Characterizing the Phenolic Compounds in Iron Walnut Oil (Juglans sigillata Dode) Across Chinese Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Physicochemical Properties
2.3. Fatty Acid Compositon
2.4. Sterols and Squalene
2.5. Polyphenol Content
2.6. Free Radical Scavenging Capacity
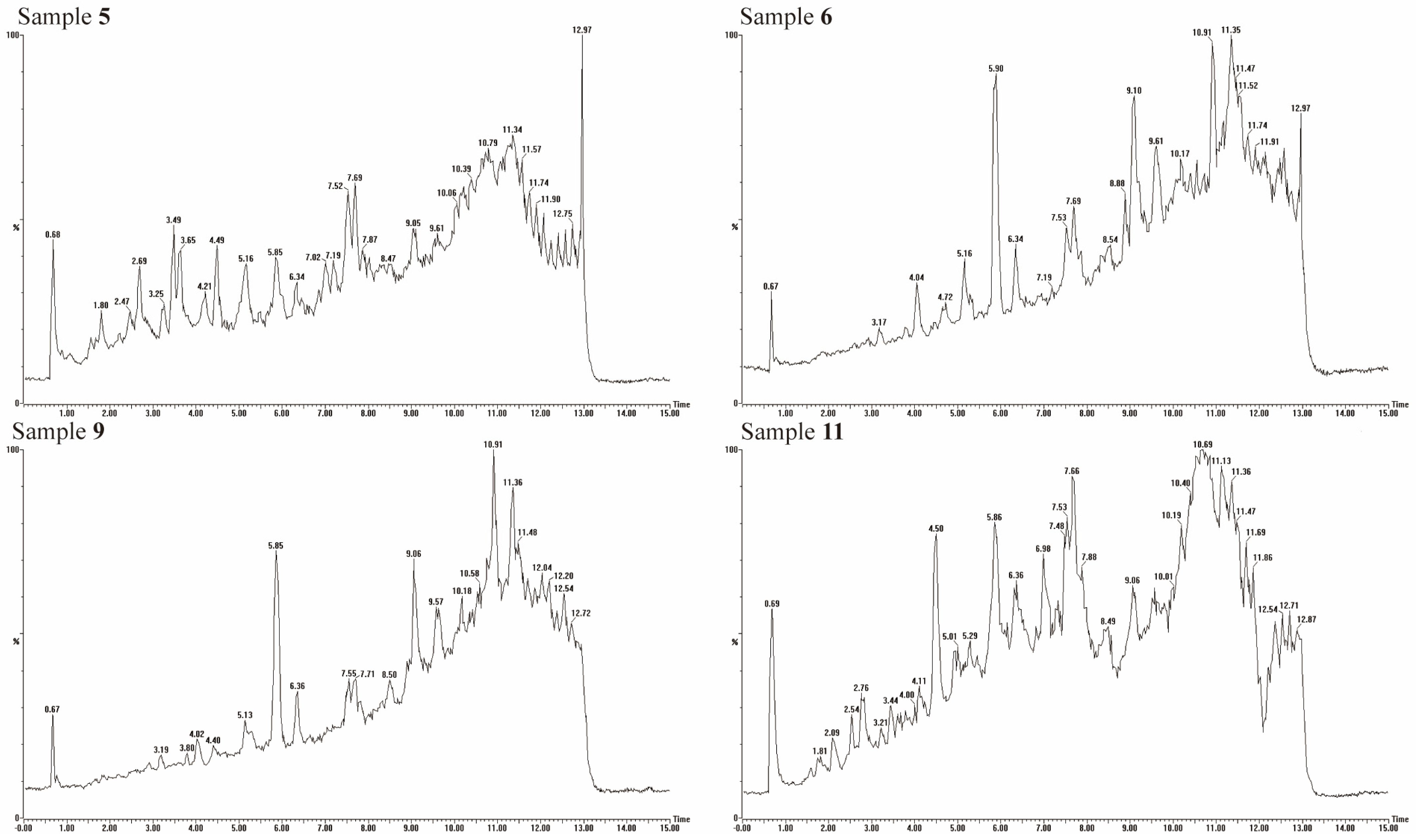
2.7. Phenolic Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Fatty Acid
3.3. Minor Compounds
3.4. Antioxidant Ability
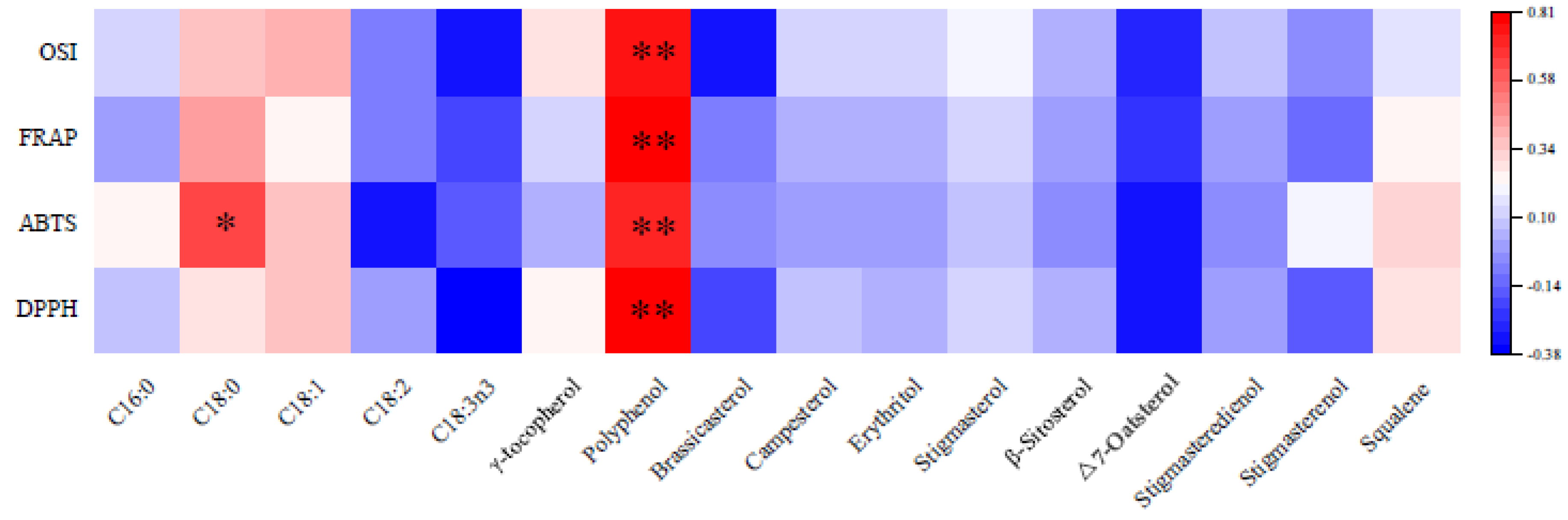
3.5. Multivariate Linear Regression and Relative Correlation Analysis
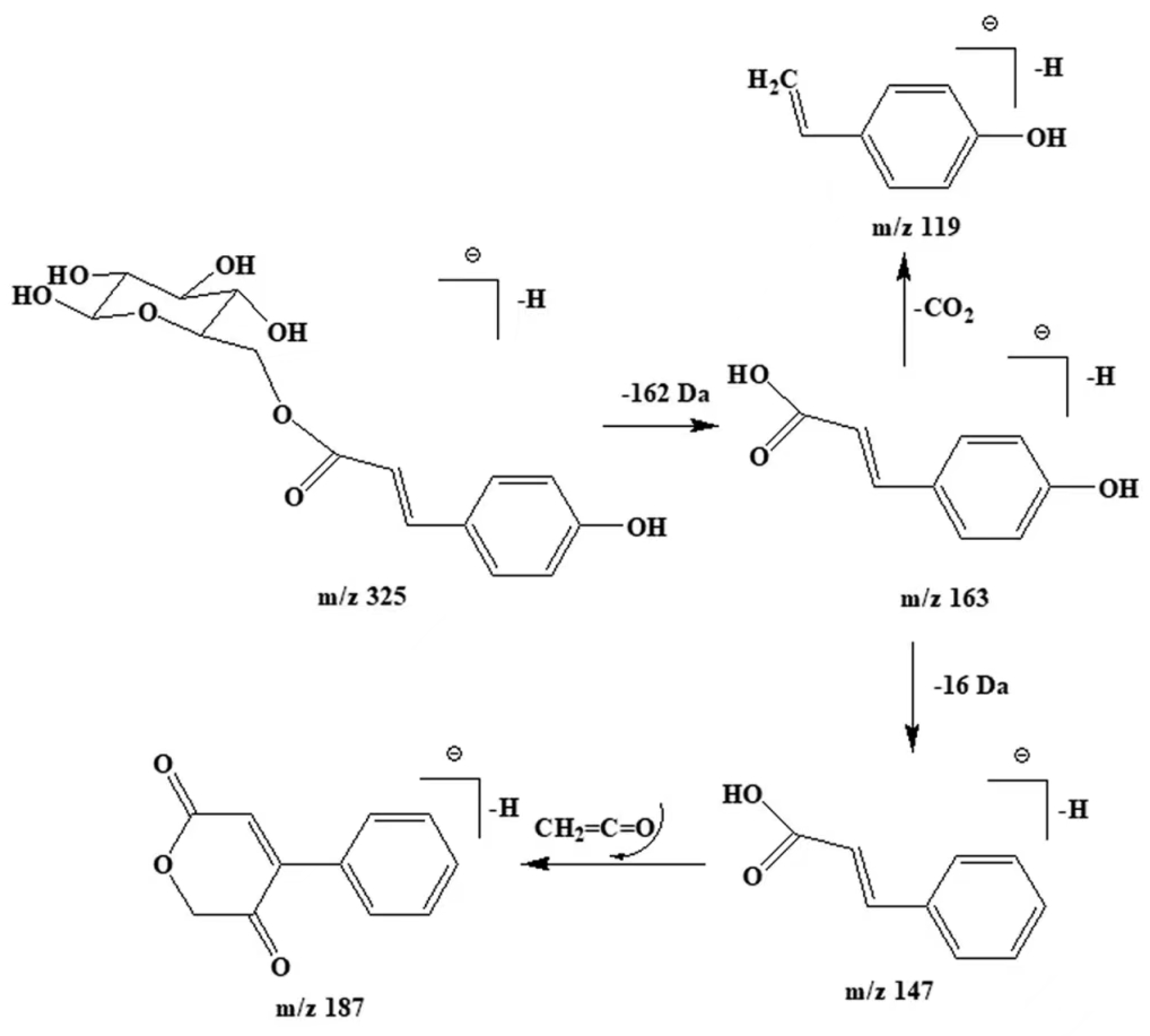
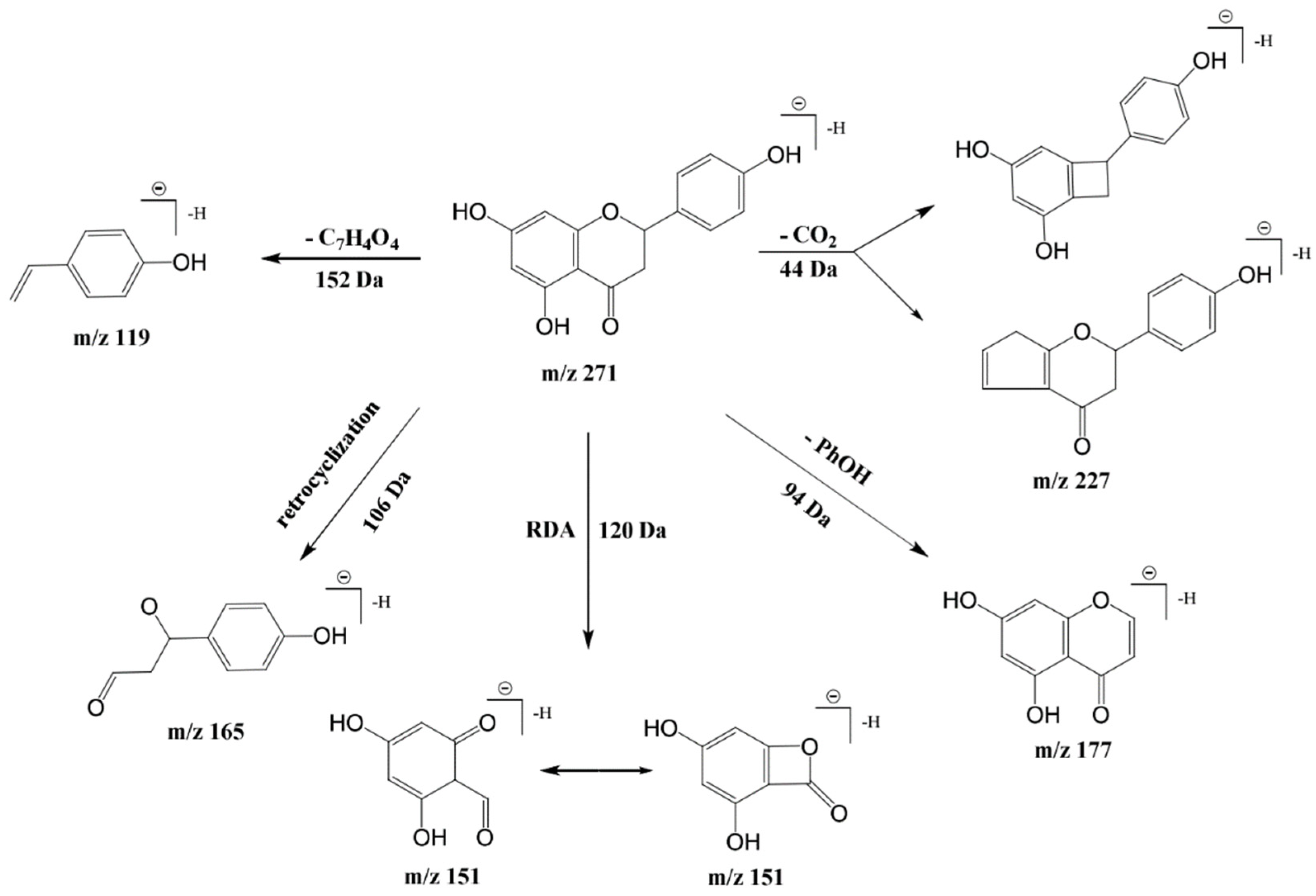
3.6. Polyphenol Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, F.; Ma, Q.; Zhang, W.; Liu, J.; Feng, Y.; Zhao, P.; Song, X.; Chen, J.; Zhang, J.; Wei, X.; et al. A genome variation map provides insights into the genetics of walnut adaptation and agronomic traits. Genome Biol. 2021, 22, 300. [Google Scholar] [CrossRef]
- Gao, P.; Jin, J.; Liu, R.; Jin, Q.; Wang, X. Chemical compositions of walnut (Juglans regia L.) oils from different cultivated regions in China. J. Am. Oil Chem. Soc. 2018, 95, 825–834. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyński, P.; Iwaniuk, P.; Rutkowska, E.; Socha, K.; Orywal, K.; Farhan, J.A.; Perkowski, M. Nutritional compounds and risk assessment of mycotoxins in ecological and conventional nuts. Food Chem. 2024, 458, 140222. [Google Scholar] [CrossRef]
- Austel, A.; Ranke, C.; Wagner, N.; Görge, J.; Ellrott, T. Weight loss with a modified Mediterranean-type diet using fat modification: A randomized controlled trial. Eur. J. Clin. Nutr. 2015, 69, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Zibaeenezhad, M.J.; Farhadi, P.; Attar, A.; Mosleh, A.; Amirmoezi, F.; Azimi, A. Effects of walnut oil on lipid profiles in hyperlipidemic type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Nutr. Diabetes 2017, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Key chemical composition of walnut (Juglans regia. L.) Oils generated with different processing methods and their cholesterol-lowering effects in HepG2 cells. Food Biosci. 2022, 45, 101436. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Yao, Y.; Feng, G.; Shu, C.; Wang, H.; Zhang, X. Walnut oil improves spatial memory in rats and increases the expression of acid-sensing ion channel genes Asic2a and Asic4. Food Sci. Nutr. 2019, 7, 293–301. