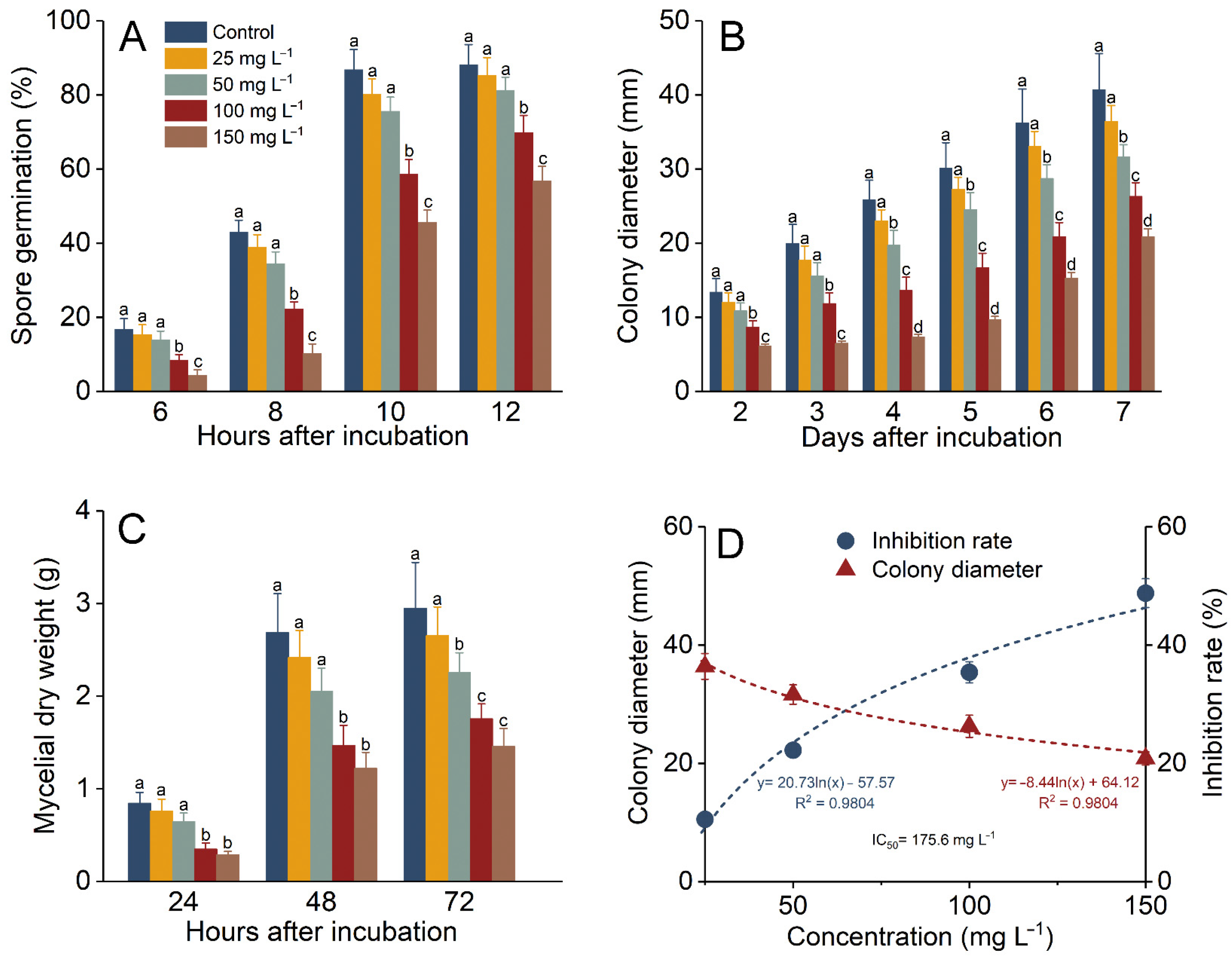
3.1. P. expansum Was Inhibited by UA In Vitro
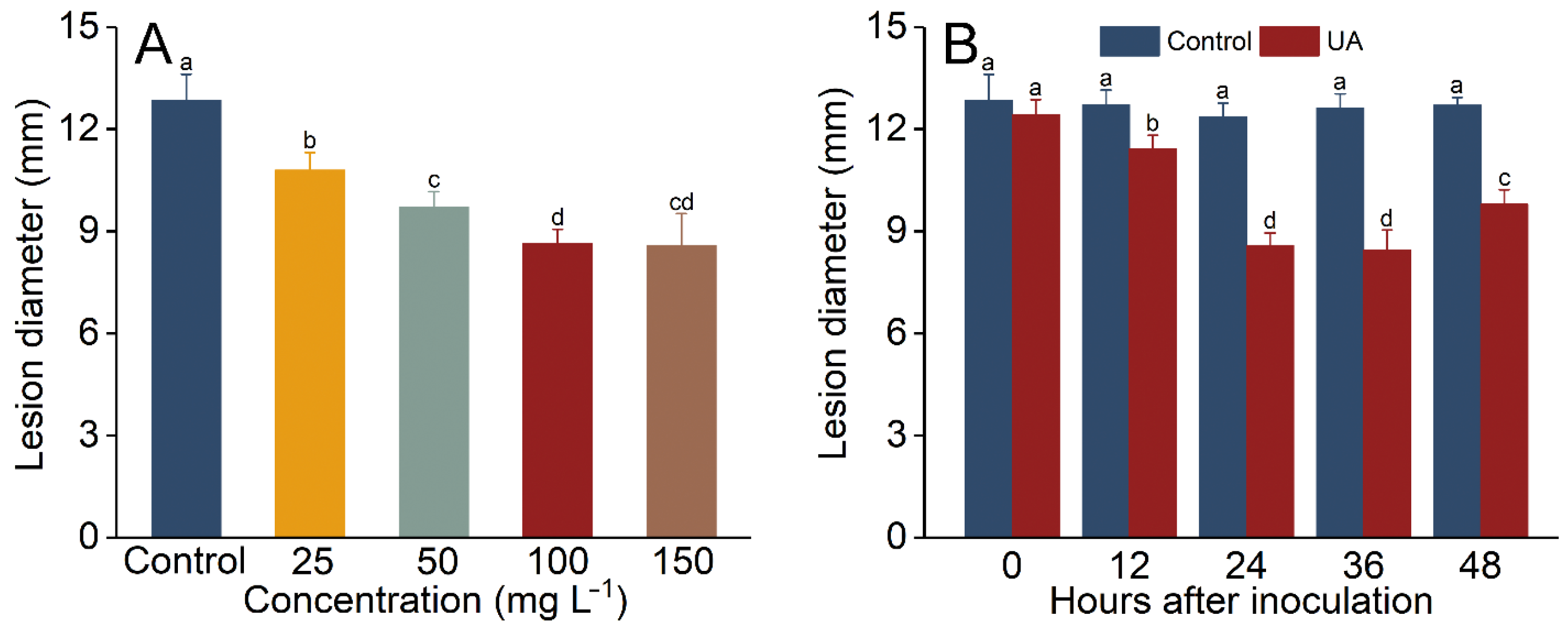
3.2. Influence of UA Concentration and Induction Time Interval on the Development of Apple Fruit Decay
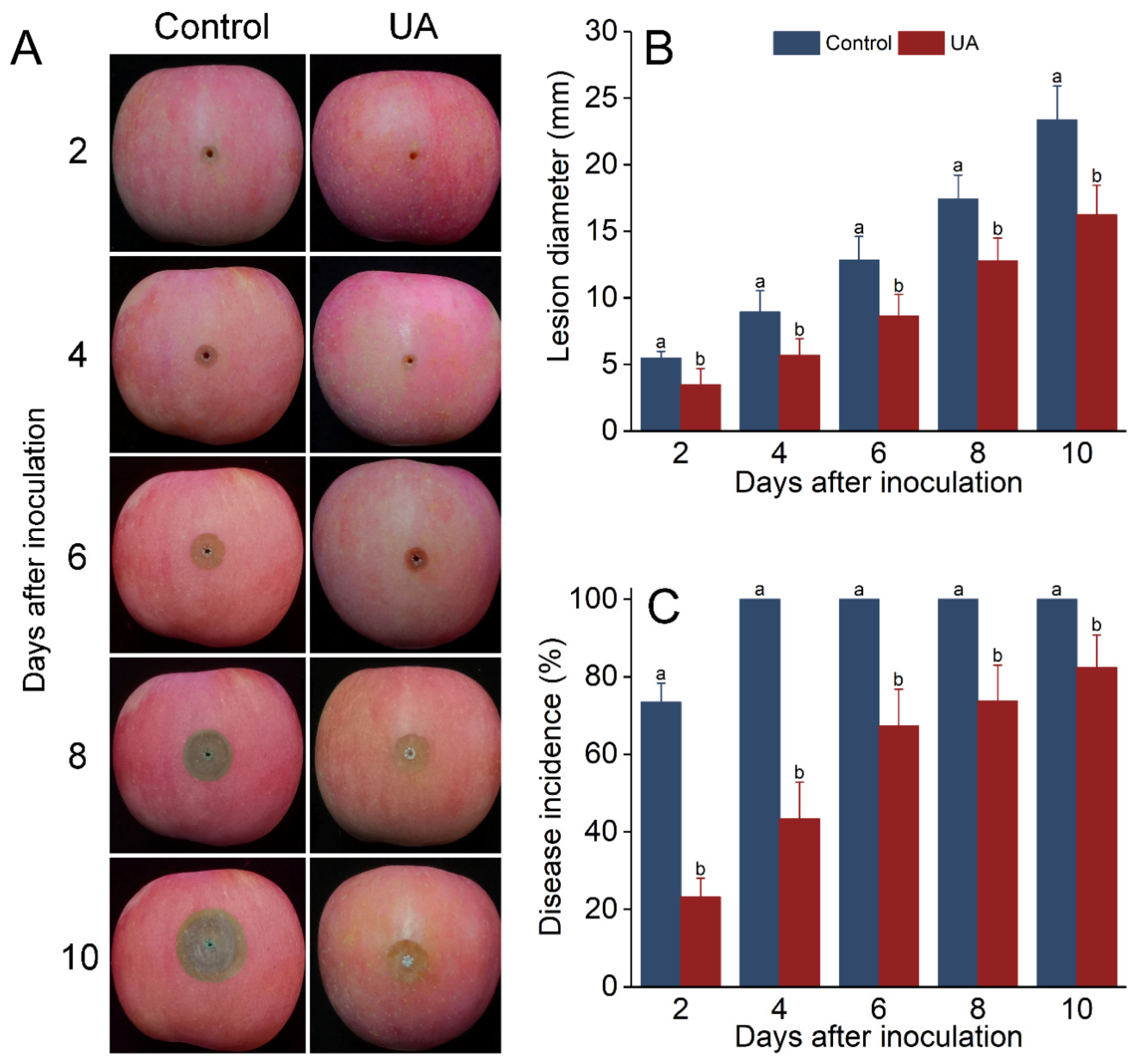
3.3. UA-Induced Resistance Offers Protection from Blue Mold Rot in Apples
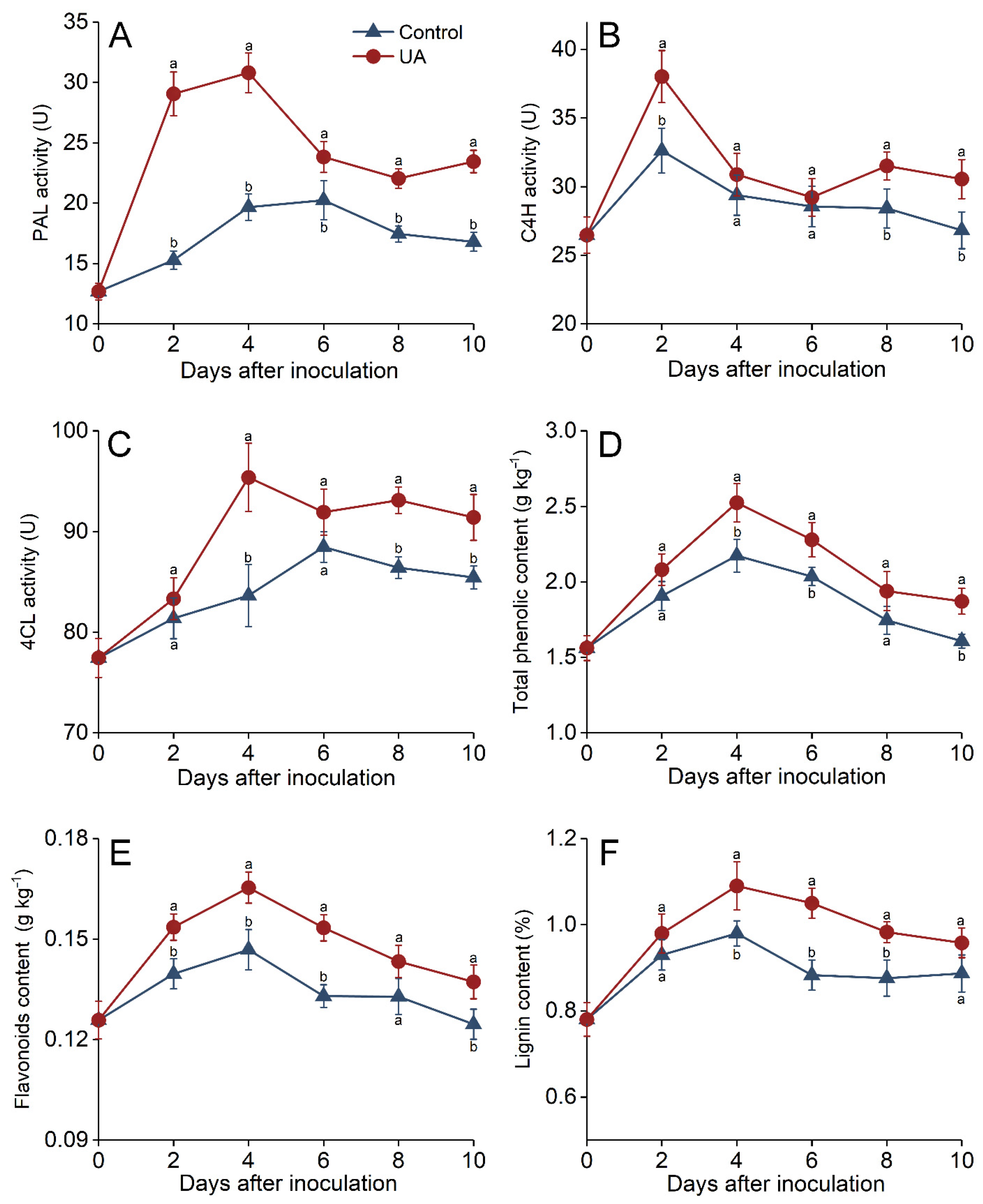
3.4. Effects of UA-Induced Resistance on Defense-Related Enzymes in Harvested Apples
3.5. Phenylpropanoid Metabolism Regulation Induced by UA
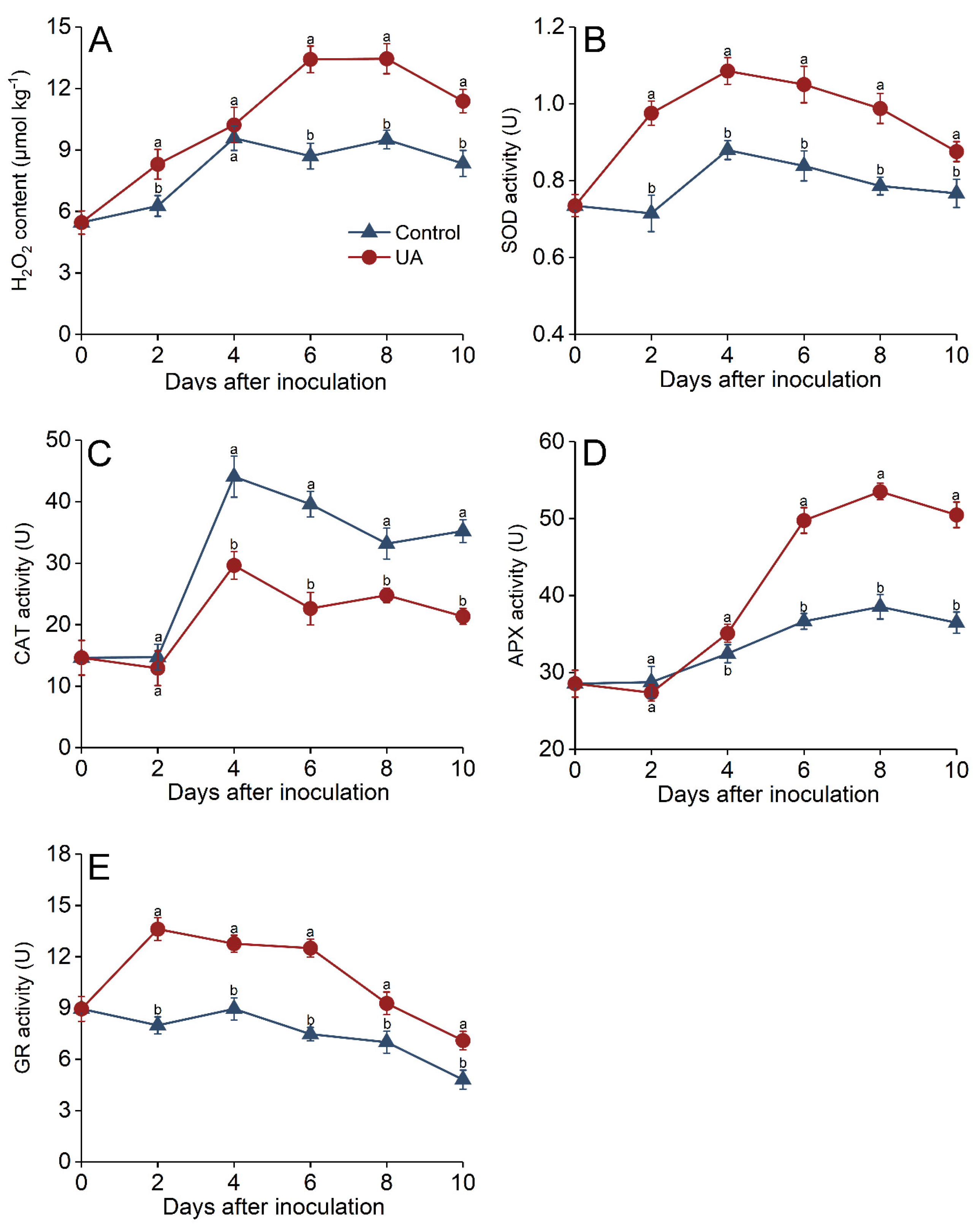
3.6. Effects of UA on Oxidative Stress Regulation in Apple Fruit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, Y.; Wei, M.; Li, C.; Chen, Y.; Lv, J.; Meng, K.; Wang, W.; Li, J. Reactive Oxygen Species Metabolism and Phenylpropanoid Pathway Involved in Disease Resistance against Penicillium expansum in Apple Fruit Induced by ϵ -Poly- l -Lysine. J. Sci. Food Agric. 2018, 98, 5082–5088. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, Y.; Li, C.; Zhao, J.; Wei, M.; Li, X.; Yang, S.; Mi, Y. Effect of Sodium Nitroprusside Treatment on Shikimate and Phenylpropanoid Pathways of Apple Fruit. Food Chem. 2019, 290, 263–269. [Google Scholar] [CrossRef]
- Gupta, S.; Saxena, S. Endophytes: Saviour of Apples from Post-Harvest Fungal Pathogens. Biol. Control 2023, 182, 105234. [Google Scholar] [CrossRef]
- Abdelhai, M.H.; Awad, F.N.; Yang, Q.; Mahunu, G.K.; Godana, E.A.; Zhang, H. Enhancement the Biocontrol Efficacy of Sporidiobolus Pararoseus Y16 against Apple Blue Mold Decay by Glycine Betaine and Its Mechanism. Biol. Control 2019, 139, 104079. [Google Scholar] [CrossRef]
- Morales, H.; Marín, S.; Ramos, A.J.; Sanchis, V. Influence of Post-Harvest Technologies Applied during Cold Storage of Apples in Penicillium expansum Growth and Patulin Accumulation: A Review. Food Control 2010, 21, 953–962. [Google Scholar] [CrossRef]
