Clean Label Approaches in Cheese Production: Where Are We?
Abstract
1. Introduction
1.1. Clean Label: What Is It?
1.2. Clean Label Applied to Cheese
2. Methodology
3. Physical Approaches
3.1. Thermal
3.1.1. Pasteurization
3.1.2. Induced Electric Field
3.1.3. Freezing
3.2. Non-Thermal
3.2.1. High Pressure Processing (HPP)
3.2.2. Pulsed Light (PL), Ultraviolet (UV), and Visible Light (VL)
3.2.3. Pulsed Electric Field (PEF)
3.2.4. Ozone (O3)
3.2.5. Filtration
4. Botanical Approach
4.1. Antimicrobial Action
4.2. Nutritional, Sensorial, and Functional Properties Improvement Potential
5. Microbiological Approaches
5.1. Protective Cultures
5.2. Postbiotics
5.2.1. Metabolites
5.2.2. Bacteriocins
5.3. Bacteriophages
6. By-Products
By-Products of the Dairy Industry
7. Natural and Edible Coatings
8. Clean Label as a Health Promoter
9. Challenges, Risks and Opportunities
9.1. Food Safety and Conservation
9.2. Sensorial Quality
9.3. Shelf-Life
9.4. SWOT Analysis
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reisman, E.; Fairbairn, M. Agri-Food Systems and the Anthropocene. Ann. Am. Assoc. Geogr. 2021, 111, 687–697. [Google Scholar] [CrossRef]
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A Review on the Beneficial Aspects of Food Processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef]
- Barkema, A.; Drabenstott, M.; Cook, M.L. The Industrialization of the U.S. Food System. 1993. Available online: https://ideas.repec.org/p/ags/famc93/265920.html (accessed on 15 November 2024).
- Haen, D. The Paradox of E-Numbers: Ethical, Aesthetic, and Cultural Concerns in the Dutch Discourse on Food Additives. J. Agric. Environ. Ethics 2014, 27, 27–42. [Google Scholar] [CrossRef]
- Van Gunst, A.; Roodenburg, A.J.C. Consumer Distrust about E-Numbers: A Qualitative Study among Food Experts. Foods 2019, 8, 178. [Google Scholar] [CrossRef]
- Rabl, V.A.; Basso, F. When Bad Becomes Worse: Unethical Corporate Behavior May Hamper Consumer Acceptance of Cultured Meat. Sustainability 2021, 13, 6770. [Google Scholar] [CrossRef]
- Pollan, M. Defense of Food: An Eater’s Manifesto; Penguin: London, UK, 2008. [Google Scholar]
- Edwards, A. Natural & Clean Label Trends; Ingredion Inc.: Westchester, IL, USA, 2013. [Google Scholar]
- Ingredion. The Clean Label Guide in Europe. 2014. Available online: https://www.ingredion.com/content/dam/ingredion/pdf-downloads/emea/87%20-%20The%20Clean%20Label%20Guide%20to%20Europe%20from%20Ingredion.pdf (accessed on 18 August 2024).
- Negowetti, N.; Ambwani, S.; Karr, S.; Rodgers, R.F.; Austin, S.B. Digging up the Dirt on “Clean” Dietary Labels: Public Health Considerations and Opportunities for Increased Federal Oversight. Int. J. Eat. Disord. 2022, 55, 39–48. [Google Scholar] [CrossRef]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean Label: Why This Ingredient but Not That One? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Singh, A.K.; Ramakanth, D.; Kumar, A.; Lee, Y.S.; Gaikwad, K.K. Active Packaging Technologies for Clean Label Food Products: A Review. J. Food Meas. Charact. 2021, 15, 4314–4324. [Google Scholar] [CrossRef]
- Curry, A. Archaeology: The Milk Revolution. Nature 2013, 500, 20–22. [Google Scholar] [CrossRef]
- Fox, P.F. Cheese: An Overview. In Cheese: Chemistry, Physics and Microbiology; Springer: Boston, MA, USA, 1993; pp. 1–36. [Google Scholar]
- Roobab, U.; Inam-Ur-Raheem, M.; Khan, A.W.; Arshad, R.N.; Zeng, X.; Aadil, R.M. Innovations in High-Pressure Technologies for the Development of Clean Label Dairy Products: A Review. Food Rev. Int. 2023, 39, 970–991. [Google Scholar] [CrossRef]
- Falih, M.A.; Altemimi, A.B.; Hamed Alkaisy, Q.; Awlqadr, F.H.; Abedelmaksoud, T.G.; Amjadi, S.; Hesarinejad, M.A. Enhancing Safety and Quality in the Global Cheese Industry: A Review of Innovative Preservation Techniques. Heliyon 2024, 10, e40459. [Google Scholar] [CrossRef]
- Biango-Daniels, M.N.; Wolfe, B.E. American Artisan Cheese Quality and Spoilage: A Survey of Cheesemakers’ Concerns and Needs. J. Dairy Sci. 2021, 104, 6283–6294. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A Natural Preservative for Food Applications—A Review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef]
- European Parliament Commission Regulation (UE) No. 1129/2011. Off. J. Eur. Union 2011, 295, 11–12.
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ Growing Appetite for Natural Foods: Perceptions towards the Use of Natural Preservatives in Fresh Fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef] [PubMed]
- Whole Foods Market, Inc. Ingredients We Don’t Allow in Our Food. Available online: http://www.wholefoodsmarket.com/about-our-products/quality-standards/food-ingredient (accessed on 19 December 2024).
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Raw and Heat-Treated Milk: From Public Health Risks to Nutritional Quality. Beverages 2017, 3, 54. [Google Scholar] [CrossRef]
- Macdonald, L.E.; Brett, J.; Kelton, D.; Majowicz, S.E.; Snedeker, K.; Sargeant, J.M. A Systematic Review and Meta-Analysis of the Effects of Pasteurization on Milk Vitamins, and Evidence for Raw Milk Consumption and Other Health-Related Outcomes. J. Food Prot. 2011, 74, 1814–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, F.; Jin, Y.; Wu, S.; Xu, X.; Yang, N. Current Applications and Challenges of Induced Electric Fields for the Treatment of Foods. Food Eng. Rev. 2022, 14, 491–508. [Google Scholar] [CrossRef]
- Zheng, Z.; Jin, Y.; Zhang, L.; Xu, X.; Yang, N. Inactivation of Microorganisms in Foods by Electric Field Processing: A Review. J. Agric. Food Res. 2024, 16, 101109. [Google Scholar] [CrossRef]
- Yang, N.; Zheng, Z.; Jin, Y.; Zhang, L.; Chitrakar, B.; Xu, X. Effect of Induced Electric Field on Microorganism Inactivation, Nutrients and Physicochemical Properties of Milk. Food Biosci. 2024, 58, 103708. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Hogenboom, J.A.; Pellegrino, L. Novel Technologies for Extending the Shelf Life of Drinking Milk: Concepts, Research Trends and Current Applications. LWT 2021, 148, 111746. [Google Scholar] [CrossRef]
- Xiang, H.; Tao, W.; Li, Y.; Mu, W.; Cheng, Y.; Shen, W.; Wang, C.; Guan, L.; Wang, K.; Sun, X.; et al. Effects of Induced Electric Field and High-Pressure Microjet Sterilization on the Physicochemical Properties of Milk. Food Sci. Anim. Prod. 2024, 2, 9240086. [Google Scholar] [CrossRef]
- Alinovi, M.; Mucchetti, G.; Wiking, L.; Corredig, M. Freezing as a Solution to Preserve the Quality of Dairy Products: The Case of Milk, Curds and Cheese. Crit. Rev. Food Sci. Nutr. 2021, 61, 3340–3360. [Google Scholar] [CrossRef] [PubMed]