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Racchi, M.L. Antixidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of different processing methods of iron walnut oils (Juglans sigillata): Lipid yield, lipid compositions, minor components, and antioxidant capacity. Eur. J. Lipid Sci. Technol. 2019, 120, 201800151. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, J.; Peng, X.; Ruan, H. Phenolic constituents from the fresh pericarps of Juglans sigillata. Nat. Prod. Res. 2021, 35, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Zhang, W.; Pan, X. Shading in fruit changes the polyphenol accumulation of pellicle by regulating activity of key enzymes and expression of their gene related polyphenol anabolism of Juglans sigillata Dode. Sci. Hortic. 2024, 329, 113017. [Google Scholar] [CrossRef]
- Li, C.; Shi, B.; Li, X.; Zhang, W.; Pan, X. Changes of phenolics contents, antioxidant activities, and enzyme activities in pellicles of Juglans sigillata Dode during fruits development. Int. J. Food Prop. 2022, 25, 2133–2145. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G.; Jankowska, A. Antioxidant properties, profile of polyphenolic compounds and tocopherol content in various walnut (Juglans regia L.) varieties. Eur. Food Res. Technol. 2019, 245, 607–616. [Google Scholar] [CrossRef]
- Balkrishna, A.; Verma, S.; Tiwari, D.; Srivastava, J.; Varshney, A. UPLC-QToF-MS based fingerprinting of polyphenolic metabolites in the bark extract of Boehmeria rugulosa Wedd. J. Mass Spectrom. 2022, 57, 4890. [Google Scholar] [CrossRef]
- Hong, C.; Chang, C.; Zhang, H.; Jin, Q.; Wu, G.; Wang, X. Identification and characterization of polyphenols in different varieties of Camellia oleifera seed cakes by UPLC-QTOF-MS. Food Res. Int. 2019, 126, 108614. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Y.; Huang, W.; Feng, X.; Yang, F.; Li, D. Separation, identification, and antioxidant activity of polyphenols from lotus seed epicarp. Molecules 2019, 24, 4007. [Google Scholar] [CrossRef]
- Man, G.; Xu, L.; Wang, Y.; Liao, X.; Xu, Z. Profiling phenolic composition in pomegranate peel from nine selected cultivars using UHPLC-QTOF-MS and UPLC-QQQ-MS. Front. Nutr. 2022, 8, 807447. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2019, 279, 279–287. [Google Scholar] [CrossRef]