- Fu, D.; Xiang, H.; Yu, C.; Zheng, X.; Yu, T. Colloidal Chitin Reduces Disease Incidence of Wounded Pear Fruit Inoculated by Penicillium expansum. Postharvest Biol. Technol. 2016, 111, 1–5. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced Resistance to Control Postharvest Decay of Fruit and Vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Prusky, D.; Romanazzi, G. Induced Resistance in Fruit and Vegetables: A Host Physiological Response Limiting Postharvest Disease Development. Annu. Rev. Phytopathol. 2023, 61, 279–300. [Google Scholar] [CrossRef] [PubMed]
- da Rocha Neto, A.C.; Luiz, C.; Maraschin, M.; Di Piero, R.M. Efficacy of Salicylic Acid to Reduce Penicillium expansum Inoculum and Preserve Apple Fruits. Int. J. Food Microbiol. 2016, 221, 54–60. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Cui, X.; Zhou, J.; Li, J.; Ai, W.; Shu, P.; Zhang, X.; Li, X.; Meng, D.; et al. SlMYC2 are Required for Methyl Jasmonate-Induced Tomato Fruit Resistance to Botrytis cinerea. Food Chem. 2020, 310, 125901. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Xu, H.; Zhu, J.; Cheng, Y.; Ge, Y. Inducing Resistance of Postharvest Fruits and Vegetables through Acibenzolar-S-Methyl Application: A Review of Implications and Mechanisms. Plant Physiol. Biochem. 2025, 220, 109542. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, H.; Wang, Y.; Lyu, L.; Prusky, D.; Ji, Y.; Zheng, X.; Bi, Y. Effect of Benzothiadiazole Treatment on Improving the Mitochondrial Energy Metabolism Involved in Induced Resistance of Apple Fruit during Postharvest Storage. Food Chem. 2020, 302, 125288. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bi, Y.; Zhang, Z.; Xie, D.; Ge, Y.; Li, W.; Wang, J.; Wang, Y. Postharvest Oxalic Acid Treatment Induces Resistance against Pink Rot by Priming in Muskmelon (Cucumis melo L.) Fruit. Postharvest Biol. Technol. 2015, 106, 53–61. [Google Scholar] [CrossRef]
- Yang, J.; Sun, C.; Fu, D.; Yu, T. Test for L-Glutamate Inhibition of Growth of Alternaria alternata by Inducing Resistance in Tomato Fruit. Food Chem. 2017, 230, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Jin, L.; Cai, Y.; Huang, Y.; Zheng, X.; Yu, T. L-Glutamate Treatment Enhances Disease Resistance of Tomato Fruit by Inducing the Expression of Glutamate Receptors and the Accumulation of Amino Acids. Food Chem. 2019, 293, 263–270. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin Isolated from Yeast Cell Wall Induces the Resistance of Tomato Fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lin, M.; Fu, D.; Yang, J.; Huang, Y.; Zheng, X.; Yu, T. Yeast Cell Wall Induces Disease Resistance against Penicillium expansum in Pear Fruit and the Possible Mechanisms Involved. Food Chem. 2018, 241, 301–307. [Google Scholar] [CrossRef]
- Žebeljan, A.; Vico, I.; Duduk, N.; Žiberna, B.; Urbanek Krajnc, A. Dynamic Changes in Common Metabolites and Antioxidants during Penicillium expansum-Apple Fruit Interactions. Physiol. Mol. Plant Pathol. 2019, 106, 166–174. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic Acid from Apple Pomace and Traditional Plants: A Valuable Triterpenoid with Functional Properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lian, Q.; Wang, L.; Shan, Y.; Li, F.; Chang, S.K.; Jiang, Y. Chemical Composition of the Cuticular Membrane in Guava Fruit (Psidium guajava L.) Affects Barrier Property to Transpiration. Plant Physiol. Biochem. 2020, 155, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gao, H.; Cao, S.; Fang, X.; Chen, H.; Xiao, S. Composition and Morphology of Cuticular Wax in Blueberry (Vaccinium spp.) Fruits. Food Chem. 2017, 219, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Fauth, M.; Schweizer, P.; Buchala, A.; Markstädter, C.; Riederer, M.; Kato, T.; Kauss, H. Cutin Monomers and Surface Wax Constituents Elicit H2O2 in Conditioned Cucumber Hypocotyl Segments and Enhance the Activity of Other H2O2Elicitors1. Plant Physiol. 1998, 117, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit Characteristics and Cuticle Triterpenes as Related to Postharvest Quality of Highbush Blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef]
- Yan, Y.; Castellarin, S.D. Blueberry Water Loss Is Related to Both Cuticular Wax Composition and Stem Scar Size. Postharvest Biol. Technol. 2022, 188, 111907. [Google Scholar] [CrossRef]
