The Overall Quality Changes of Chinese Sauced Ducks at Different Stages During Processing and Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sauced Duck Preparation
2.3. Experimental Group Design
2.4. Determination of Edible Indexes
2.5. Fatty Acid Analysis
2.6. Sensory Evaluation
2.7. Microbial Community Analysis
2.8. Gas Chromatography–Ion Mobility Spectrometry Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Physical Changes of Samples Across Different Stages Were Analyzed
3.2. The Microbial Biomarkers of Samples Were Identified at Different Stages
3.3. The Quantitative and Qualitative Analyses of Flavor Compounds of Samples at Different Stages Were Performed
3.4. The Biomarker 3-Carene Was Clarified Through Correlation Analysis
3.5. The Potential Chemical Reactions Were Predicted Based on the Previous Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | amino acid |
| ANOSIM | analysis of similarities |
| CTAB/SDS | cetyltrimethylammonium bromide/sodium docecyl sulfate-based |
| FA | free fatty acid |
| GC-IMS | gas chromatography–ion mobility spectrometry |
| GC-MS | gas chromatography–mass spectrometry |
| HCl | hydrochloric acid |
| LDA | linear discriminant analysis |
| MDA | malondialdehyde |
| MgO | magnesium oxide |
| MRPP | multi-response permutation procedure |
| MUFA | mono-unsaturated fatty acid |
| NaCl | sodium chloride |
| PCA | principal component analysis |
| PCoA | principal coordinate analysis |
| PLS-DA | partial least squares–discriminant analysis |
| PUFA | poly-unsaturated fatty acid |
| SFA | saturated fatty acid |
| Stage A | samples collected after marinating and before cooking |
| Stage B | samples collected after cooking and before drying |
| Stage C | samples collected after drying and before storage |
| Stage D | samples stored after 5 d |
| Stage E | samples stored after 10 d |
| Stage F | samples stored after 15 d |
| TBARS | thiobarbituric acid reactive substance |
| TPC | total plate count |
| T-VBN | total volatile basic nitrogen |
| UPGMA | unweighted pair group method with arithmetic mean |
| UFA | unsaturated fatty acid |
References
- Lyu, W.; Yang, H.; Li, N.; Lu, L.; Yang, C.; Jin, P.; Xiao, Y. Molecular characterization, developmental expression, and modulation of occludin by early intervention with Clostridium butyricum in Muscovy ducks. Poultry Sci. 2021, 100, 101271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, J.; Wang, S.; Yu, X.; Zhang, Q.; Zhu, C. Influence of heating temperatures and storage on the odor of duck meat and identification of characteristic odorous smell compounds. Food Chem. X 2024, 21, 101242. [Google Scholar] [CrossRef]