- Tang, C.; Tao, G.; Wang, Y.; Liu, Y.; Li, J. Identification of α-tocopherol and its oxidation products by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2020, 68, 669–677. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qin, C.; Wang, X.; Ding, N. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, X.; Li, J.; Wang, F.; Mi, J.; Shi, Y.; He, F.; Chen, L.; Zhang, F.; Wan, X. Chemical compositions of walnut (Juglans spp.) oil: Combined effects of genetic and climatic factors. Forests 2022, 13, 962. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Castilho, P.C. Phytochemical profile, chemotaxonomic studies, and in vitro antioxidant activities of two endemisms from Madeira Archipelago: Melanoselinum decipiens and Monizia edulis (Apiaceae). Chem. Biodivers. 2016, 13, 1290–1306. [Google Scholar] [CrossRef]
- Verardo, G.; Duse, I.; Callea, A. Analysis of underivatized oligosaccharides by liquid chromatography/electrospray ionization tandem mass spectrometry with post-column addition of formic acid. Rapid Commun. Mass Spectrom. 2009, 23, 1607–1618. [Google Scholar] [CrossRef]
- Gruz, J.; Novák, O.; Strnad, M. Rapid analysis of phenolic acids in beverages by UPLC–MS/MS. Food Chem. 2008, 111, 789–794. [Google Scholar] [CrossRef]
- Said, R.B.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Bai, L.; Masukawa, N.; Yamaki, M.; Takagi, S. A polyphenol and two bibenzyls from Pleione bulbocodioides. Phytochemistry 1998, 47, 1637–1640. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.Å.; Smeds, A.I.; Sjöholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2007, 43, 97–107. [Google Scholar] [CrossRef]
- Wang, H.; Feng, F. Identification of components in Zhi-Zi-Da-Huang decoction by HPLC coupled with electrospray ionization tandem mass spectrometry, photodiode array and fluorescence detectors. J. Pharm. Biomed. Anal. 2009, 49, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv. ‘Criolla’) by ultra-performance liquid chromatography with diode array and mass spectrometric detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, R.; Mansur, S.; Lai, H.; Ubul, A.; Sun, G.; Huang, G.; Aisa, H.A. Qualitative analysis of polyphenols in macroporous resin pretreated pomegranate husk extract by HPLC-QTOF-MS: Qualitative analysis of polyphenols in pomegranate husk extract. Phytochem. Anal. 2017, 28, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV (mg/g) | 0.39 ± 0.01 h | 0.42 ± 0.00 g | 0.41 ± 0.00 g | 0.46 ± 0.00 f | 0.54 ± 0.01 d | 0.54 ± 0.00 d | 0.62 ± 0.01 c | 0.62 ± 0.01 c | 0.48 ± 0.00 e | 0.63 ± 0.01 c | 0.74 ± 0.03 a | 0.68 ± 0.00 b |