- Digvijay; Kelly, A.L.; Lamichhane, P. Ice Crystallization and Structural Changes in Cheese during Freezing and Frozen Storage: Implications for Functional Properties. Crit. Rev. Food Sci. Nutr. 2025, 65, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, N.; Canada, J.; Sousa, I. Effect of Freezing on the Rheological, Chemical and Colour Properties of Serpa Cheese. J. Dairy. Res. 2011, 78, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Pax, A.P.; Ong, L.; Pax, R.A.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. Industrial Freezing and Tempering for Optimal Functional Properties in Thawed Mozzarella Cheese. Food Chem. 2023, 405, 134933. [Google Scholar] [CrossRef] [PubMed]
- Kateh, S.; Purnomo, E.H.; Hasanah, U. Meta-analysis: Microbial Inactivation in Milk Using High-pressure Processing (HPP). Int. J. Food Sci. Technol. 2024, 59, 4185–4193. [Google Scholar] [CrossRef]
- Lawrence, I.; Jung, S. HPP as an Innovation Tool for Healthy Foods. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–200. [Google Scholar]
- Ozaybi, N. High-Pressure Processing of Milk and Dairy Products: Latest Update. Processes 2024, 12, 2073. [Google Scholar] [CrossRef]
- Liu, G.; Carøe, C.; Qin, Z.; Munk, D.M.E.; Crafack, M.; Petersen, M.A.; Ahrné, L. Comparative Study on Quality of Whole Milk Processed by High Hydrostatic Pressure or Thermal Pasteurization Treatment. LWT 2020, 127, 109370. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of High Pressure Processing on the Safety, Shelf Life and Quality of Raw Milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Zagorska, J.; Galoburda, R.; Raita, S.; Liepa, M. Inactivation and Recovery of Bacterial Strains, Individually and Mixed, in Milk after High Pressure Processing. Int. Dairy J. 2021, 123, 105147. [Google Scholar] [CrossRef]
- Alpas, H.; Bozoglu, F. The Combined Effect of High Hydrostatic Pressure, Heat and Bacteriocins on Inactivation of Foodborne Pathogens in Milk and Orange Juice. World J. Microbiol. Biotechnol. 2000, 16, 387–392. [Google Scholar] [CrossRef]
- Serna-Hernandez, S.O.; Escobedo-Avellaneda, Z.; García-García, R.; de Rostro-Alanis, M.J.; Welti-Chanes, J. High Hydrostatic Pressure Induced Changes in the Physicochemical and Functional Properties of Milk and Dairy Products: A Review. Foods 2021, 10, 1867. [Google Scholar] [CrossRef]
- Inácio, R.S.; Rodríguez-Alcalá, L.M.; Pimentel, L.L.; Saraiva, J.A.; Gomes, A.M.P. Evolution of Qualitative and Quantitative Lipid Profiles of High-Pressure-Processed Serra Da Estrela Cheese throughout Storage. Appl. Sci. 2023, 13, 5927. [Google Scholar] [CrossRef]
- Delgado-Martínez, F.J.; Carrapiso, A.I.; Contador, R.; Ramírez, M.R. Volatile Compounds and Sensory Changes after High Pressure Processing of Mature “Torta Del Casar” (Raw Ewe’s Milk Cheese) during Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2019, 52, 34–41. [Google Scholar] [CrossRef]
- Nuñez, M.; Calzada, J.; Olmo, A. del High Pressure Processing of Cheese: Lights, Shadows and Prospects. Int. Dairy J. 2020, 100, 104558. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The Efficacy and Safety of High-pressure Processing of Food. EFSA J. 2022, 20, 7128. [Google Scholar] [CrossRef]
- Proulx, J.; Hsu, L.C.; Miller, B.M.; Sullivan, G.; Paradis, K.; Moraru, C.I. Pulsed-Light Inactivation of Pathogenic and Spoilage Bacteria on Cheese Surface. J. Dairy Sci. 2015, 98, 5890–5898. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, F.E.; Plazzotta, S.; Conte, A.; Manzocco, L. Effect of Pulsed Light on Microbial Inactivation, Sensory Properties and Protein Structure of Fresh Ricotta Cheese. LWT 2021, 139, 110556. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Cristianini, M.; Anjos, C.A.R. Packaging Aspects for Processing and Quality of Foods Treated by Pulsed Light. J. Food Process Preserv. 2020, 44, 14902. [Google Scholar] [CrossRef]
- Mandal, R.; Mohammadi, X.; Wiktor, A.; Singh, A.; Pratap Singh, A. Applications of Pulsed Light Decontamination Technology in Food Processing: An Overview. Appl. Sci. 2020, 10, 3606. [Google Scholar] [CrossRef]
- Lacivita, V.; Conte, A.; Lyng, J.G.; Arroyo, C.; Zambrini, V.A.; Del Nobile, M.A. High Intensity Light Pulses to Reduce Microbial Load in Fresh Cheese. J. Dairy Res. 2018, 85, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hospital, X.F.; Arias, K.; Hierro, E. Application of Pulsed Light to Sliced Cheese: Effect on Listeria Inactivation, Sensory Quality and Volatile Profile. Food Bioprocess Technol. 2016, 9, 1335–1344. [Google Scholar] [CrossRef]
- Dos Anjos, C.; Sellera, F.P.; de Freitas, L.M.; Gargano, R.G.; Telles, E.O.; Freitas, R.O.; Baptista, M.S.; Ribeiro, M.S.; Lincopan, N.; Pogliani, F.C.; et al. Inactivation of Milk-Borne Pathogens by Blue Light Exposure. J. Dairy Sci. 2020, 103, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Keklik, N.M.; Elik, A.; Salgin, U.; Demirci, A.; Koçer, G. Inactivation of Staphylococcus aureus and Escherichia coli O157:H7 on Fresh Kashar Cheese with Pulsed Ultraviolet Light. Food Sci. Technol. Int. 2019, 25, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.-H.; Ha, J.-W. The Synergistic Bactericidal Effect of Simultaneous 222 Nm Krypton-Chlorine Excilamp and 307 Nm UVB Light Treatment on Sliced Cheese and Its Mechanisms. Food Microbiol. 2024, 122, 104552. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.-E.; Lee, S.-Y. Antibacterial Effect and Mechanisms of Action of 460–470 nm Light-Emitting Diode against Listeria monocytogenes and Pseudomonas fluorescens on the Surface of Packaged Sliced Cheese. Food Microbiol. 2020, 86, 103314. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet Radiation: An Interesting Technology to Preserve Quality and Safety of Milk and Dairy Foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Delorme, M.M.; Pimentel, T.C.; Freitas, M.Q.; da Cunha, D.T.; Silva, R.; Guimarães, J.T.; Scudino, H.; Esmerino, E.A.; Duarte, M.C.K.H.; Cruz, A.G. Consumer Innovativeness and Perception about Innovative Processing Technologies: A Case Study with Sliced Prato Cheese Processed by Ultraviolet Radiation. Int. J. Dairy Technol. 2021, 74, 768–777. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Barreto Crespo, M.T.; Pereira, V.J. Small but Powerful: Light-Emitting Diodes for Inactivation of Aspergillus Species in Real Water Matrices. Water Res. 2020, 168, 115108. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Lobacz, A.; Tarapata, J.; Zulewska, J. UV Light Application as a Mean for Disinfection Applied in the Dairy Industry. Appl. Sci. 2021, 11, 7285. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-W.; Back, K.