- Shu, C.; Zhao, H.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Antifungal Efficacy of Ursolic Acid in Control of Alternaria alternata Causing Black Spot Rot on Apple Fruit and Possible Mechanisms Involved. Sci. Hortic. 2019, 256, 108636. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, L.; Xiao, S.; Chen, H.; Han, Y.; Niu, B.; Wu, W.; Gao, H. Ursolic Acid, the Main Component of Blueberry Cuticular Wax, Inhibits Botrytis cinerea Growth by Damaging Cell Membrane Integrity. Food Chem. 2023, 415, 135753. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Transcription Combined Metabolic Analysis Reveals the Mechanism of Potassium Phosphate Enhances Resistance to Walnut Anthracnose (Colletotrichum gloeosporioides). Postharvest Biol. Technol. 2024, 218, 113165. [Google Scholar] [CrossRef]
- Mahlo, S.M.; Eloff, J.N. Acetone Leaf Extracts of Breonadia salicina (Rubiaceae) and Ursolic Acid Protect Oranges against Infection by Penicillium Species. S. Afr. J. Bot. 2014, 93, 48–53. [Google Scholar] [CrossRef]
- Shaik, A.B.; Ahil, S.B.; Govardhanam, R.; Senthi, M.; Khan, R.; Sojitra, R.; Kumar, S.; Srinivas, A. Antifungal Effect and Protective Role of Ursolic Acid and Three Phenolic Derivatives in the Management of Sorghum Grain Mold Under Field Conditions. Chem. Biodivers. 2016, 13, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Shou, J.; Wang, J.; Hu, W.; Hannan, F.; Mwamba, T.M.; Farooq, M.A.; Zhou, W.; Islam, F. Ursolic Acid Limits Salt-Induced Oxidative Damage by Interfering With Nitric Oxide Production and Oxidative Defense Machinery in Rice. Front. Plant Sci. 2020, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Han, Y.; Chen, H.; Wu, W.; Fang, X.; Gao, H. Effects of cuticular wax on disease resistance of postharvest blueberry. J. Chin. Inst. Food Sci. Technol. 2021, 21, 205–213. [Google Scholar] [CrossRef]
- Zhang, S. Ursolic Acid, a Natural Endogenous Compound, Inhibits Browning in Fresh-Cut Apples. Postharvest Biol. Technol. 2025, 219, 113228. [Google Scholar] [CrossRef]
