Pandanus Amaryllifolius Roxb. Polyphenol Extract Alleviates NAFLD via Regulating Gut Microbiota and AMPK/AKT/mTOR Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PAE
2.3. Determination of the Composition of PAE
2.4. Animals and Experimental Design
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Serum and Liver Biochemical Analysis
2.7. Histological Analysis
2.8. Untargeted Metabolomics of Liver
2.9. Western Blot Analysis
2.10. 16 S rRNA Analysis of Gut Microbiota
2.11. Statistical Analysis
3. Results and Discussion
3.1. Compounds of PAE
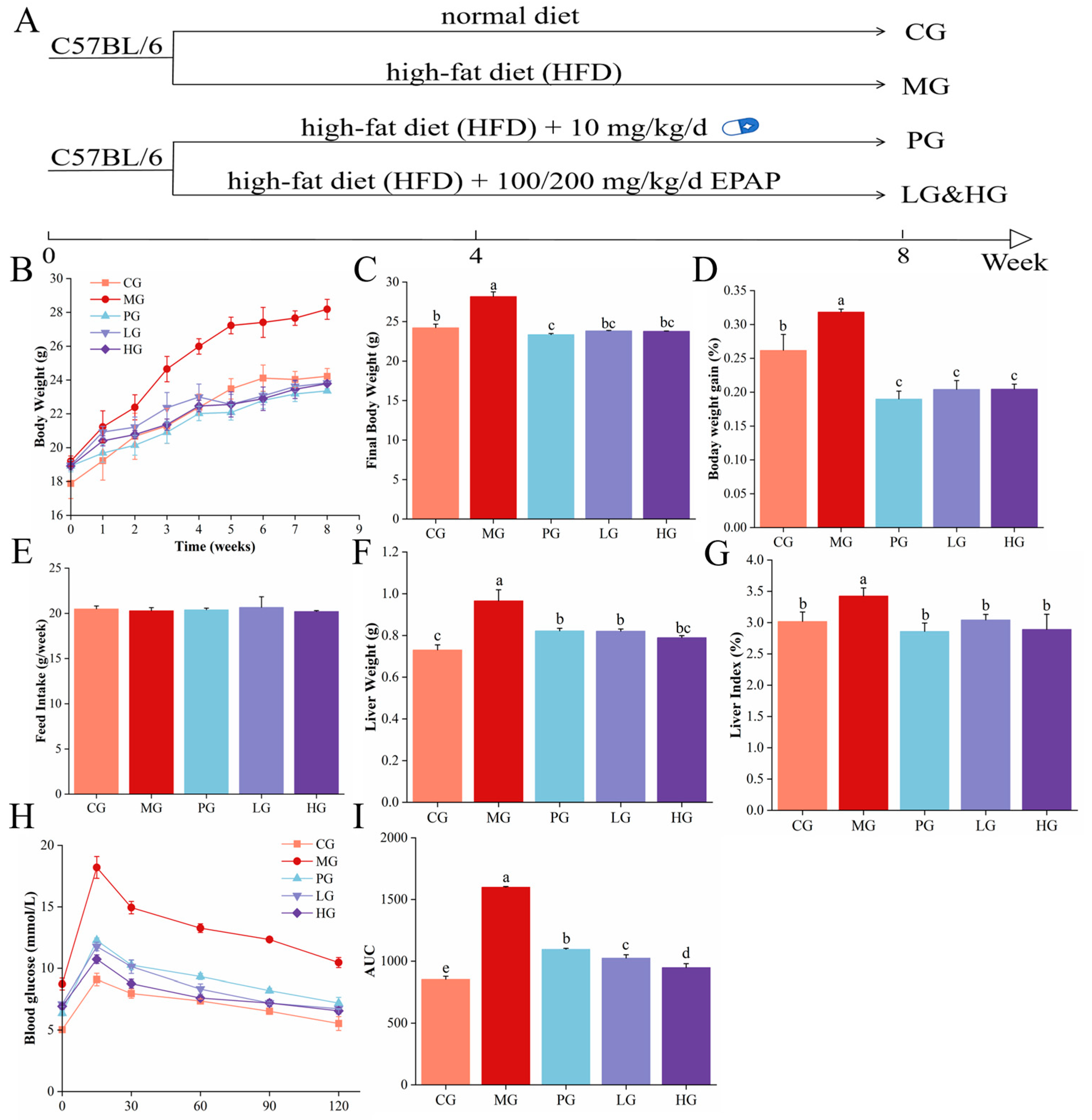