- Arshad, M.S.; Kwon, J.H.; Ahmad, R.S.; Ameer, K.; Ahmad, S.; Jo, Y. Influence of e-beam irradiation on microbiological and physicochemical properties and fatty acid profile of frozen duck meat. Food Sci. Nutr. 2020, 8, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, J.; Wu, Y.; Wang, S.; Chen, Y. A possible systematic culinary approach for spent duck meat: Sous-vide cuisine and its optimal cooking condition. Poultry Sci. 2023, 102, 102636. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiao, Y.; Hu, D.; Liu, J.; Chen, W.; Ren, D. The safety evaluation of chilled pork from online platform in China. Food Control 2019, 96, 244–250. [Google Scholar] [CrossRef]
- Luo, Q.; Shi, R.; Liu, Y.; Huang, L.; Chen, W.; Wang, C. Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice. Int. J. Mol. Sci. 2022, 23, 3710. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Xu, L.; Gu, T.; Sun, Y.; Xia, Q.; He, J.; Pan, D.; Lu, L. Investigation into the characteristic volatile flavor of old duck. Food Chem. X 2023, 20, 100899. [Google Scholar] [CrossRef]
- Xu, L.; He, J.; Duan, M.; Chang, Y.; Gu, T.; Tian, Y.; Cai, Z.; Zeng, T.; Lu, L. Effects of lactic acid bacteria-derived fermented feed on the taste and quality of duck meat. Food Res. Int. 2023, 174, 113679. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lyu, W.; Zeng, T.; Wang, W.; Chen, Q.; Zhao, J.; Zhang, G.; Lu, L.; Yang, H.; Xiao, Y. Duck gut metagenome reveals the microbiome signatures linked to intestinal regional, temporal development, and rearing condition. iMeta 2024, 3, e198. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Dzieciol, M.; Pinior, B.; Neubauer, V.; Metzler-Zebeli, B.U.; Wagner, M.; Schmitz-Esser, S. High diversity of viable bacteria isolated from lymph nodes of slaughter pigs and its possible impacts for food safety. J. Appl. Microbiol. 2015, 119, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Frimpong, E.B.; Ye, K.; Li, C.; Zhou, G. Assessment of quality characteristics and bacterial community of modified atmosphere packaged chilled pork loins using 16S rRNA amplicon sequencing analysis. Food Res. Int. 2021, 145, 110412. [Google Scholar] [CrossRef] [PubMed]