- Shu, C.; Sun, X.; Cao, J.; Droby, S.; Jiang, W. Antifungal Efficiency and Mechanisms of Ethyl Ferulate against Postharvest Pathogens. Int. J. Food Microbiol. 2024, 417, 110710. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Wang, Y.; Bai, X.; Qi, Q.; Xu, M.; Zhou, T. Dissecting Inhibitory Effect of Boric Acid on Virulence and Patulin Production of Penicillium expansum. Postharvest Biol. Technol. 2016, 117, 187–196. [Google Scholar] [CrossRef]
- Li, J.; Lei, H.; Song, H.; Lai, T.; Xu, X.; Shi, X. 1-Methylcyclopropene (1-MCP) Suppressed Postharvest Blue Mold of Apple Fruit by Inhibiting the Growth of Penicillium expansum. Postharvest Biol. Technol. 2017, 125, 59–64. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Jiao, W.; Li, X.; Wang, X.; Cao, J.; Jiang, W. Chlorogenic Acid Induces Resistance against Penicillium expansum in Peach Fruit by Activating the Salicylic Acid Signaling Pathway. Food Chem. 2018, 260, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yong, D.; Zhang, Y.; Shi, X.; Li, B.; Li, G.; Liang, W.; Wang, C. Streptomyces Rochei A-1 Induces Resistance and Defense-Related Responses against Botryosphaeria dothidea in Apple Fruit during Storage. Postharvest Biol. Technol. 2016, 115, 30–37. [Google Scholar] [CrossRef]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense Responses, Induced by p-Coumaric Acid and Methyl p-Coumarate, of Jujube (Ziziphus jujuba Mill.) Fruit against Black Spot Rot Caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Cao, J.; Jiang, W.; Zhao, Y. Effects of Preharvest Oligochitosan Sprays on Postharvest Fungal Diseases, Storage Quality, and Defense Responses in Jujube (Zizyphus jujuba Mill. Cv. Dongzao) Fruit. Sci. Hortic. 2012, 142, 196–204. [Google Scholar] [CrossRef]
- Milosevic, N.; Slusarenko, A.J. Active Oxygen Metabolism and Lignification in the Hypersensitive Response in Bean. Physiol. Mol. Plant Pathol. 1996, 49, 143–158. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Wei, M.; Li, X.; Tang, Q.; Duan, B. Effect of Trisodium Phosphate Treatment on Black Spot of Apple Fruit and the Roles of Anti-Oxidative Enzymes. Physiol. Mol. Plant Pathol. 