| PV (g/100 g) | 0.06 ± 0.00 g | 0.07 ± 0.00 f | 0.06 ± 0.00 g | 0.09 ± 0.00 cd | 0.09 ± 0.00 c | 0.08 ± 0.00 de | 0.09 ± 0.00 cd | 0.08 ± 0.00 de | 0.07 ± 0.00 ef | 0.07 ± 0.00 f | 0.24 ± 0.00 a | 0.23 ± 0.00 b |
| Fatty acid (%) | ||||||||||||
| C12:0 | 0.05 ± 0.01 f | 0.08 ± 0.01 e | 0.05 ± 0.00 f | 0.14 ± 0.01 b | 0.05 ± 0.00 f | 0.05 ± 0.00 f | 0.12 ± 0.01 c | 0.10 ± 0.01 d | 0.05 ± 0.00 f | 0.16 ± 0.01 a | 0.05 ± 0.00 f | 0.05 ± 0.00 f |
| C13:0 | 0.11 ± 0.00 a | 0.11 ± 0.01 a | 0.10 ± 0.00 ab | 0.08 ± 0.01 c | 0.10 ± 0.00 ab | 0.10 ± 0.01 ab | 0.10 ± 0.00 ab | 0.11 ± 0.01 ab | 0.09 ± 0.00 bc | 0.09 ± 0.00 bc | 0.10 ± 0.01 ab | 0.10 ± 0.01 ab |
| C14:0 | N.D. c | 0.03 ± 0.01 ab | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.03 ± 0.01 ab | 0.02 ± 0.00 b | 0.03 ± 0.01 c | 0.03 ± 0.01 ab | N.D. c | 0.03 ± 0.00 ab | 0.03 ± 0.01 ab | N.D. c |
| C15:0 | N.D. c | N.D. c | 0.03 ± 0.01 b | N.D. c | N.D. c | N.D. c | 0.02 ± 0.00 b | N.D. c | N.D. c | 0.02 ± 0.00 b | 0.04 ± 0.01 a | N.D. c |
| C16:0 | 2.92 ± 0.03 i | 9.89 ± 0.15 a | 3.38 ± 0.19 h | 5.89 ± 0.02 f | 6.15 ± 0.12 e | 5.45 ± 0.02 g | 5.73 ± 0.08 f | 5.85 ± 0.02 f | 5.37 ± 0.01 g | 6.40 ± 0.01 d | 7.68 ± 0.02 c | 7.92 ± 0.09 b |
| C16:1 | 0.04 ± 0.01 c | 0.13 ± 0.01 b | 0.05 ± 0.01 c | 0.06 ± 0.00 c | 0.05 ± 0.01 c | 0.06 ± 0.01 c | 0.07 ± 0.00 c | 0.05 ± 0.00 c | 0.06 ± 0.00 c | 0.12 ± 0.01 b | 0.20 ± 0.02 a | 0.19 ± 0.04 a |
| C17:0 | N.D. c | 0.05 ± 0.01 a | N.D. c | 0.04 ± 0.00 ab | 0.06 ± 0.01 a | 0.05 ± 0.00 ab | 0.04 ± 0.01 ab | 0.05 ± 0.01 ab | 0.06 ± 0.01 a | 0.04 ± 0.01 ab | 0.04 ± 0.00 ab | 0.04 ± 0.01 b |
| C17:1 | 0.03 ± 0.00 a | 0.03 ± 0.01 a | N.D. b | 0.03 ± 0.01 a | N.D. b | 0.04 ± 0.00 a | 0.04 ± 0.02 a | 0.03 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | N.D. b |
| C18:0 | 1.07 ± 0.00 g | 2.25 ± 0.03 f | 1.09 ± 0.03 g | 3.00 ± 0.00 c | 3.72 ± 0.03 a | 2.49 ± 0.01 d | 2.97 ± 0.03 c | 3.11 ± 0.01 b | 2.49 ± 0.04 d | 2.48 ± 0.00 d | 2.44 ± 0.01 de | 2.40 ± 0.02 e |
| C18:1 | 15.80 ± 0.03 g | 21.87 ± 1.02 ab | 17.62 ± 0.00 f | 19.91 ± 0.02 c | 18.84 ± 0.03 de | 22.42 ± 0.01 a | 22.39 ± 0.04 a | 21.26 ± 0.03 b | 22.43 ± 0.00 a | 20.41 ± 0.01 c | 19.08 ± 0.04 d | 18.38 ± 0.04 e |
| C18:2 | 68.81 ± 0.01 a | 61.05 ± 1.11 e | 64.99 ± 0.16 b | 62.15 ± 0.02 c | 61.26 ± 0.08 de | 61.95 ± 0.04 cd | 59.14 ± 0.09 f | 51.97 ± 0.01 g | 62.00 ± 0.04 cd | 61.80 ± 0.01 cde | 61.28 ± 0.02 de | 61.87 ± 0.07 cd |
| C18:3n6 | 0.04 ± 0.00b cd | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.04 ± 0.01 bcd | 0.05 ± 0.01 bc | N.D. e | 0.03 ± 0.00 cd | 0.07 ± 0.01 a | 0.04 ± 0.01 cd | 0.03 ± 0.00 d | 0.04 ± 0.00 cd | N.D. e |