-H.; Kim, Y.-H.; Kang, D.-H. Efficacy of UV-C Irradiation for Inactivation of Food-Borne Pathogens on Sliced Cheese Packaged with Different Types and Thicknesses of Plastic Films. Food Microbiol. 2016, 57, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ghasemi, N.; Zare, F.; Duley, J.A.; Cowley, D.M.; Shaw, P.N.; Koorts, P.; Bansal, N. Nanosecond Pulsed Electric Field Treatment of Human Milk: Effects on Microbiological Inactivation, Whey Proteome and Bioactive Protein. Food Chem. 2023, 406, 135073. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Nabilah, U.U.; Sitanggang, A.B.; Dewanti-Hariyadi, R.; Sugiarto, A.T.; Purnomo, E.H. Meta-analysis: Microbial Inactivation in Milk Using Pulsed Electric Field. Int. J. Food Sci. Technol. 2022, 57, 5750–5763. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive Review on Pulsed Electric Field in Food Preservation: Gaps in Current Studies for Potential Future Research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality Aspects and Safety of Pulsed Electric Field (PEF) Processing on Dairy Products: A Comprehensive Review. Food Rev. Int. 2022, 38, 96–117. [Google Scholar] [CrossRef]
- Emanuel, E.; Dubrovin, I.; Pogreb, R.; Pinhasi, G.A.; Cahan, R. Resuscitation of Pulsed Electric Field-Treated Staphylococcus aureus and Pseudomonas putida in a Rich Nutrient Medium. Foods 2021, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Šalaševičius, A.; Uždavinytė, D.; Visockis, M.; Ruzgys, P.; Šatkauskas, S. Effect of Pulsed Electric Field (PEF) on Bacterial Viability and Whey Protein in the Processing of Raw Milk. Appl. Sci. 2021, 11, 11281. [Google Scholar] [CrossRef]
- de Oliveira Souza, S.M.; de Alencar, E.R.; Ribeiro, J.L.; de Aguiar Ferreira, M. Inactivation of Escherichia coli O157:H7 by Ozone in Different Substrates. Braz. J. Microbiol. 2019, 50, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.; Eramo, V.; Lembo, M.; Forniti, R.; Carboni, C.; Botondi, R. Effects of Gaseous Ozone Treatment on the Mite Pest Control and Qualitative Properties during Ripening Storage of Pecorino Cheese. J. Sci. Food Agric. 2023, 103, 2124–2133. [Google Scholar] [CrossRef]
- Botondi, R.; Lembo, M.; Carboni, C.; Eramo, V. The Use of Ozone Technology: An Eco–Friendly Method for the Sanitization of the Dairy Supply Chain. Foods 2023, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of Foodborne Bacteria Biofilms by Aqueous and Gaseous Ozone. Front. Microbiol. 2018, 9, 02024. [Google Scholar] [CrossRef] [PubMed]
- Makardij, A.; Chen, X.D.; Farid, M.M. Microfiltration and Ultrafiltration of Milk. Food Bioprod. Process. 1999, 77, 107–113. [Google Scholar] [CrossRef]
- Head, L.E.; Bird, M.R. The Removal of Psychrotropic Spores from Milk Protein Isolate Feeds Using Tubular Ceramic Microfilters. J. Food Process Eng. 2013, 36, 113–124. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Rodríguez-González, O.; Jayaram, S.; Griffiths, M.W. Microbial Inactivation and Shelf Life Comparison of ‘Cold’ Hurdle Processing with Pulsed Electric Fields and Microfiltration, and Conventional Thermal Pasteurisation in Skim Milk. Int. J. Food Microbiol. 2011, 144, 379–386. [Google Scholar] [CrossRef] [PubMed]
- France, T.C.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. Cold Microfiltration as an Enabler of Sustainable Dairy Protein Ingredient Innovation. Foods 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Dupas, C.; Métoyer, B.; El Hatmi, H.; Adt, I.; Mahgoub, S.A.; Dumas, E. Plants: A Natural Solution to Enhance Raw Milk Cheese Preservation? Food Res. Int. 2020, 130, 108883. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.C.L.P.; Saldanha Nandi, R.D.; Scandorieiro, S.; Gonçalves, M.C.; Reis, G.F.; Dibo, M.; Medeiros, L.P.; Panagio, L.A.; Fagan, E.P.; Takayama Kobayashi, R.K.; et al. Antimicrobial Effect of Origanum vulgare (L.) Essential Oil as an Alternative for Conventional Additives in the Minas Cheese Manufacture. LWT 2022, 157, 113063. [Google Scholar] [CrossRef]
- Schuh, J.; Battisti, P.; Gargetti, A.; Zapparoli, A.; Balsan, T.I.; Gilioli, A.; Zanetti, V.C.; Foralosso, F.B.; Vargas Junior, Á.; Fronza, N.; et al. Basil, Marjoram, Nutmeg and Oregano Essential Oils as Natural Preservatives of Quark-Type Cheese. Food Sci. Technol. 2022, 42, 31322. [Google Scholar] [CrossRef]
- Ávila, M.; Calzada, J.; Muñoz-Tébar, N.; Sánchez, C.; Elguea-Culebras, G.O.; Carmona, M.; Molina, A.; Berruga, M.I.; Garde, S. Inhibitory Activity of Aromatic Plant Extracts against Dairy-Related Clostridium Species and Their Use to Prevent the Late Blowing Defect of Cheese. Food Microbiol. 2023, 110, 104185. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Ciric, A.; Soković, M.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Foeniculum vulgare Mill. as Natural Conservation Enhancer and Health Promoter by Incorporation in Cottage Cheese. J. Funct. Foods 2015, 12, 428–438. [Google Scholar] [CrossRef]
- Saraiva, C.; Silva, A.C.; García-Díez, J.; Cenci-Goga, B.; Grispoldi, L.; Silva, A.F.; Almeida, J.M. Antimicrobial Activity of Myrtus communis L. and Rosmarinus officinalis L. Essential Oils against Listeria monocytogenes in Cheese. Foods 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Hlebová, M.; Foltinová, D.; Vešelényiová, D.; Medo, J.; Šramková, Z.; Tančinová, D.; Mrkvová, M.; Hleba, L. The Vapor Phase of Selected Essential Oils and Their Antifungal Activity In Vitro and In Situ against Penicillium commune, a Common Contaminant of Cheese. Foods 2022, 11, 3517. [Google Scholar] [CrossRef]
- Licon, C.C.; Moro, A.; Librán, C.M.; Molina, A.M.; Zalacain, A.; Berruga, M.I.; Carmona, M. Volatile Transference and Antimicrobial Activity of Cheeses Made with Ewes’ Milk Fortified with Essential Oils. Foods 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Pasha, I.; Jahangir Chughtai, M.F.; Ali, Z.; Bukhari, H.; Zuhair, M. Application of Essential Oils in Food Industry: Challenges and Innovation. J. Essent. Oil Res. 2022, 34, 97–110. [Google Scholar] [CrossRef]
- Garde, S.; Arias, R.; Gaya, P.; Nuñez, M. Occurrence of Clostridium spp. in Ovine Milk and Manchego Cheese with Late Blowing Defect: Identification and Characterization of Isolates. Int. Dairy J. 2011, 21, 272–278. [Google Scholar] [CrossRef]
- Kaya, H.I.; Simsek, O.; Akgunoglu, O. Diversity of Clostridium spp. Causing Late Blowing in Kaşar Cheese and Their Behaviour against Various Antimicrobials. Int. Dairy J. 2023, 139, 105560. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of Essential Oils in Dairy Products: A Review of Their Functions and Potential Benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.; Salama, H.H.; Sayed, R.S. Plant Extracts and Essential Oils in the Dairy Industry: A Review. Foods Raw Mater. 2023, 11, 321–337. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential Application of Herbs and Spices and Their Effects in Functional Dairy Products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef]