2019, 106, 226–231. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Tian, S.-P.; Xu, Y. Effects of High Oxygen Concentration on Pro- and Anti-Oxidant Enzymes in Peach Fruits during Postharvest Periods. Food Chem. 2005, 91, 99–104. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of Ascorbate Peroxidase in Spinach Chloroplasts; Its Inactivation in Ascorbate-Depleted Medium and Reactivation by Monodehydroascorbate Radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of Quality and Antioxidant Metabolism of Sweet Cherry Fruit by Near-Freezing Temperature Storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of Glutathione Reductase in Crude Tissue Homogenates Using 5,5′-Dithiobis(2-Nitrobenzoic Acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Yuan, S.; Ding, X.; Zhang, Y.; Cao, J.; Jiang, W. Characterization of Defense Responses in the ‘Green Ring’ and ‘Red Ring’ on Jujube Fruit upon Postharvest Infection by Alternaria alternata and the Activation by the Elicitor Treatment. Postharvest Biol. Technol. 2019, 149, 166–176. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The Effect of Temperature on Phenolic Content in Wounded Carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tian, Y.; Wang, T.; Huang, X.; Zhou, L.; Shengming, L.; Chen, G.; Che, Z. Semisynthesis, Anti-Oomycete and Anti-Fungal Activities of Ursolic Acid Ester Derivatives. Nat. Prod. Res. 2024, 38, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Meng, K.; Tian, Z.; Sun, J.; Li, H.; Zhang, Z.; Soloveva, V.; Li, H.; Fu, G.; Xia, Q.; et al. Triterpenoids Manipulate a Broad Range of Virus-Host Fusion via Wrapping the HR2 Domain Prevalent in Viral Envelopes. Sci. Adv. 2018, 4, eaau8408. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid.-Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Romanazzi, G.; Murolo, S.; Feliziani, E. Effects of an Innovative Strategy to Contain Grapevine Bois Noir: Field Treatment with Resistance Inducers. Phytopathology 2013, 103, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Jin, L.; Cai, Y.; Zheng, X.; Yu, T. (1→3)-β-D-Glucan from Yeast Cell Wall: Characteristic and Potential Application in Controlling Postharvest Disease of Pear. Postharvest Biol. Technol. 2019, 154, 105–114. [Google Scholar] [CrossRef]