- Senapati, M.; Sahu, P.P. Meat quality assessment using Au patch electrode Ag-SnO2/SiO2/Si MIS capacitive gas sensor at room temperature. Food Chem. 2020, 324, 126893. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, K.; Ren, D.; Xiao, Y. Prevalence and characterization of Salmonella from meat in slaughterhouses in Hangzhou, China. Int. J. Food Microbiol. 2022, 371, 109649. [Google Scholar] [CrossRef]
- Zhou, H.; Cui, W.; Gao, Y.; Li, P.; Pu, X.; Wang, Y.; Wang, Z.; Xu, B. Analysis of the volatile compounds in Fuliji roast chicken during processing and storage based on GC-IMS. Curr. Res. Food Sci. 2022, 5, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, X.; Huang, A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef]
- Khan, M.A.; Ali, S.; Abid, M.; Cao, J.; Jabbar, S.; Tume, R.K.; Zhou, G. Improved duck meat quality by application of high pressure and heat: A study of water mobility and compartmentalization, protein denaturation and textural properties. Food Res. Int. 2014, 62, 926–933. [Google Scholar] [CrossRef]
- Geeraerts, W.; De Vuyst, L.; Leroy, F. Mapping the dominant microbial species diversity at expiration date of raw meat and processed meats from equine origin, an underexplored meat ecosystem, in the Belgian retail. Int. J. Food Microbiol. 2019, 289, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. LWT 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, C.; Cao, Y.; Zhao, Y.; Lu, S.; Wu, D.; Guan, R. Improving quality of sea buckthorn juice by high-pressure processing. LWT 2023, 185, 115149. [Google Scholar] [CrossRef]
- Escalante-Valdez, M.J.; Guardado-Félix, D.; Serna-Saldívar, S.O.; Barrera-Arellano, D.; Chuck-Hernández, C. Effects of Post Anthesis Foliar Application of Sodium Selenite to Soybeans (Glycine max): Lipid Composition and Oil Stability. Biomolecules 2019, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.; Andrade, M.J.; García, C.; Rondán, J.J.; Núñez, F. Effects of preservative agents on quality attributes of dry-cured fermented sausages. Foods 2020, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Wang, P.; Luan, D.; Sun, J.; Huang, M.; Wang, B.; Zheng, Y. Quality changes of duck meat during thermal sterilization processing caused by microwave, stepwise retort, and general retort heating. Front. Nutr. 2022, 9, 1016942. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, C.S.; de Sousa, P.H.M.; Nunes, F.M. Cooking beyond flavors: Exploring the chemical reactions in the preparation of duck in tucupi of Pará-Brazil. Int. J. Gastron. Food Sci. 2024, 36, 100949. [Google Scholar] [CrossRef]