- Solhi, P.; Azadmard-Damirchi, S.; Hesari, J.; Hamishehkar, H. Production of the Processed Cheese Containing Tomato Powder and Evaluation of Its Rheological, Chemical and Sensory Characteristics. J. Food Sci. Technol. 2020, 57, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Christaki, S.; Moschakis, T.; Kyriakoudi, A.; Biliaderis, C.G.; Mourtzinos, I. Recent Advances in Plant Essential Oils and Extracts: Delivery Systems and Potential Uses as Preservatives and Antioxidants in Cheese. Trends Food Sci. Technol. 2021, 116, 264–278. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Martins, A.P.L.; Tavaria, F.K.; Dias, J.; Santos, M.T.; Alvarenga, N.; Pintado, M.E. Impact of LAB from Serpa PDO Cheese in Cheese Models: Towards the Development of an Autochthonous Starter Culture. Foods 2023, 12, 701. [Google Scholar] [CrossRef]
- Bintsis, T.; Papademas, P. The Application of Protective Cultures in Cheese: A Review. Fermentation 2024, 10, 117. [Google Scholar] [CrossRef]
- Eren-Vapur, U.; Cinar, A.; Altuntas, S. Protective Culture: Is It a Solution to Improve the Quality of Culture-free White Cheese? J. Food Process Preserv. 2022, 46, 16432. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Viale, S.; Deplano, M.; Mereu, F.; Blažić, M.; Cosentino, S. Inhibitory Effect of Lactiplantibacillus plantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model. Foods 2022, 11, 715. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Zhang, T.; Liu, Y.; Lü, X.; Kuipers, O.P.; Yi, Y. (Meta)Genomics -Assisted Screening of Novel Antibacterial Lactic Acid Bacteria Strains from Traditional Fermented Milk from Western China and Their Bioprotective Effects on Cheese. LWT 2023, 175, 114507. [Google Scholar] [CrossRef]
- Meloni, M.P.; Piras, F.; Siddi, G.; Migoni, M.; Cabras, D.; Cuccu, M.; Nieddu, G.; McAuliffe, O.; De Santis, E.P.L.; Scarano, C. Effect of Commercial and Autochthonous Bioprotective Cultures for Controlling Listeria monocytogenes Contamination of Pecorino Sardo Dolce PDO Cheese. Foods 2023, 12, 3797. [Google Scholar] [CrossRef] [PubMed]
- Suárez, N.; Weckx, S.; Minahk, C.; Hebert, E.M.; Saavedra, L. Metagenomics-Based Approach for Studying and Selecting Bioprotective Strains from the Bacterial Community of Artisanal Cheeses. Int. J. Food Microbiol. 2020, 335, 108894. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Kim, G.-B. Inhibition of Listeria Monocytogenes in Fresh Cheese Using a Bacteriocin-Producing Lactococcus lactis CAU2013 Strain. Food Sci. Anim. Resour. 2022, 42, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Martín, I.; Rodríguez, A.; Córdoba, J.J. Application of Selected Lactic-Acid Bacteria to Control Listeria monocytogenes in Soft-Ripened “Torta Del Casar” Cheese. LWT 2022, 168, 113873. [Google Scholar] [CrossRef]
- Ewida, R.M.; Hasan, W.S.; Elfaruk, M.S.; Alayouni, R.R.; Hammam, A.R.A.; Kamel, D.G. Occurrence of Listeria Spp. in Soft Cheese and Ice Cream: Effect of Probiotic Bifidobacterium Spp. on Survival of Listeria monocytogenes in Soft Cheese. Foods 2022, 11, 3443. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, X.; Li, L.; Liu, D.; Zhao, F.; Liu, Y.; Wu, S.; Lü, X.; Wu, G.; Yi, Y. Bacteriocinogenic Lacticaseibacillus paracasei Strains from Inner Mongolian Fermented Milk Efficiently Control Pathogenic Bacteria in Model Cheddar-like Cheese. Food Biosci. 2024, 57, 103516. [Google Scholar] [CrossRef]
- Ahmed, W.I.; Kamar, A.M.; Hamad, G.M.; Mehany, T.; El-Desoki, W.I.; Ali, E.; Simal-Gandara, J. Biocontrol of Bacillus cereus by Lactobacillus plantarum in Kareish Cheese and Yogurt. LWT 2023, 183, 114946. [Google Scholar] [CrossRef]
- Hassan, H.; St-Gelais, D.; Gomaa, A.; Fliss, I. Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices. Foods 2021, 10, 898. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Alía, A.; Martínez-Blanco, M.; Lozano-Ojalvo, D.; Córdoba, J.J. Control of Listeria monocytogenes Growth and Virulence in a Traditional Soft Cheese Model System Based on Lactic Acid Bacteria and a Whey Protein Hydrolysate with Antimicrobial Activity. Int. J. Food Microbiol. 2022, 361, 109444. [Google Scholar] [CrossRef]
- Callon, C.; Arliguie, C.; Montel, M.-C. Control of Shigatoxin-Producing Escherichia coli in Cheese by Dairy Bacterial Strains. Food Microbiol. 2016, 53, 63–70. [Google Scholar] [CrossRef]
- Lawton, M.R.; Jencarelli, K.G.; Kozak, S.M.; Alcaine, S.D. Short Communication: Evaluation of Commercial Meat Cultures to Inhibit Listeria monocytogenes in a Fresh Cheese Laboratory Model. J. Dairy Sci. 2020, 103, 1269–1275. [Google Scholar] [CrossRef]
- Makki, G.M.; Kozak, S.M.; Jencarelli, K.G.; Alcaine, S.D. Evaluation of the Efficacy of Commercial Protective Cultures to Inhibit Mold and Yeast in Cottage Cheese. J. Dairy Sci. 2021, 104, 2709–2718. [Google Scholar] [CrossRef]
- Rendueles, C.; Duarte, A.C.; Escobedo, S.; Fernández, L.; Rodríguez, A.; García, P.; Martínez, B. Combined Use of Bacteriocins and Bacteriophages as Food Biopreservatives. A Review. Int. J. Food Microbiol. 2022, 368, 109611. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.V.; Martins, E.; Moreira, I.M.F.B.; de Carvalho, A.F. Strategies for the Development of Bioprotective Cultures in Food Preservation. Int. J. Microbiol. 2022, 2022, 6264170. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, M.; Liu, Y.; Gunther, N.W.; Pepe, T.; Anastasio, A.; Qi, P.X.; Tomasula, P.M.; Renye, J.A. Anti-Listerial Activity of Thermophilin 110 and Pediocin in Fermented Milk and Whey. Food Control 2021, 125, 107941. [Google Scholar] [CrossRef]
- Tsuda, H. Production of Reuterin by Lactobacillus coryniformis and Its Antimicrobial Activities. J. Dairy Res. 2023, 90, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Biron, E.; Ben Said, L.; Subirade, M.; Fliss, I. Bacteriocin-Based Synergetic Consortia: A Promising Strategy to Enhance Antimicrobial Activity and Broaden the Spectrum of Inhibition. Microbiol. Spectr. 2022, 10, e0040621. [Google Scholar] [CrossRef]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; del Peláez-Martínez, M.C.; Sacristán-Pérez-Minayo, G.; Gutiérrez-Fernández, Á.J.; de la Torre, A.H. The Effect of the Pediocin PA-1 Produced by Pediococcus Acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish Dry-Fermented Sausages and Frankfurters. Food Control 2010, 21, 679–685. [Google Scholar] [CrossRef]