- Romanazzi, G.; Gabler, F.M.; Smilanick, J.L. Preharvest Chitosan and Postharvest UV Irradiation Treatments Suppress Gray Mold of Table Grapes. Plant Dis. 2006, 90, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Goodman, R.M. Systemic Acquired Resistance and Induced Systemic Resistance in Conventional Agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Conrath, U.; Pieterse, C.M.J.; Mauch-Mani, B. Priming in Plant–Pathogen Interactions. Trends Plant Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef]
- Kumari, M.; Kamat, S.; Dixit, R.; Pandey, S.; Giri, V.P.; Mishra, A. Chapter 14—Microbial Formulation Approaches in Postharvest Disease Management. In Food Security and Plant Disease Management; Kumar, A., Droby, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 279–305. ISBN 978-0-12-821843-3. [Google Scholar]
- Durrant, W.E.; Dong, X. Systemic Acquired Resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, X.; Chen, M.; Fu, Y.; Xiang, M.; Chen, J. Effect of Exogenous Methyl Jasmonate Treatment on Disease Resistance of Postharvest Kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wan, Y.; Qin, G.; Xu, Y. Induction of Defense Responses against Alternaria Rot by Different Elicitors in Harvested Pear Fruit. Appl. Microbiol. Biotechnol. 2006, 70, 729. [Google Scholar] [CrossRef]
- Yang, W.; Guo, M.; Zhang, W.; Cheng, S.; Chen, G. Methyl Salicylate and Methyl Jasmonate Induce Resistance to Alternaria Tenuissima by Regulating the Phenylpropane Metabolism Pathway of Winter Jujube. Postharvest Biol. Technol. 2023, 204, 112440. [Google Scholar] [CrossRef]