| C18:3n3 | 10.86 ± 0.01 c | 4.16 ± 0.23 i | 12.41 ± 0.03 b | 8.53 ± 0.01 f | 9.41 ± 0.02 d | 7.20 ± 0.03 h | 9.04 ± 0.02 e | 17.23 ± 0.04 a | 7.17 ± 0.05 h | 8.30 ± 0.00 g | 8.88 ± 0.00 e | 8.96 ± 0.01 de |
| C20:0 | 0.23 ± 0.02 a | 0.16 ± 0.01 b | N.D. g | 0.06 ± 0.01 ef | 0.11 ± 0.00 c | N.D. g | 0.09 ± 0.01 d | 0.07 ± 0.01 de | 0.05 ± 0.02 f | 0.07 ± 0.01 def | N.D. g | N.D. g |
| C20:1 | 0.03 ± 0.01 g | 0.16 ± 0.00 cde | 0.22 ± 0.03 ab | 0.15 ± 0.00 de | 0.19 ± 0.00 bc | 0.18 ± 0.01 cd | 0.24 ± 0.00 a | 0.15 ± 0.01 de | 0.18 ± 0.01 cd | 0.13 ± 0.01 ef | 0.13 ± 0.03 ef | 0.10 ± 0.01 f |
| SFA | 4.38 ± 0.05 g | 12.57 ± 0.16 a | 4.66 ± 0.22 f | 9.23 ± 0.03 d | 10.21 ± 0.16 c | 8.16 ± 0.04 e | 9.10 ± 0.13 d | 9.30 ± 0.03 d | 8.09 ± 0.07 e | 9.29 ± 0.00 d | 10.36 ± 0.04 bc | 10.51 ± 0.11 b |
| MUFA | 15.90 ± 0.05 f | 22.19 ± 1.04 a | 21.41 ± 0.23 b | 20.15 ± 0.01 c | 19.08 ± 0.03 de | 22.69 ± 0.02 a | 22.74 ± 0.03 a | 21.49 ± 0.02 b | 22.71 ± 0.02 a | 20.69 ± 0.02 c | 19.44 ± 0.03 d | 18.76 ± 0.06 e |
| PUFA | 79.68 ± 0.00 a | 65.21 ± 0.88 g | 74.21 ± 0.61 b | 70.68 ± 0.03 cd | 70.67 ± 0.10 cd | 69.15 ± 0.07 e | 68.18 ± 0.11 f | 69.20 ± 0.05 e | 69.17 ± 0.01 e | 70.11 ± 0.01 d | 70.16 ± 0.01 d | 70.83 ± 0.06 c |
| Tocopherol (mg/g) | ||||||||||||
| α-tocopherol | N.D. h | 78.58 ± 0.17 a | N.D. h | 3.57 ± 0.12 g | 26.36 ± 0.76 b | 11.78 ± 0.00 d | 5.09 ± 0.62 fg | 7.96 ± 0.30 e | 23.74 ± 2.85 c | N.D. h | 11.78 ± 0.97 d | 6.00 ± 0.66 ef |
| β-tocopherol | N.D. d | N.D. d | N.D. d | N.D. d | 2.26 ± 0.41 b | 4.84 ± 0.09 a | N.D. d | 1.24 ± 0.36 c | 1.15 ± 0.82 c | 1.75 ± 0.31 bc | 1.65 ± 0.23 bc | N.D. d |
| γ-tocopherol | 329.73 ± 2.35 d | 440.27 ± 4.85 a | 355.54 ± 1.33 b | 308.72 ± 2.91 e | 327.45 ± 0.61 d | 343.22 ± 2.24 c | 245.79 ± 3.79 g | 263.92 ± 2.40 f | 305.77 ± 3.16 e | 350.15 ± 2.59 bc | 304.22 ± 5.50 e | 333.95 ± 4.29 d |
| δ-tocopherol | 3.54 ± 0.79 e | 6.19 ± 0.92 d | 13.35 ± 0.93 c | 12.27 ± 0.50 c | 26.78 ± 0.65 a | 7.23 ± 0.37 d | 17.81 ± 1.41 b | 16.97 ± 1.10 b | 6.98 ± 0.61 d | 18.63 ± 0.64 b | 16.84 ± 0.34 b | 18.38 ± 0.71 b |
| Total tocopherol | 333.27 ± 3.14 ef | 525.05 ± 3.76 a | 368.89 ± 2.25 c | 324.56 ± 2.28 f | 382.85 ± 1.23 b | 367.07 ± 1.96 cd | 268.68 ± 5.82 h | 290.09 ± 3.56 g | 337.64 ± 5.79 e | 370.54 ± 3.54 c | 334.48 ± 6.58 e | 358.33 ± 4.33 d |