- Ryan, A.; Patel, P.; O’Connor, P.M.; Ross, R.P.; Hill, C.; Hudson, S.P. Pharmaceutical Design of a Delivery System for the Bacteriocin Lacticin 3147. Drug Deliv. Transl. Res. 2021, 11, 1735–1751. [Google Scholar] [CrossRef]
- El-Ziney, M.G.; Debevere, J.M. The Effect of Reuterin on Listeria monocytogenes and Escherichia coli O157:H7 in Milk and Cottage Cheese. J. Food Prot. 1998, 61, 1275–1280. [Google Scholar] [CrossRef]
- Ávila, M.; Gómez-Torres, N.; Hernández, M.; Garde, S. Inhibitory Activity of Reuterin, Nisin, Lysozyme and Nitrite against Vegetative Cells and Spores of Dairy-Related Clostridium Species. Int. J. Food Microbiol. 2014, 172, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Thangaraj, P.; Kim, J.-H. Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases. Foods 2023, 13, 89. [Google Scholar] [CrossRef]
- Charneco, G.O.; de Waal, P.P.; van Rijswijck, I.M.H.; van Peij, N.N.M.E.; van Sinderen, D.; Mahony, J. Bacteriophages in the Dairy Industry: A Problem Solved? Annu. Rev. Food Sci. Technol. 2023, 14, 367–385. [Google Scholar] [CrossRef]
- Gómez-Galindo, M.; Truchado, P.; Allende, A.; Gil, M.I. Optimization of the Use of a Commercial Phage-Based Product as a Control Strategy of Listeria monocytogenes in the Fresh-Cut Industry. Foods 2023, 12, 3171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, L.; Xiang, P.; Zhang, T.; Peng, L.; Zou, L.; Li, Q. Phages in Fermented Foods: Interactions and Applications. Fermentation 2023, 9, 201. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Rios-Velasco, C.; Acosta-Muñiz, C.H. Incorporation of A511 Bacteriophage in a Whey Protein Isolate-Based Edible Coating for the Control of Listeria monocytogenes in Cheese. Food Packag. Shelf Life 2023, 37, 101095. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Pinto, C.A.; Ferreira, V.; Brandão, T.R.S.; Saraiva, J.M.A.; Castro, S.M.; Teixeira, P. Non-Thermal Approach to Listeria monocytogenes Inactivation in Milk: The Combined Effect of High Pressure, Pediocin PA-1 and Bacteriophage P100. Food Microbiol. 2020, 86, 103315. [Google Scholar] [CrossRef]
- Ávila, M.; Sánchez, C.; Calzada, J.; Mayer, M.J.; Berruga, M.I.; López-Díaz, T.M.; Narbad, A.; Garde, S. Isolation and Characterization of New Bacteriophages Active against Clostridium tyrobutyricum and Their Role in Preventing the Late Blowing Defect of Cheese. Food Res. Int. 2023, 163, 112222. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.; Kim, J.; Ryu, S.; Bai, J. Characterization of KMSP1, a Newly Isolated Virulent Bacteriophage Infecting Staphylococcus aureus, and Its Application to Dairy Products. Int. J. Food Microbiol. 2023, 390, 110119. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; López, A.; Sáez-Orviz, S.; Marcet, I.; García, P.; Rendueles, M.; Díaz, M. Effectiveness of Bacteriophages Incorporated in Gelatine Films against Staphylococcus aureus. Food Control 2021, 121, 107666. [Google Scholar] [CrossRef]
- Wongyoo, R.; Sunthornthummas, S.; Sawaengwong, T.; Surachat, K.; Rangsiruji, A.; Atithep, T.; Sarawaneeyaruk, S.; Doi, K.; Nantavisai, K.; Insian, K.; et al. Isolation of Bacteriophages Specific to Pseudomonas mosselii for Controlling Milk Spoilage. Int. Dairy J. 2023, 145, 105674. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Viñas, I.; Alegre, I. Evaluation of Commercial Anti-Listerial Products for Improvement of Food Safety in Ready-to-Eat Meat and Dairy Products. Antibiotics 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Garde, S.; Landete, J.M.; Calzada, J.; Baker, D.J.; Evans, R.; Narbad, A.; Mayer, M.J.; Ávila, M. Identification, Activity and Delivery of New LysFA67 Endolysin to Target Cheese Spoilage Clostridium tyrobutyricum. Food Microbiol. 2024, 117, 104401. [Google Scholar] [CrossRef]
- Jolicoeur, A.P.; Lemay, M.-L.; Beaubien, E.; Bélanger, J.; Bergeron, C.; Bourque-Leblanc, F.; Doré, L.; Dupuis, M.-È.; Fleury, A.; Garneau, J.E.; et al. Longitudinal Study of Lactococcus Phages in a Canadian Cheese Factory. Appl. Environ. Microbiol. 2023, 89, e0042123. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.L.; Lacorte, G.A.; Isidorio, W.R.; Landgraf, M.; de Melo Franco, B.D.G.; Pinto, U.M.; Hoffmann, C. High Level of Interaction between Phages and Bacteria in an Artisanal Raw Milk Cheese Microbial Community. mSystems 2023, 8, e0056422. [Google Scholar] [CrossRef]
- Marcó, M.B.; Moineau, S.; Quiberoni, A. Bacteriophages and Dairy Fermentations. Bacteriophage 2012, 2, 149–158. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed]
- Gowe, C. Review on Potential Use of Fruit and Vegetables By-Products as a Valuable Source of Natural Food Additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Martins, V.; Rocha, F.; Soković, M.D.; Barata, A.M.; Carvalho, A.M.; Barros, L.; Ferreira, I.C.F.R. Valorisation of Table Tomato Crop By-Products: Phenolic Profiles and in Vitro Antioxidant and Antimicrobial Activities. Food Bioprod. Process. 2020, 124, 307–319. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and Antioxidant Properties of Tomato Processing Byproducts and Their Correlation with the Biochemical Composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; et al. Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants 2021, 10, 306. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are Olive Pomace Powders a Safe Source of Bioactives and Nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of Cheese Whey Using Microbial Fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- León-López, A.; Pérez-Marroquín, X.A.; Estrada-Fernández, A.G.; Campos-Lozada, G.; Morales-Peñaloza, A.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications. Polymers 2022, 14, 1258. [Google Scholar] [CrossRef] [PubMed]
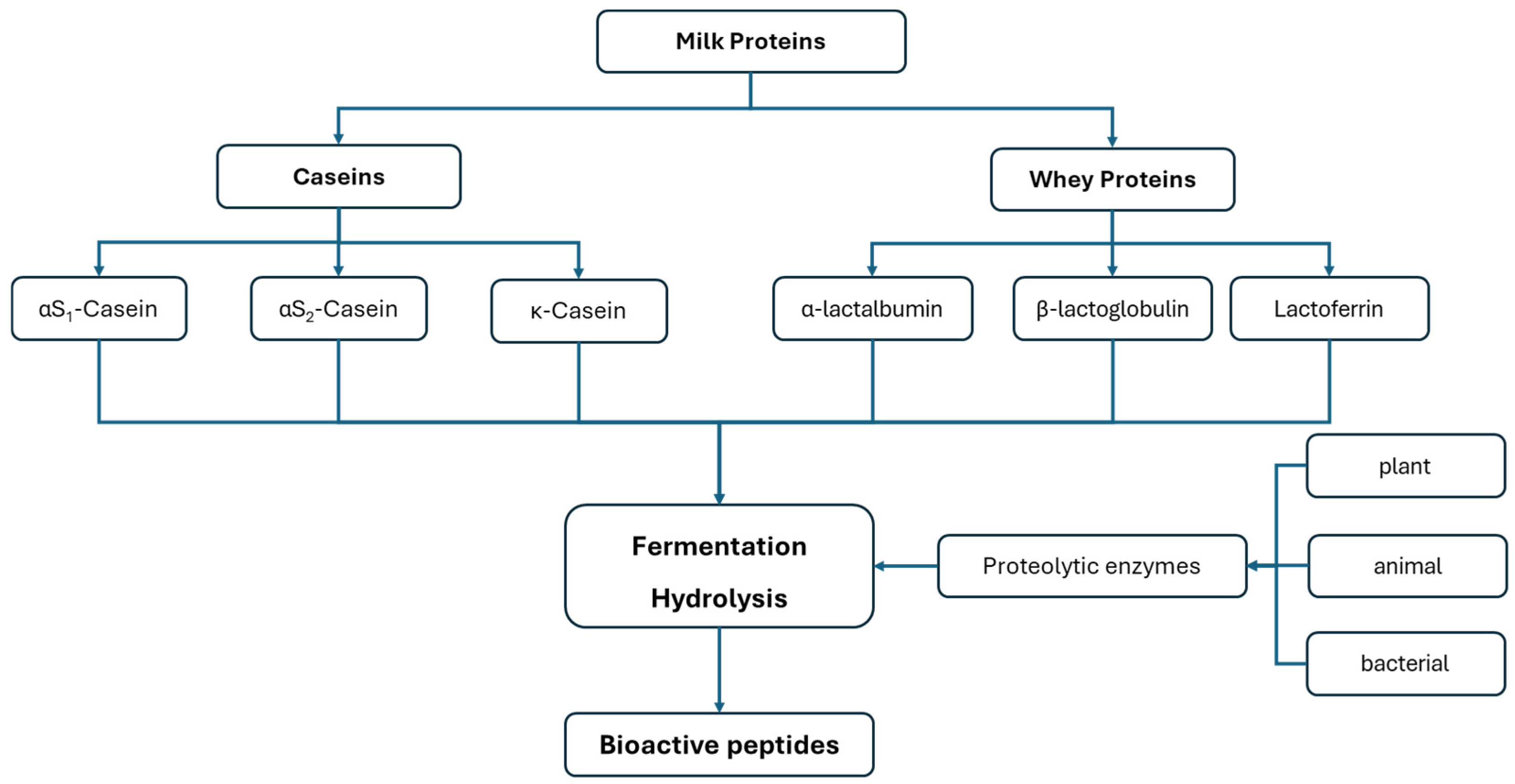
- Madureira, A.R.; Tavares, T.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Invited Review: Physiological Properties of Bioactive Peptides Obtained from Whey Proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Szwajkowska, M.; Teter, A.; Barłowska, J.; Król, J.; Litwińczuk, Z. Bovine Milk Protein as the Source of Bioactive Peptides Influencing the Consumers’ Immune System. Anim. Sci. Pap. Rep. 2011, 29, 269–280. [Google Scholar]
- Lahov, E.; Regelson, W. Antibacterial and Immunostimulating Casein-Derived Substances from Milk: Casecidin, Isracidin Peptides. Food Chem. Toxicol. 1996, 34, 131–145. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Pistillo, B.R.; Caputo, L.; Favia, P.; Baruzzi, F. Bovine Lactoferrin and Lactoferricin on Plasma-Deposited Coating against Spoilage Pseudomonas spp. Innov. Food Sci. Emerg. Technol. 2013, 20, 215–222. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-Derived Antimicrobial Peptides Generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef]