3.2. PAE Improved Body Weight Parameters in NAFLD Mice
3.3. PAE Maintained Glucose Homeostasis
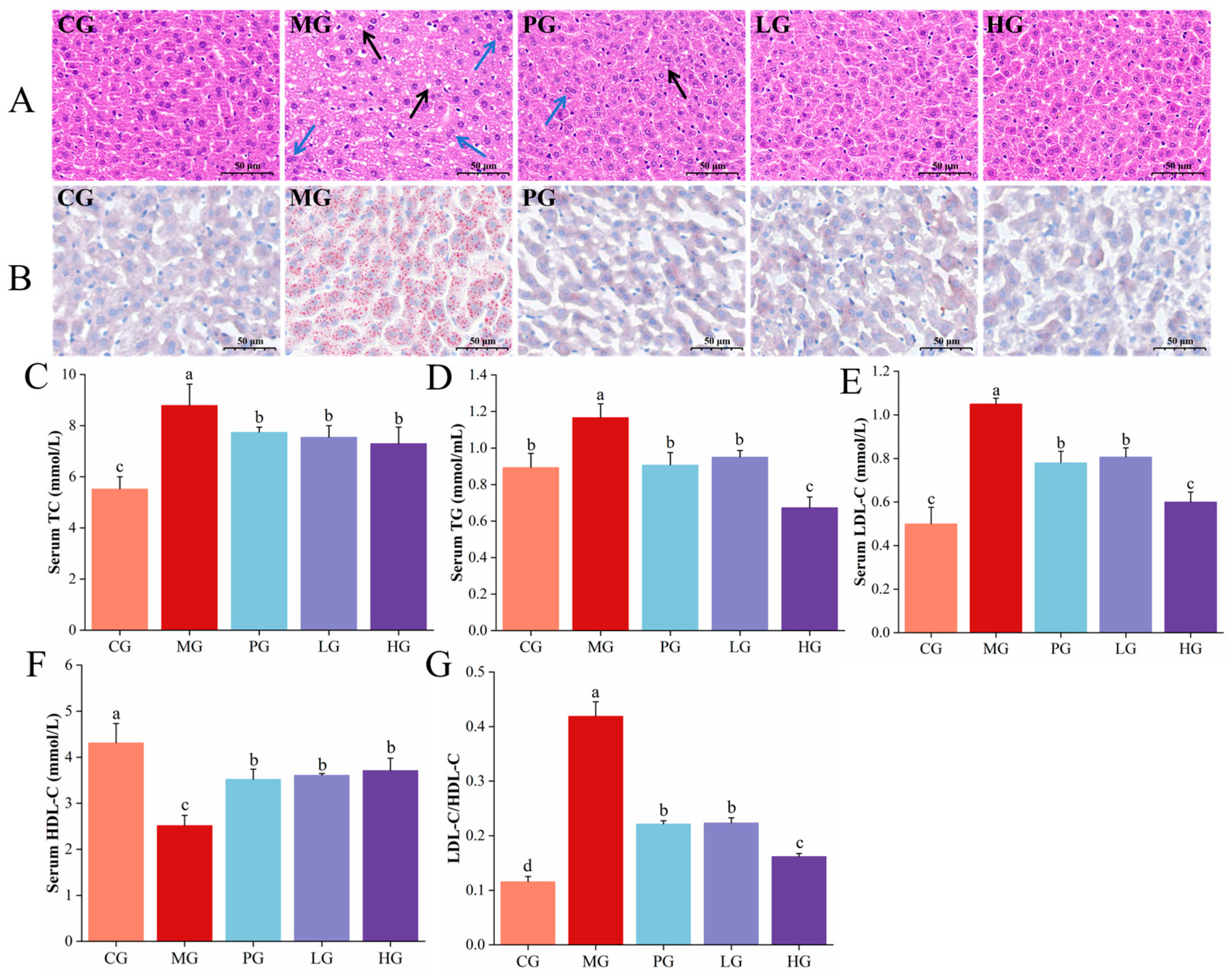
3.4. Pathological Observations of Liver
3.5. PAE Improved HFD-Induced Lipid Accumulation
3.6. PAE Ameliorated HFD-Induced Liver Damage in NAFLD Mice
3.7. PAE Alleviated HFD-Induced Oxidative Damage of NAFLD Mice