- Liu, R.; Kong, F.; Xing, S.; He, Z.; Bai, L.; Sun, J.; Tan, X.; Zhao, D.; Zhao, G.; Wen, J. Dominant changes in the breast muscle lipid profiles of broiler chickens with wooden breast syndrome revealed by lipidomics analyses. J. Anim. Sci. Biotechnol. 2022, 13, 93. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Song, Y.; Qiang, Y.; Han, D.; Zhang, C. Changes provoked by altitudes and cooking methods in physicochemical properties, volatile profile, and sensory characteristics of yak meat. Food Chem. X 2023, 20, 101019. [Google Scholar] [CrossRef] [PubMed]

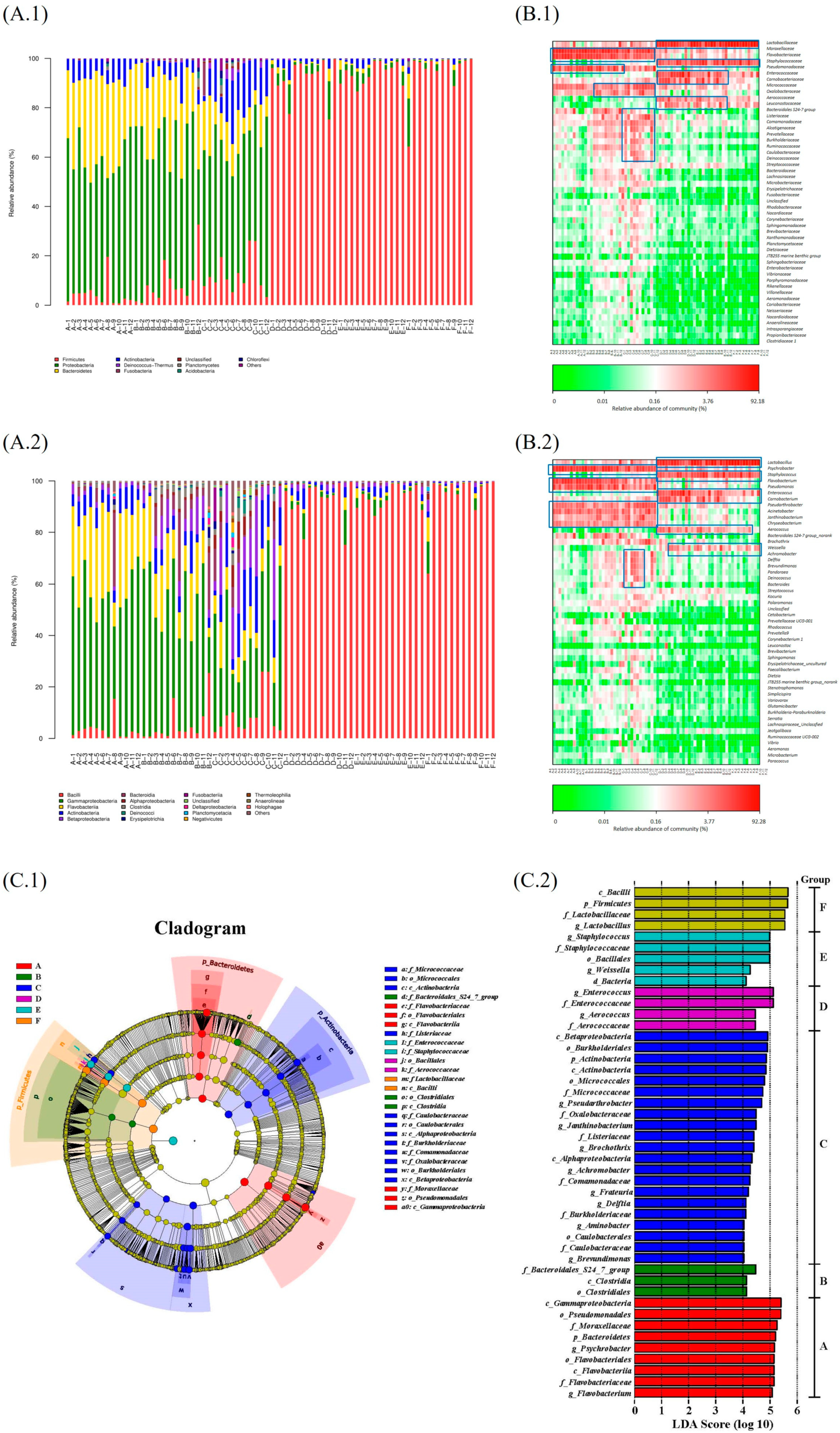

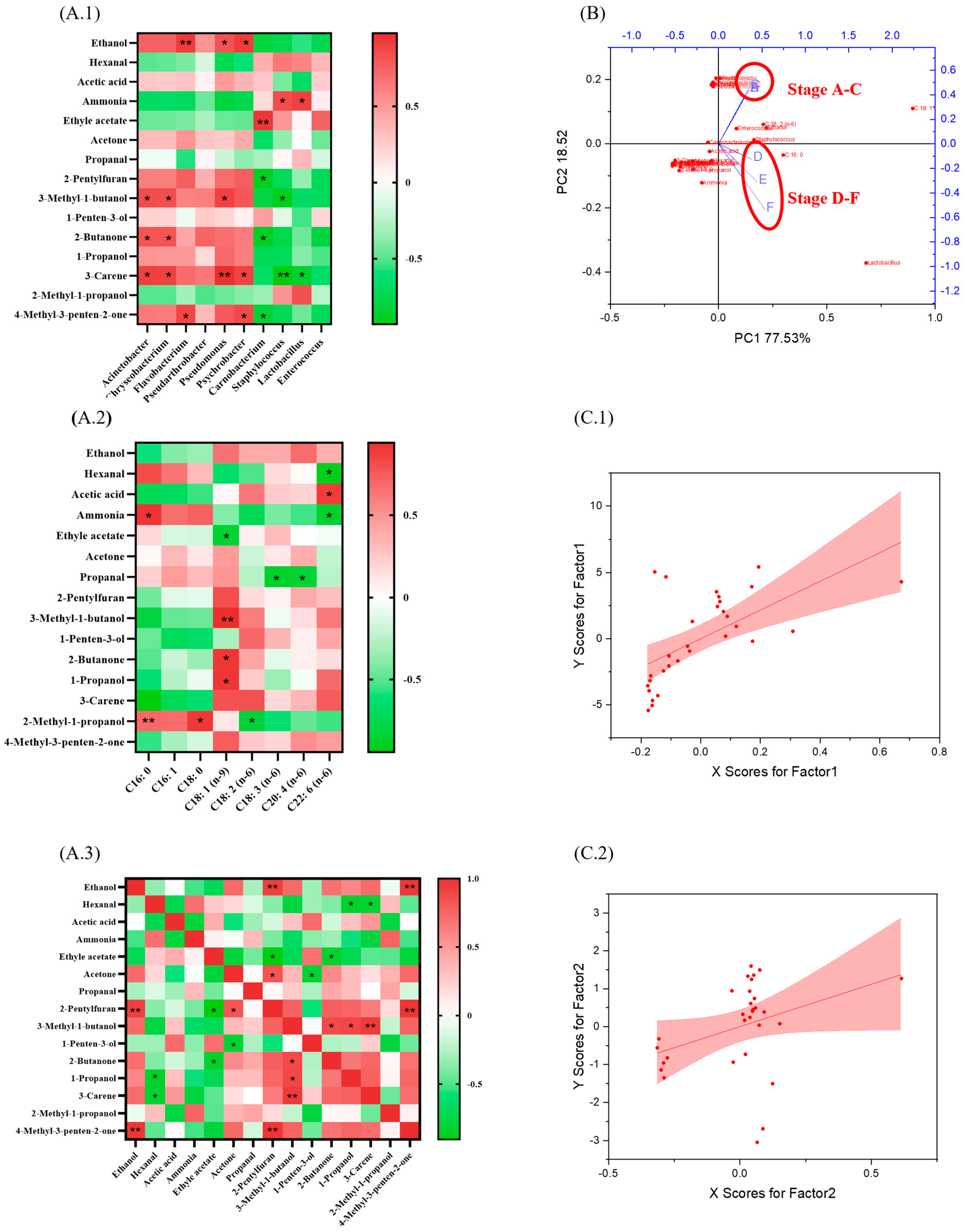
| Index | Stage | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| L* | 32.1 ± 0.3 a | 48.2 ± 0.8 f | 46.5 ± 0.3 e | 44.3 ± 0.2 d | 42.5 ± 0.1 c | 41.3 ± 0.1 b |
| a* | 8.2 ± 0.9 a | 10.5 ± 0.3 b | 11.2 ± 0.6 c | 11.5 ± 0.1 c | 12.6 ± 0.5 d | 12.1 ± 0.3 d |
| b* | 5.8 ± 0.6 a | 6.0 ± 1.2 b | 7.0 ± 0.4 c | 7.8 ± 0.6 d | 8.1 ± 0.3 d | 8.2 ± 0.3 d |
| T-VBN (mg/100 g) | 5.92 ± 0.42 a | 6.85 ± 0.25 b | 7.12 ± 0.92 b | 20.25 ± 0.89 c | 24.58 ± 0.43 d | 48.20 ± 1.06 e |
| TBARS (mg/kg) | 0.16 ± 0.00 a | 0.27 ± 0.03 b | 0.58 ± 0.05 c | 0.96 ± 0.08 d | 1.37 ± 0.02 e | 2.13 ± 0.15 f |
| TPC (log10 CFU/g) | 1.82 ± 0.02 a | 2.24 ± 0.06 b | 2.20 ± 0.05 b | 6.85 ± 0.01 c | 7.82 ± 0.12 d | 8.45 ± 0.08 e |
| No. | Compound | CAS | Formula | Description |
|---|---|---|---|---|
| Aldehydes (n = 15) | ||||
| 1 | Benzaldehyde | C100527 | C7H6O | Bitter almond, Burnt sugar, Cherry, Malt, Roasted pepper |
| 2 | Nonanal | C124196 | C9H18O | Fat, Floral, Green, Lemon |
| 3 | (E)-2-Heptenal | C18829555 | C7H12O | Almond, Fat, Fruit |
| 4 | Heptanal | C111717 | C7H14O | Citrus, Fat, Green, Nut |
| 5 | Hexanal | C66251 | C6H12O | Apple, Fat, Fresh, Green, Oil |
| 6 | Pentanal | C110623 | C5H10O | Almond, Bitter, Malt, Oil, Pungent |
| 7 | Acetal | C105577 | C6H14O2 | Creamy, Fruit, Pleasant, Tropical fruit |
| 8 | Butanal | C123728 | C4H8O | Banana, Green, Pungent |
| 9 | 2-Methylpropanal | C78842 | C4H8O | Burnt, Caramel, Cocoa, Green, Malt |
| 10 | Propanal | C123386 | C3H6O | Floral, Pungent, Solvent |
| 11 | Acetaldehyde | C75070 | C2H4O | Floral, Green apple |
| 12 | Methional | C3268493 | C4H8OS | Cooked potato, Soy |
| 13 | 3-Methyl-2-butenal | C107868 | C5H8O | Almond, Roasted |