- Alvarenga, N.; Fernandes, J.; Gomes, S.; Baltazar, T.; Fiates, V.; Fidalgo, L.G.; Santos, T.; Conceição, C.; Dias, J. Impact of Different Cynara cardunculus L. Extracts on the Physicochemical, Microbial, and Sensory Properties of Serpa Cheese. Int. Dairy J. 2025, 162, 106159. [Google Scholar] [CrossRef]
- Tavares, T.G.; Malcata, F.X. Whey Proteins as Source of Bioactive Peptides against Hypertension. In Bioactive Food Peptides in Health and Disease; IntechOpen: Rijeka, Croatia, 2013; Volume 75. [Google Scholar]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant Activity of Whey Protein Hydrolysates in Milk Beverage System. J. Food Sci. Technol. 2014, 52, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, A.; Dettling, C.; Thomas, U.; Hunziker, P. Isolation and Characterization of Four Bactericidal Domains in the Bovine β-Lactoglobulin. Biochim. Biophys. Acta (BBA)—General. Subj. 2001, 1526, 131–140. [Google Scholar] [CrossRef]
- Tavares, L.; Souza, H.K.S.; Gonçalves, M.P.; Rocha, C.M.R. Physicochemical and Microstructural Properties of Composite Edible Film Obtained by Complex Coacervation between Chitosan and Whey Protein Isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- Pires, A.F.; Díaz, O.; Cobos, A.; Pereira, C.D. A Review of Recent Developments in Edible Films and Coatings-Focus on Whey-Based Materials. Foods 2024, 13, 2638. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Fu, Y.; Dudley, E.G. Antimicrobial-coated Films as Food Packaging: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar] [CrossRef]
- Alipour, A.; Rahaiee, S.; Rajaei Litkohi, H.; Jamali, S.N.; Jafari, S.M. Development and Optimization of Whey Protein- Lepidium Perfoliatum Gum Packaging Films: An Approach towards Antimicrobial and Biodegradable Films. Ind. Crops Prod. 2023, 196, 116447. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of Whey Protein Edible Films Containing Plant Essential Oils on Microbial Inactivation of Sliced Kasar Cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Sogut, E.; Filiz, B.E.; Seydim, A.C. Whey Protein Isolate- and Carrageenan-Based Edible Films as Carriers of Different Probiotic Bacteria. J. Dairy Sci. 2022, 105, 4829–4842. [Google Scholar] [CrossRef]
- Odila Pereira, J.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible Films as Carrier for Lactic Acid Bacteria. LWT 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Pereira, C.D.; Varytskaya, H.; Łydzińska, O.; Szkolnicka, K.; Gomes, D.; Pires, A. Effect of Sheep’s Whey Edible Coatings with a Bioprotective Culture, Kombucha Tea or Oregano Essential Oil on Cheese Characteristics. Foods 2024, 13, 4132. [Google Scholar] [CrossRef]
- Ramazanidoroh, F.; Hosseininezhad, M.; Shahrampour, D.; Wu, X. Edible Packaging as a Functional Carrier of Prebiotics, Probiotics, and Postbiotics to Boost Food Safety, Quality, and Shelf Life. Probiotics Antimicrob. Proteins 2024, 16, 1327–1347. [Google Scholar] [CrossRef]
- Sharafi, H.; Divsalar, E.; Rezaei, Z.; Liu, S.-Q.; Moradi, M. The Potential of Postbiotics as a Novel Approach in Food Packaging and Biopreservation: A Systematic Review of the Latest Developments. Crit. Rev. Food Sci. Nutr. 2024, 64, 12524–12554. [Google Scholar] [CrossRef]
- Das, P.P.; Prathapan, R.; Ng, K.W. Advances in Biomaterials Based Food Packaging Systems: Current Status and the Way Forward. Biomater. Adv. 2024, 164, 213988. [Google Scholar] [CrossRef] [PubMed]
- Banasaz, S.; Ferraro, V. Keratin from Animal By-Products: Structure, Characterization, Extraction and Application—A Review. Polymers 2024, 16, 1999. [Google Scholar] [CrossRef] [PubMed]
- Bora, R.; Chutia, H.; Monika; Changmai, M.; Mahanta, C.L.; Katiyar, V.; Ghosh, T. Natural Fiber–Based Composite for Food Packaging. In Agro-Waste Derived Biopolymers and Biocomposites; Wiley: Hoboken, NJ, USA, 2024; pp. 33–68. [Google Scholar]
- Nikoo, M.; Gavlighi, H.A. Natural Antioxidants and Flavorings for Clean Label Foods. In The Age of Clean Label Foods; Springer International Publishing: Cham, Switzerland, 2022; pp. 73–102. [Google Scholar]
- Hjerpsted, J.; Tholstrup, T. Cheese and Cardiovascular Disease Risk: A Review of the Evidence and Discussion of Possible Mechanisms. Crit. Rev. Food Sci. Nutr. 2016, 56, 1389–1403. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.W.; Dhaun, N.; Bailey, M.A. The Impact of Excessive Salt Intake on Human Health. Nat. Rev. Nephrol. 2022, 18, 321–335. [Google Scholar] [CrossRef]
- Khan, A.W.; Roobab, U.; Wang, Z.; Raza, M.M.; Nawazish, H.; Islam, F.; Aadil, R.M. Salt Reduction in Food Products: A Systematic Review of Clean-Label Ingredients and Non-Thermal Technologies. Trends Food Sci. Technol. 2024, 153, 104695. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S.; Baloda, S.; Singh, N.; Singh, S.; Bhuker, A.; Singh, P.; Yashveer, S.; et al. Identification and Detection of Bioactive Peptides in Milk and Dairy Products: Remarks about Agro-Foods. Molecules 2020, 25, 3328. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q.; Mársico, E.T.; Campelo, P.H.; Pimentel, T.C.; Cristina Silva, M.; et al. Probiotic Fermented Whey-Milk Beverages: Effect of Different Probiotic Strains on the Physicochemical Characteristics, Biological Activity, and Bioactive Peptides. Food Res. Int. 2023, 164, 112396. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Batista, P.; Gomes, J.E.G.; da Silva, R.; Pintado, M.M. Screening of Novel Bioactive Peptides from Goat Casein: In Silico to In Vitro Validation. Int. J. Mol. Sci. 2022, 23, 2439. [Google Scholar] [CrossRef]
- Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides. Nutrients 2022, 14, 3001. [Google Scholar] [CrossRef]
- Islam, M.Z.; Jahan, N.; Liza, R.I.; Sojib, M.S.I.; Hasan, M.S.; Ferdous, T.; Islam, M.A.; Rashid, M.H. Newly Characterized Lactiplantibacillus plantarum Strains Isolated from Raw Goat Milk as Probiotic Cultures with Potent Cholesterol-Lowering Activity. J. Agric. Food Res. 2022, 10, 100427. [Google Scholar] [CrossRef]
- Verraes, C.; Vlaemynck, G.; Van Weyenberg, S.; De Zutter, L.; Daube, G.; Sindic, M.; Uyttendaele, M.; Herman, L. A Review of the Microbiological Hazards of Dairy Products Made from Raw Milk. Int. Dairy J. 2015, 50, 32–44. [Google Scholar] [CrossRef]
- EFSA Official European Union Website. EFSA Foodborne Outbreaks—Dashboard. Available online: https://www.efsa.europa.eu/en/microstrategy/FBO-dashboard (accessed on 5 October 2024).