| Polyphenol (mg/g) | 4.10 ± 0.35 j | 1.22 ± 0.00 k | 10.01 ± 0.41 f | 6.45 ± 0.35 i | 62.91 ± 0.21 a | 55.15 ± 0.48 b | 12.10 ± 0.21 e | 22.24 ± 0.28 d | 42.98 ± 0.28 c | 7.62 ± 0.07 h | 42.76 ± 0.69 c | 8.51 ± 0.07 g |
| Sterols (mg/g) | ||||||||||||
| Brassicasterol | 70.83 ± 1.00 c | 62.11 ± 0.36 f | 73.42 ± 3.41 b | 66.21 ± 0.05 e | 69.29 ± 0.86 cd | 66.64 ± 0.13 de | 143.01 ± 1.05 a | 67.10 ± 1.15 de | 66.28 ± 0.13 e | 67.84 ± 0.53 de | 66.18 ± 0.49 e | 66.39 ± 0.28 e |
| Campesterol | 145.11 ± 2.55 d | 1135.52 ± 1.38 a | 213.53 ± 1.17 c | 78.40 ± 0.71 g | 113.01 ± 0.35 e | 66.12 ± 0.62 i | 265.72 ± 1.54 b | 216.61 ± 4.35 c | 71.93 ± 0.41 h | 76.27 ± 1.76 g | 94.48 ± 1.44 f | 61.35 ± 1.66 j |
| Campestanol | N.D. b | 79.82 ± 2.01 a | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b |
| Stigmasterol | 12.57 ± 0.28 e | 331.53 ± 1.42 a | 6.61 ± 0.11 h | 12.02 ± 0.60 ef | 34.87 ± 1.57 c | 10.71 ± 0.73 f | 26.11 ± 0.46 d | 54.34 ± 0.82 b | 7.84 ± 0.06 gh | 7.64 ± 0.16 gh | 10.60 ± 0.11 f | 8.55 ± 0.4 g |
| Ergost-5-en-3β-ol | N.D. b | 32.81 ± 0.64 a | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b | N.D. b |
| Erythritol | 20.53 ± 0.59 b | 85.28 ± 2.59 a | 17.18 ± 0.36 cd | 18.77 ± 0.54 bc | 17.85 ± 1.06 cd | 10.49 ± 0.43 f | 18.10 ± 0.81 cd | 18.20 ± 0.15 cd | 9.77 ± 0.07 f | 14.52 ± 0.36 e | 16.27 ± 0.36 de | 9.90 ± 0.68 f |
| β-Sitosterol | 1730.98 ± 2.65 c | 3737.97 ± 6.26 a | 1960.08 ± 3.60 b | 1358.05 ± 3.53 h | 1393.35 ± 4.28 g | 1170.97 ± 3.32 j | 1690.97 ± 2.21 d | 1452.22 ± 2.14 f | 1166.11 ± 3.44 j | 1275.03 ± 1.45 i | 1602.59 ± 4.32 e | 1153.29 ± 4.58 k |
| △5-Avenasterol | 348.29 ± 0.73 b | 418.24 ± 1.84 a | 327.42 ± 1.14 c | 234.46 ± 1.22 e | 199.16 ± 0.53 f | 112.18 ± 1.62 j | 181.62 ± 2.31 h | 313.59 ± 0.43 d | 101.19 ± 1.74 k | 144.94 ± 1.98 i | 187.19 ± 0.49 g | 187.86 ± 1.73 g |
| Stigmasteredienol | 32.56 ± 0.28 c | 115.03 ± 0.70 a | 27.91 ± 0.39 d | 23.74 ± 0.02 e | 21.68 ± 0.20 f | 13.89 ± 1.13 h | 23.37 ± 0.13 e | 36.30 ± 0.44 b | 14.05 ± 0.97 h | 13.67 ± 0.92 h | 17.60 ± 0.56 g | 16.58 ± 0.32 g |
| Stigmasterenol | 71.91 ± 0.28 i | 203.58 ± 1.46 b | 168.36 ± 0.67 d | 191.01 ± 1.37 c | 157.16 ± 2.15 e | 117.38 ± 1.90 h | 125.09 ± 2.45 g | 275.90 ± 1.10 a | 141.71 ± 1.45 f | 142.12 ± 0.16 f | 191.41 ± 0.18 c | 192.18 ± 0.50 c |
| △7-Oatsterol | N.D. c | 54.55 ± 2.23 a | N.D. c | N.D. c | N.D. c | N.D. c | N.D. c | 16.45 ± 0.12 b | N.D. c | N.D. c | N.D. c | N.D. c |