| 14 | 2-Methyl-2-pentenal | C623369 | C6H10O | Fruit |
| 15 | Octanal | C124130 | C8H16O | Citrus, Fat, Green, Oil, Pungent |
| Esters (n = 17) | ||||
| 1 | Methyl octanoate | C111115 | C9H18O2 | Fruit, Orange, Wax, Wine |
| 2 | (E)-2-Octenal | C2548870 | C8H14O | Dandelion, Fat, Fruit, Grass, Green, Spice |
| 3 | Hexyl propionate | C2445763 | C9H18O2 | Fruit |
| 4 | 1-Penten-3-ol | C616251 | C5H10O | Butter, Fish, Green, Oxidized, Wet earth |
| 5 | Methyl 2-methylbutanoate | C868575 | C6H12O2 | Apple, Fruit, Green apple, Strawberry |
| 6 | Ethyl acetate | C141786 | C4H8O2 | Aromatic, Brandy, Grape |
| 7 | Methyl acetate | C79209 | C3H6O2 | Ester, Green |
| 8 | Ethyl propanoate | C105373 | C5H10O2 | Apple, Pineapple, Rum, Strawberry |
| 9 | Propyl acetate | C109604 | C5H10O2 | Celery, Floral, Pear, Red fruit |
| 10 | Ethyl 3-methylbutanoate | C108645 | C7H14O2 | Apple, Fruit, Pineapple, Sour |
| 11 | Ethyl 2-methylbutanoate | C7452791 | C7H14O2 | Apple, Ester, Green apple, Kiwi, Strawberry |
| 12 | Isoamyl acetate | C123922 | C7H14O2 | Apple, Banana, Pear |
| 13 | Ethyl butanoate | C105544 | C6H12O2 | Apple, Butter, Cheese, Pineapple, Strawberry |
| 14 | Butyl acetate | C123864 | C6H12O2 | Apple, Banana |
| 15 | Pentyl acetate | C628637 | C7H14O2 | Apple, Banana, Pear |
| 16 | Ethyl hexanoate | C123660 | C8H16O2 | Apple peel, Brandy, Fruit gum, Overripe fruit, Pineapple |
| 17 | Ethyl pentanoate | C539822 | C7H14O2 | Apple, Dry fish, Herb, Nut, Yeast |
| Alcohols (n = 9) | ||||
| 1 | 1-Octen-3-ol | C3391864 | C8H16O | Cucumber, Earth, Fat, Floral, Mushroom |
| 2 | 1-Hexanol | C111273 | C6H14O | Banana, Flower, Grass, Herb |
| 3 | Acetoin | C513860 | C4H8O2 | Butter, Creamy, Green pepper |
| 4 | 1-Pentanol | C71410 | C5H12O | Balsamic, Fruit, Green, Pungent, Yeast |
| 5 | 3-Methyl-1-butanol | C123513 | C5H12O | Burnt, Cocoa, Floral, Malt |
| 6 | 1-Butanol | C71363 | C4H10O | Fruit |
| 7 | 2-Methyl-1-propanol | C78831 | C4H10O | Apple, Bitter, Cocoa, Wine |
| 8 | 1-Propanol | C71238 | C3H8O | Alcohol, Candy, Pungent |
| 9 | 2-Propanol | C67630 | C3H8O | Floral |
| Ketones (n = 8) | ||||
| 1 | 1-Hydroxy-2-propanone | C116096 | C3H6O2 | Butter, Herb, Malt, Pungent |
| 2 | 2-Heptanone | C110430 | C7H14O | Blue cheese, Fruit, Green, Nut, Spice |
| 3 | 1-Penten-3-one | C1629589 | C5H8O | Fish, Green, Mustard, Pungent |
| 4 | 2-Pentanone | C107879 | C5H10O | Fruit, Pungent |
| 5 | 2-Butanone | C78933 | C4H8O | Fragrant, Fruit, Pleasant |