- Mohapatra, R.K.; Mishra, S.; Tuglo, L.S.; Sarangi, A.K.; Kandi, V.; AL Ibrahim, A.A.; Alsaif, H.A.; Rabaan, A.A.; Zahan, M.K. Recurring Food Source-based Listeria Outbreaks in the United States: An Unsolved Puzzle of Concern? Health Sci. Rep. 2024, 7, e1863. [Google Scholar] [CrossRef] [PubMed]
- Nájera, A.I.; Nieto, S.; Barron, L.J.R.; Albisu, M. A Review of the Preservation of Hard and Semi-Hard Cheeses: Quality and Safety. Int. J. Environ. Res. Public Health 2021, 18, 9789. [Google Scholar] [CrossRef] [PubMed]
- Mileriene, J.; Serniene, L.; Kondrotiene, K.; Lauciene, L.; Kasetiene, N.; Sekmokiene, D.; Andruleviciute, V.; Malakauskas, M. Quality and Nutritional Characteristics of Traditional Curd Cheese Enriched with Thermo-coagulated Acid Whey Protein and Indigenous Lactococcus lactis Strain. Int. J. Food Sci. Technol. 2021, 56, 2853–2863. [Google Scholar] [CrossRef]
- Song, X.; Zheng, Y.; Zhou, X.; Deng, Y. Quark Cheese Processed by Dense-Phase Carbon Dioxide: Shelf-Life Evaluation and Physiochemical, Rheological, Microstructural and Volatile Properties Assessment. Foods 2022, 11, 2340. [Google Scholar] [CrossRef]
- Choi, D.; Bedale, W.; Chetty, S.; Yu, J. Comprehensive Review of Clean-label Antimicrobials Used in Dairy Products. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13263. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, T.; Le Loir, Y.; Jeantet, R. Breakthrough Innovations in Industrial Cheesemaking Processes. Curr. Opin. Food Sci. 2025, 62, 101267. [Google Scholar] [CrossRef]
- Cao, Y.; Miao, L. Consumer Perception of Clean Food Labels. Br. Food J. 2023, 125, 433–448. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Dove, M.; Balasubramanian, A.; Narayanan, B.G. Transparency as a Way of Attaining Quality, Safety and Optimal Food Purchases. SocioEcon. Chall. 2020, 4, 48–62. [Google Scholar] [CrossRef]
- Grant, K.R.; Gallardo, R.K.; McCluskey, J.J. Consumer Preferences for Foods with Clean Labels and New Food Technologies. Agribusiness 2021, 37, 764–781. [Google Scholar] [CrossRef]
- Uddin, A.; Gallardo, R.K. Consumers’ Willingness to Pay for Organic, Clean Label, and Processed with a New Food Technology: An Application to Ready Meals. Int. Food Agribus. Manag. Rev. 2021, 24, 563–580. [Google Scholar] [CrossRef]
- Jamaluddin, F.; Noranizan, M.A.; Mohamad Azman, E.; Mohamad, A.; Yusof, N.L.; Sulaiman, A. A Review of Clean-Label Approaches to Chilli Paste Processing. Int. J. Food Sci. Technol. 2022, 57, 763–773. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Garric, G.; Harel-Oger, M.; Chatel, M.; Lê, S.; Mounier, J.; Valence, F.; Coton, E. Antifungal Activity of Lactic Acid Bacteria Combinations in Dairy Mimicking Models and Their Potential as Bioprotective Cultures in Pilot Scale Applications. Front. Microbiol. 2018, 9, 01787. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.; Sharma, M.; Inbaraj, B.S. Functional Clean-label Starch: Sustainable Production Technologies and Food Applications. Starch-Stärke 2024, 76, 2400157. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Hurley, R.A. Graduate Student Literature Review: A Systematic Review of Articles Influencing United States Retail Cheese Packaging, Labeling, and Market Trends Related to Cheese in the Marketplace and during Consumption. J. Dairy Sci. 2024, 107, 10244–10255. [Google Scholar] [CrossRef]
- Jadhav, S.P.; Shah, U.B.; Shelke, K. Current Facts about Clean Label Food Products. In Food Intolerances; CRC Press: Boca Raton, MA, USA, 2024; pp. 162–200. [Google Scholar]
- Oštarić, F.; Antunac, N.; Cubric-Curik, V.; Curik, I.; Jurić, S.; Kazazić, S.; Kiš, M.; Vinceković, M.; Zdolec, N.; Špoljarić, J.; et al. Challenging Sustainable and Innovative Technologies in Cheese Production: A Review. Processes 2022, 10, 529. [Google Scholar] [CrossRef]
- Shekhar, C. One Health Approach to Sustainable Dairy Production, Dairy Food Safety and Security. In The Microbiology, Pathogenesis and Zoonosis of Milk Borne Diseases; Elsevier: Amsterdam, The Netherlands, 2024; pp. 421–441. [Google Scholar]
- Yu, M.; Watson, S. Market Overview of Health and Wellness Food Products. In Flavor-Associated Applications in Health and Wellness Food Products; Springer International Publishing: Cham, Switzerland, 2024; pp. 3–19. [Google Scholar]
- Malak-Rawlikowska, A.; Majewski, E.; Arfini, F.; Bellassen, V. Food Labelling as a Way to Address Trust and Information Asymmetry Issues in Premium Cheese Supply Chains. In Trust and Supply Chains; Routledge: New York, NY, USA, 2024; pp. 129–145. [Google Scholar]
- de Freitas Netto, S.V.; Sobral, M.F.F.; Ribeiro, A.R.B.; Soares, G.R.d.L. Concepts and Forms of Greenwashing: A Systematic Review. Environ. Sci. Eur. 2020, 32, 19. [Google Scholar] [CrossRef]


| Target | Treatment | Effect | Ref. |
|---|---|---|---|
| Escherichia coli | 600 MPa, 5 min, 40 °C | −3.00 log CFU·mL−1 | [36] |
| 600 MPa, 5 min, 25 °C | −6.80 log CFU·mL−1 | [37] | |
| 250 MPa, 10 min, 25 °C | −6.39 log CFU·mL−1 | [33] | |
| Listeria monocytogenes | 600 MPa, 10 min, 25 °C | −5.91 log CFU·mL−1 | [37] |
| Pseudomonas spp. | 600 MPa, 3 min, 25 °C | Total elimination | [37] |
| Staphylococcus aureus | 600 MPa, 25 min, 25 °C | −4.70 log CFU·mL−1 | [38] |
| Strain: 765 | 345 MPa, 5 min, 50 °C | >−8.0 CFU·mL−1 | [39] |
| Target | Matrix | Treatment | Effect | Ref. |
|---|---|---|---|---|
| Enterobacteriaceae | Mozzarella fresh | PL, 7.02 J·cm−2, 2 s | Total elimination | [49] |
| Fresh ricotta | PL, 1.03 J·cm−2, 5 s | Delaying spoilage | [46] | |
| Escherichia coli | Milk | VL, 413 nm, 720 J·cm−2, 2 h | >−5 log | [51] |
| Strain: O157:H7 | Fresh kashar | UV, 100–11,000 nm, 45 s | −3.02 log CFU·cm−2 | [52] |
| Listeria spp. | Gouda, slice | PL, 0.90 J·cm−2, 5 s | −3 log CFU·cm−2 | [50] |
| PL, 12.0 J·cm−2, 3 pulses·s−1, 360 µs | −3.37 log CFU·cm−2 | [45] | ||
| Listeria monocytogenes | Sliced | UV, 222 and 307 nm, 80 s | −3.20 log CFU·g−1 | [53] |
| Packaged | VL, 460–470 nm, 4 d | Total elimination | [54] | |
| Pseudomonas spp. | Fresh ricotta | PL, 3.10 J·cm−2, 5 s | Delaying spoilage | [46] |
| Pseudomonas fluorescens | Mozzarella fresh | PL, 7.02 J·cm−2, 2 s | Total elimination | [49] |
| Packaged | VL, 460–470 nm, 4 d | Total elimination | [54] | |
| Pseudomonas aeruginosa | Milk | VL, 413 nm, 720 J·cm−2, 2 h | >−5 log | [51] |
| Salmonella typhimurium | Sliced | UV, 222 and 307 nm, 80 s | −3.50 log CFU·g−1 | [53] |
| Milk | VL, 413 nm, 720 J·cm−2, 2 h | >−5 log | [51] | |
| Staphylococcus aureus | Milk | VL, 413 nm, 720 J·cm−2, 2 h | >−5 log | [51] |
| Fresh kashar | UV, 100–11,000 nm, 45 s | −1.62 log CFU·cm−2 | [52] |
| Target | Botanical Species | 1 | 2 | 3 | Dose | Result | Ref. |
|---|---|---|---|---|---|---|---|