| Squalene (mg/g) | 6.87 ± 0.56 f | 9.65 ± 0.26 d | 27.16 ± 1.12 a | 2.03 ± 0.10 g | 17.87 ± 0.36 b | 6.10 ± 0.10 f | 2.42 ± 0.03 g | 6.07 ± 0.09 f | 6.02 ± 0.16 f | 8.45 ± 0.48 e | 14.10 ± 0.14 c | 14.33 ± 0.32 c |
| OSI (h) | 1.77 ± 0.04 k | 4.56 ± 0.01 d | 3.45 ± 0.07 e | 2.87 ± 0.01 g | 6.45 ± 0.06 a | 6.11 ± 0.00 b | 2.14 ± 0.06 j | 3.05 ± 0.06 f | 5.38 ± 0.07 c | 2.33 ± 0.00 i | 2.84 ± 0.04 g | 2.58 ± 0.06 h |
| Free radical scavenging capacity (μmol TE/100 g) | ||||||||||||
| DPPH | 360.24 ± 3.57 g | 442.95 ± 4.28 c | 429.02 ± 3.57 d | 370.62 ± 9.27 fg | 519.65 ± 7.13 a | 493.64 ± 6.42 b | 389.64 ± 2.14 e | 377.59 ± 1.43 f | 488.13 ± 2.14 b | 390.06 ± 3.57 e | 423.94 ± 5.71 d | 367.82 ± 3.57 fg |
| ABTS | 213.58 ± 1.32 i | 440.84 ± 6.59 c | 431.08 ± 1.32 d | 390.44 ± 1.32 g | 683.26 ± 1.32 a | 475.09 ± 2.64 b | 410.53 ± 5.28 ef | 408.69 ± 1.32 f | 468.41 ± 7.92 b | 402.48 ± 2.64 f | 419.23 ± 7.91 e | 368.88 ± 2.64 h |
| FRAP | 70.27 ± 0.00 gh | 98.40 ± 3.26 d | 90.48 ± 1.63 e | 73.58 ± 1.63 g | 158.70 ± 2.85 a | 118.86 ± 0.41 b | 87.31 ± 1.22 e | 81.73 ± 3.26 f | 113.04 ± 2.04 c | 66.72 ± 0.81 h | 78.55 ± 1.22 f | 71.90 ± 3.26 g |
| Dependent Variable | R2 | Adjusted R2 | Variable | R | Standard Error | t | Significance (Two-Tails p) | Equation |
|---|---|---|---|---|---|---|---|---|
| DPPH | 0.844 | 0.810 | (constant) | 3.113 × 10−16 | 0.126 | 0.000 | 1.000 | Y = 3.113 × 10−16 + 0.904 (Polyphenol) + 0.439 (γ-Tocopherol) |
| Polyphenol | 0.904 | 0.134 | 6.724 | 0.000 | ||||
| γ-Tocopherol | 0.439 | 0.134 | 3.265 | 0.010 | ||||
| ABTS | 0.516 | 0.467 | (constant) | −1.585 × 10−16 | 0.211 | 0.000 | 1.000 | Y = −1.585 × 10−16 + 0.718 (Polyphenol) |
| Polyphenol | 0.718 | 0.220 | 3.264 | 0.009 | ||||
| FRAP | 0.612 | 0.573 | (constant) | 2.788 × 10−16 | 0.189 | 0.000 | 1.000 | Y = 2.788 × 10−16 + 0.782 (Polyphenol) |
| Polyphenol | 0.782 | 0.197 | 3.973 | 0.003 | ||||
| OSI | 0.796 | 0.750 | (constant) | −3.083 × 10−16 | 0.144 | 0.000 | 1.000 | Y = −3.083 × 10−16 + 0.862 (Polyphenol) + 0.471 (γ-Tocopherol) |
| Polyphenol | 0.862 | 0.154 | 5.594 | 0.000 | ||||
| γ-Tocopherol | 0.471 | 0.154 | 3.061 | 0.014 |
| No. | Retention Time (min) | MW | [M-H] (m/z) | Fragment Irons | Compounds | Molecular Formula | Samples |
|---|---|---|---|---|---|---|---|
| 1 | 0.725 | 342 | 341 | 179, 131, 119, 113, 89, 71 | Hexose polymer | 5, 6, 9, 11 | |
| 2 | 0.932 | 170 | 169 | 137, 87 | Gallic acid | C7H6O5 | 5, 6, 9, 11 |
| 3 | 1.516 | 138 | 137 | 93 | P-hydroxybenzoic acid | C7H6O3 | 5, 6, 9, 11 |