- Li, M.; Qu, X.; Gong, D.; Huang, T.; Wang, Y.; Yang, Y.; Gao, Z.; Zhang, Z.; Sun, J.; Hu, M. Induced Resistance to Control Postharvest Stem-End Rot by Methyl Jasmonate in Mango Fruit. Physiol. Mol. Plant Pathol. 2024, 134, 102426. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Liang, L.; Chen, Y.; Chen, J.; Guo, S.; Zhang, X.; Zhao, L.; Zhang, H. Alginate Oligosaccharide Induces Resistance against Penicillium expansum in Pears by Priming Defense Responses. Plant Physiol. Biochem. 2025, 220, 109531. [Google Scholar] [CrossRef]
- Zhuo, R.; Li, B.; Tian, S. Alginate Oligosaccharide Improves Resistance to Postharvest Decay and Quality in Kiwifruit (Actinidia deliciosa Cv. Bruno). Hortic. Plant J. 2022, 8, 44–52. [Google Scholar] [CrossRef]
- Vilanova, L.; Wisniewski, M.; Norelli, J.; Viñas, I.; Torres, R.; Usall, J.; Phillips, J.; Droby, S.; Teixidó, N. Transcriptomic Profiling of Apple in Response to Inoculation with a Pathogen (ss) and a Non-Pathogen (Penicillium digitatum). Plant Mol. Biol. Report. 2014, 32, 566–583. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Its Integration into Metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Saberi Riseh, R.; Fathi, F.; Lagzian, A.; Vatankhah, M.; Kennedy, J.F. Modifying Lignin: A Promising Strategy for Plant Disease Control. Int. J. Biol. Macromol. 2024, 271, 132696. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, C.; Jiao, W.; Cui, K.; Cao, J.; Jiang, W. Ursolic Acid Induces Multifaceted Defense Responses Against Postharvest Blue Mold Rot in Apple Fruit. Foods 2025, 14, 761. https://doi.org/10.3390/foods14050761
Shu C, Jiao W, Cui K, Cao J, Jiang W. Ursolic Acid Induces Multifaceted Defense Responses Against Postharvest Blue Mold Rot in Apple Fruit. Foods. 2025; 14(5):761. https://doi.org/10.3390/foods14050761
Chicago/Turabian StyleShu, Chang, Wenxiao Jiao, Kuanbo Cui, Jiankang Cao, and Weibo Jiang. 2025. "Ursolic Acid Induces Multifaceted Defense Responses Against Postharvest Blue Mold Rot in Apple Fruit" Foods 14, no. 5: 761. https://doi.org/10.3390/foods14050761
APA StyleShu, C., Jiao, W., Cui, K., Cao, J., & Jiang, W. (2025). Ursolic Acid Induces Multifaceted Defense Responses Against Postharvest Blue Mold Rot in Apple Fruit. Foods, 14(5), 761. https://doi.org/10.3390/foods14050761