| 6 | Acetone | C67641 | C3H6O | Pungent |
| 7 | Cyclopentanone | C120923 | C5H8O | Mint, Cool |
| 8 | Dihydro-2(3H)-furanone | C96480 | C4H6O2 | Caramel, Cheese, Roasted nut |
| Acids (n = 4) | ||||
| 1 | Propanoic acid | C79094 | C3H6O2 | Fat, Fruit, Pungent, Silage, Soy |
| 2 | Butanoic acid | C107926 | C4H8O2 | Butter, Cheese, Sour |
| 3 | Acetic acid | C64197 | C2H4O2 | Acid, Fruit, Pungent, Sour, Vinegar |
| 4 | 2-Methylpropanoic acid | C79312 | C4H8O2 | Burnt, Butter, Cheese, Sweat |
| Others (n = 12) | ||||
| 1 | 2,6-Dimethylpyrazine | C108509 | C6H8N2 | Cocoa, Coffee, Green, Roast beef, Roasted nut |
| 2 | Methylpyrazine | C109080 | C5H6N2 | Cocoa, Green, Hazelnut, Popcorn, Roasted |
| 3 | 2-Pentylfuran | C3777693 | C9H14O | Butter, Floral, Fruit, Green bean |
| 4 | 3-Carene | C13466789 | C10H16 | Lemon |
| 5 | Dimethyl disulfide | C624920 | C2H6S2 | Cabbage, Garlic, Onion |
| 6 | alpha-Pinene | C80568 | C10H16 | Cedarwood, Pine, Sharp |
| 7 | Dimethyl sulfide | C75183 | C2H6S | Cabbage, Organic, Sulfur, Wet earth |
| 8 | Furfural | C98011 | C5H4O2 | Almond, Baked potatoes, Bread, Burnt, Spice |
| 9 | Dipropyl disulfide | C629196 | C6H14S2 | Cooked meat, Garlic, Onion, Pungent, Sulfur |
| 10 | 3-Ethylpyridine | C536787 | C7H9N | Nuts |
| 11 | 2,5-Dimethylpyrazine | C123320 | C6H8N2 | Cocoa, Roast neef, Roasted nut |
| 12 | 2-Acetylfuran | C1192627 | C6H6O2 | Balsamic, Cocoa, Coffee |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, K.; Cai, J.; Pan, D.; Chen, B.; Fan, J.; Ren, D.; Xiao, Y. The Overall Quality Changes of Chinese Sauced Ducks at Different Stages During Processing and Storage. Foods 2025, 14, 834. https://doi.org/10.3390/foods14050834
Yao K, Cai J, Pan D, Chen B, Fan J, Ren D, Xiao Y. The Overall Quality Changes of Chinese Sauced Ducks at Different Stages During Processing and Storage. Foods. 2025; 14(5):834. https://doi.org/10.3390/foods14050834
Chicago/Turabian StyleYao, Kaiyong, Jie Cai, Daodong Pan, Bindan Chen, Jinghui Fan, Daxi Ren, and Yingping Xiao. 2025. "The Overall Quality Changes of Chinese Sauced Ducks at Different Stages During Processing and Storage" Foods 14, no. 5: 834. https://doi.org/10.3390/foods14050834
APA StyleYao, K., Cai, J., Pan, D., Chen, B., Fan, J., Ren, D., & Xiao, Y. (2025). The Overall Quality Changes of Chinese Sauced Ducks at Different Stages During Processing and Storage. Foods, 14(5), 834. https://doi.org/10.3390/foods14050834