| 4 | 2.129 | 164 | 163 | 117 | P-coumaric acid | C10H10O3 | 5, 6, 9, 11 |
| 5 | 2.475 | 194 | 193 | 178, 134 | Ferulic acid | C10H10O4 | 5, 6, 11 |
| 6 | 2.580 | 181 | 180 | 178, 136, 124 | Pine cypress alcohol | C10H12O3 | 5, 6, 9, 11 |
| 7 | 2.687 | 182 | 181 | 137, 109 | High vanillic acid | C9H10O4 | 5, 6 |
| 8 | 2.696 | 198 | 197 | 182, 167, 153 | Eugenoic acid | C9H10O5 | 5, 6 |
| 9 | 2.908 | 174 | 173 | 145 | Hydroquinone | C10H6O3 | 5, 6, 9, 11 |
| 10 | 3.246 | 264 | 263 | 219, 204, 201, 153 | Abscisic acid | C15H20O4 | 5, 6, 9, 11 |
| 11 | 3.478 | 426 | 425 | 425, 263, 219, 204, 201, 153 | Abscisic acid hexoside | 5, 6, 9, 11 | |
| 12 | 3.75 | 168 | 167 | 123, 108 | Vanillic acid | C8H8O4 | 6, 9, 11 |
| 13 | 3.823 | 180 | 179 | 163, 135, 117 | Caffeic acid | C9H8O4 | 5, 6, 9, 11 |
| 14 | 4.044 | 272 | 271 | 177,165,151,119,107 | Naringenin | C15H12O5 | 5, 6, 9 |
| 15 | 4.50 | 302 | 301 | 257, 229, 185 | Tannic acid | C14H6O8 | 5, 6, 9, 11 |
| 16 | 5.890 | 354 | 353 | 191, 179, 135 | Chlorogenic acid | C16H18O9 | 5,6 |
| 17 | 6.006 | 358 | 357 | 151, 136, 128 | Pine ester alcohol | C20H22O6 | 5, 6, 9, 11 |
| 18 | 6.689 | 492 | 491 | 329, 311,149,135 | Caffeoyl dihydroxyphenyl lactyl tartaric acid | 5, 6, 9 | |
| 19 | 6.954 | 326 | 325 | 163,147, 187, 146, 119 | Coumaryl hexose | C15H18O8 | 5, 6, 9, 11 |
| 20 | 7.523 | 302 | 301 | 179, 151 | Quercetin | C15H10O7 | 5, 6, 9, 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Chang, K.; Wang, S.; Zheng, Y.; Yin, J.; Zhang, X.; Reaney, M.J.T. Characterizing the Phenolic Compounds in Iron Walnut Oil (Juglans sigillata Dode) Across Chinese Regions. Foods 2025, 14, 899. https://doi.org/10.3390/foods14050899
Gao P, Chang K, Wang S, Zheng Y, Yin J, Zhang X, Reaney MJT. Characterizing the Phenolic Compounds in Iron Walnut Oil (Juglans sigillata Dode) Across Chinese Regions. Foods. 2025; 14(5):899. https://doi.org/10.3390/foods14050899
Chicago/Turabian StyleGao, Pan, Kairui Chang, Shu Wang, Yuling Zheng, Jiaojiao Yin, Xinghe Zhang, and Martin J. T. Reaney. 2025. "Characterizing the Phenolic Compounds in Iron Walnut Oil (Juglans sigillata Dode) Across Chinese Regions" Foods 14, no. 5: 899. https://doi.org/10.3390/foods14050899
APA StyleGao, P., Chang, K., Wang, S., Zheng, Y., Yin, J., Zhang, X., & Reaney, M. J. T. (2025). Characterizing the Phenolic Compounds in Iron Walnut Oil (Juglans sigillata Dode) Across Chinese Regions. Foods, 14(5), 899. https://doi.org/10.3390/foods14050899