| Aspergillus flavus | Oregano (Origanum vulgare L.) | EO | milk | R | 0.02% (V/V) | Inhibited (15 days) | [79] |
| Bacillus cereus | Basil (Ocimum basilicum L.) | EO | curd | F | MIC: 0.08 mg·mL−1 | [80] | |
| Marjoram (Origanum manjerona L.) | EO | curd | F | MIC: 0.31 mg·mL−1 | [80] | ||
| Oregano (Origanum vulgare L.) | EO | curd | F | MIC: 0.08 mg·mL−1 | [80] | ||
| Clostridium spp. | Oregano (Origanum vulgare L.) | EE | milk | R | MIC: 35.2 μL·mL−1 | [81] | |
| EO | milk | R | MIC: 0.06 mg·mL−1 | [81] | |||
| Clostridium beijerinckii | Marjoram (Origanum majorana L.) | EO | milk | R | MIC: 0.04 mg·mL−1 | [81] | |
| Savory (Satureja montana L.) | EO | milk | R | MIC: 0.12 mg·mL−1 | [81] | ||
| Clostridium. tyrobutyricum | Hyssop (Hyssopus officinalis L.) | EE | milk | R | MIC: 23.4 μL·mL−1 | [81] | |
| Spanish Lavender (Lavandula stoechas L.) | EE | milk | R | MIC: 31.3 μL·mL−1 | [81] | ||
| Marjoram (Origanum majorana L.) | EE | milk | R | MIC: 125 μL·mL−1 | [81] | ||
| Savory (Satureja montana L.) | EE | milk | R | MIC: 11.7.6 μL·mL−1 | [81] | ||
| Tarragon (Artemisia dracunculus L.) | EE | milk | R | MIC: 15.6 μL·mL−1 | [81] | ||
| Escherichia coli | Oregano (Origanum vulgare L.) | EO | milk | R | 0.02% (V/V) | Elimination after 3 days | [80] |
| Fennel (Foeniculum vulgare Mill.) | D | curd | F | MIC: 1.00 mg·mL−1 | [82] | ||
| Marjoram (Origanum majorana L.) | EO | curd | R | MIC: 1.25 mg·mL−1 | [80] | ||
| Basil (Ocimum basilicum L.) | EO | curd | F | MIC: 0.075 mg·mL−1 | [80] | ||
| Listeria monocytogenes | Myrtle (Myrtus communis L.) | EO | milk | R | MIC: 31.25 μL·mL−1 | 1–2 log CFU/g reduction relative to control | [83] |
| Basil (Ocimum basilicum L.) | EO | curd | F | MIC: 1.25 mg·mL−1 | [80] | ||
| Rosemary (Rosmarinus officinalis L.) | EO | milk | R | MIC: 0.40 μL·mL−1 | 1–2 log CFU/g reduction relative to control | [83] | |
| Oregano (Origanum vulgare L.) | EO | curd | F | MIC: 0.62 mg·mL−1 | [80] | ||
| Marjoram (Origanum majorana L.) | EO | curd | F | MIC: 2.5 mg·mL−1 | [80] | ||
| Penicillium sp. | Caraway (Carum carvi L.) | EO | closed atmosphere | R | MIC: 0.250 μL·mL−1 | [84] | |
| Fennel (Foeniculum vulgare Mill.) | D | curd | F | MIC: 0.40 mg·mL−1 | [82] | ||
| Litsea (Litsea cubeba Lour. Per.) | EO | closed atmosphere | R | MIC: 0.016 μL·mL−1 | [84] | ||
| Marjoram (Origanum majorana L.) | EO | closed atmosphere | R | MIC: 0.250 μL·mL−1 | [84] | ||
| Thyme (Thymus vulgaris L.) | EO | closed atmosphere | R | MIC: 0.063 μL·mL−1 | [84] | ||
| Red thyme (Thymus serpyllum L.) | EO | closed atmosphere | R | MIC: 0.125 μL·mL−1 | [84] | ||
| Penicillium verrucosum | Thyme (Thymus vulgaris L.) | EO | milk | R | 0.025 mg·g−1 | Total inhibition (4 months) | [85] |
| Staphylococcus aureus | Oregano (Origanum vulgare L.) | EO | milk | R | 0.02% (V/V) | −107 CFU·g−1 after 3 h | [79] |
| EO | curd | F | MIC: 0.60 mg·mL−1 | [80] | |||
| EO | milk | F | 0.01% (V/V) | Total elimination after 7 days | [86] | ||
| Ginger (Zingiber officinale Roscoe) | EO | milk | F | 0.01% (V/V) | Total elimination after 14 days | [86] | |
| Basil (Ocimum basilicum L.) | EO | curd | R | MIC: 0.075 mg·mL−1 | [80] | ||
| Marjoram (Origanum manjerona L.) | EO | curd | F | MIC: 1.25 mg·mL−1 | [80] |
| Target | Protective Culture | Action | Ref. |
|---|---|---|---|
| Listeria monocytogenes | Lactococcus lactis, Lactiplantibacillus plantarum | Lowered counts by 3–4 log units compared to the control | [97] |
| Companilactobacillus crustorum; Lactiplantibacillus plantarum; Limosilactobacillus fermentum | −0.52 log units units compared to the control | [98] | |
| Lactobacillus delbruekki ssp. sunkii | −0.3–1.8 log CFU·g−1, non-detectable after 90 days of ripening | [99] | |
| Enterococcus faecium CRL1879 | Undetectable up to 30 days of ripening with initial inoculation of 103 CFU·mL−1 without organoleptic changes | [100] | |
| Lactococcus lactis CAU2013 | Reduce the growth by 1 log unit | [101] | |
| Lacticaseibacillus casei 116; Lactococcus garvieae 151 | −3.57 log CFU·g−1 after 90 days of ripening | [102] | |
| Bifidobacterium breve; Bifidobacterium animalis | −10.29 log CFU·g−1 after 21 days | [103] | |
| Bacillus cereus | Lacticaseibacillus paracasei | Decrease the counts in Kareish cheese | [104] |
| Lactiplantibacillus plantarum | In vitro IZD: 3.2 ± 0.61 mm | [105] | |
| Clostridium tyrobutyricum | Lactococcus lactis spp. lactis 32 | −0.6 log units units compared to the control | [106] |
| Target | Bacteriocin | Action | Ref. |
|---|---|---|---|
| Listeria ivanovii | Pediocin PA-1 | MIC: 0.09 μg·mL−1 | [118] |
| Reuterin | MIC: 250 μg·mL−1 | ||
| Nisin | MIC: 1.56 μg·mL−1 | ||
| Listeria monocytogenes | Pediocin | −2 log, 30 d, 4 °C | [119] |
| >−4 log CFU·mL−1 | [116] | ||
| Thermophilin 110 | ≥640 AU·mL−1, inhibited growth | [116] | |
| Lacticin 3147 | MIC: 0.99 μg·mL−1 | [120] | |
| Reuterin | 150 units·g−1, 3 d, −4.8 log CFU·mL−1 | [121] | |
| Clostridium tyrobutyricum | Reuterin | MIC: 4.06 mM | [122] |
| Nisin | MIC: 6.25 μg·mL−1 | [122] | |
| Salmonella enterica | Reuterin | MIC: 125 μg·mL−1 | [118] |
| Escherichia coli O157:H7 | Reuterin | 150 units·g−1, 7 d, undetectable | [121] |
| Target | Phage | Action | Note | Ref. |
|---|---|---|---|---|
| Listeria monocytogenes | A511 | Bacterial counts reduced 0.86 log CFU·g−1 | In a whey protein isolate-based edible coating | [127] |
| P100 | Eliminated when inoculated with levels of 104 CFU·mL−1 | Combined effect with HPP | [128] | |
| Clostridium tyrobutyricum | FA67 | Late blowing defect on day 14 of ripening | [129] | |
| Staphylococcus aureus | KMSP1 | −8.8 CFU·mL−1 in milk −4.3 CFU·cm−2 in sliced cheddar | [130] | |
| phiIPLA-RODI | Reduction and control | Gelatine films remained | [131] | |
| Pseudomonas mosselii | ΦC106 Φ21A | Total elimination in milk | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, J.; Gomes, S.; Reboredo, F.H.; Pintado, M.E.; Amaral, O.; Dias, J.; Alvarenga, N. Clean Label Approaches in Cheese Production: Where Are We? Foods 2025, 14, 805. https://doi.org/10.3390/foods14050805
Fernandes J, Gomes S, Reboredo FH, Pintado ME, Amaral O, Dias J, Alvarenga N. Clean Label Approaches in Cheese Production: Where Are We? Foods. 2025; 14(5):805. https://doi.org/10.3390/foods14050805
Chicago/Turabian StyleFernandes, Jaime, Sandra Gomes, Fernando H. Reboredo, Manuela E. Pintado, Olga Amaral, João Dias, and Nuno Alvarenga. 2025. "Clean Label Approaches in Cheese Production: Where Are We?" Foods 14, no. 5: 805. https://doi.org/10.3390/foods14050805
APA StyleFernandes, J., Gomes, S., Reboredo, F. H., Pintado, M. E., Amaral, O., Dias, J., & Alvarenga, N. (2025). Clean Label Approaches in Cheese Production: Where Are We? Foods, 14(5), 805. https://doi.org/10.3390/foods14050805