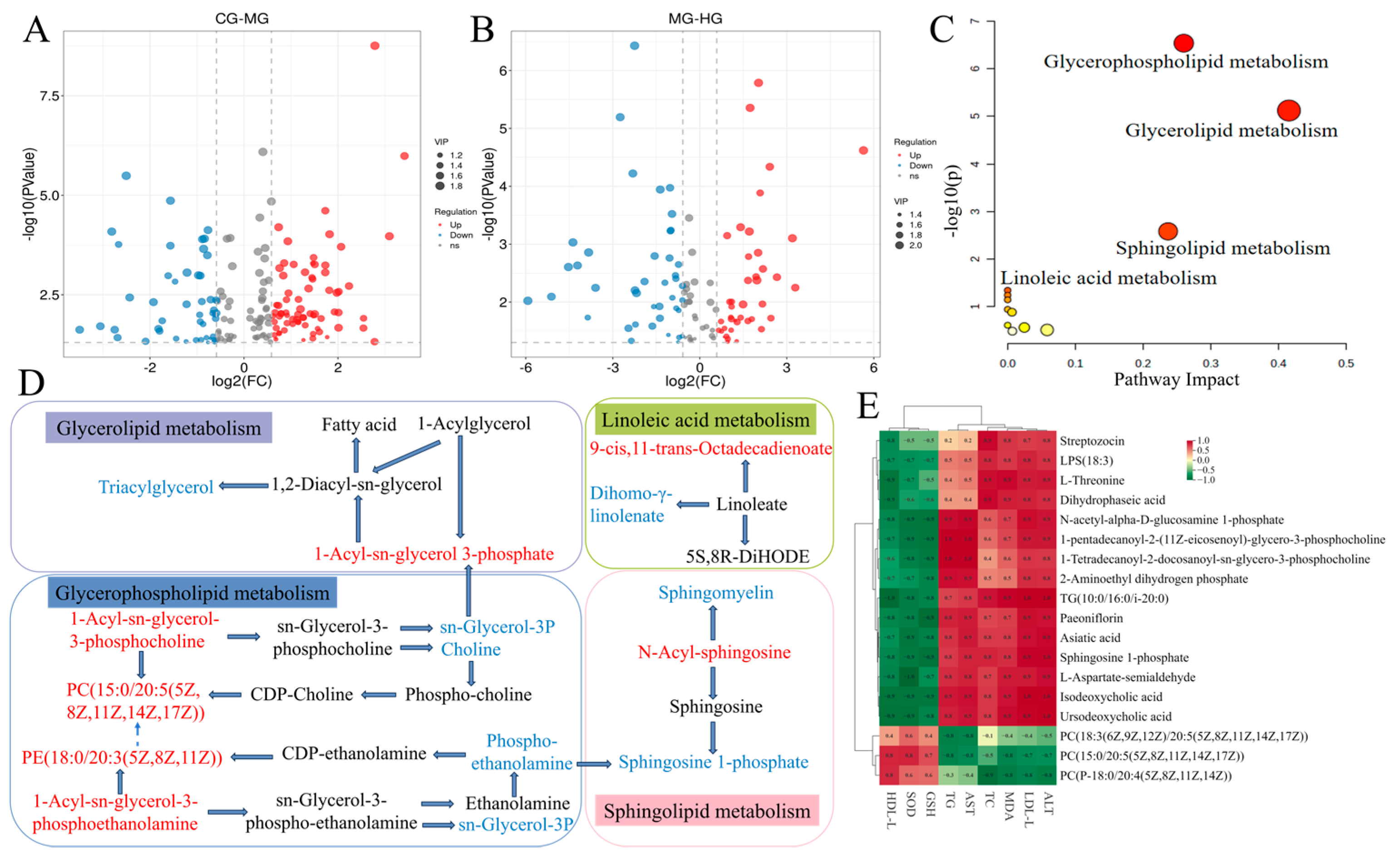
3.8. Liver Metabolomics Analysis
3.9. Potential Relationships Between Differential Metabolites and Biochemical Indices
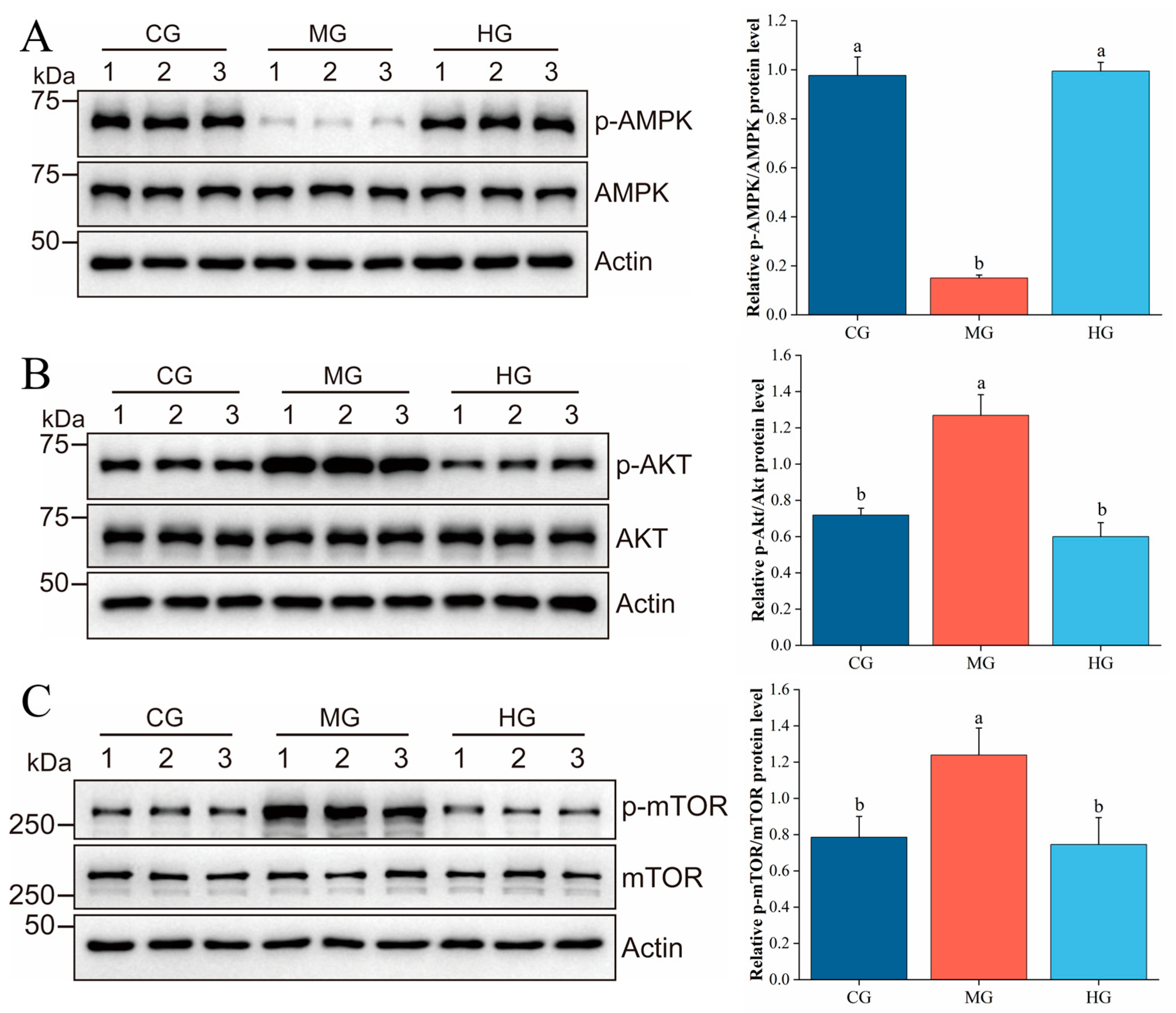
3.10. PAE Suppresses Hepatic Lipid Accumulation via Regulating the AMPK/AKT/mTOR Pathway
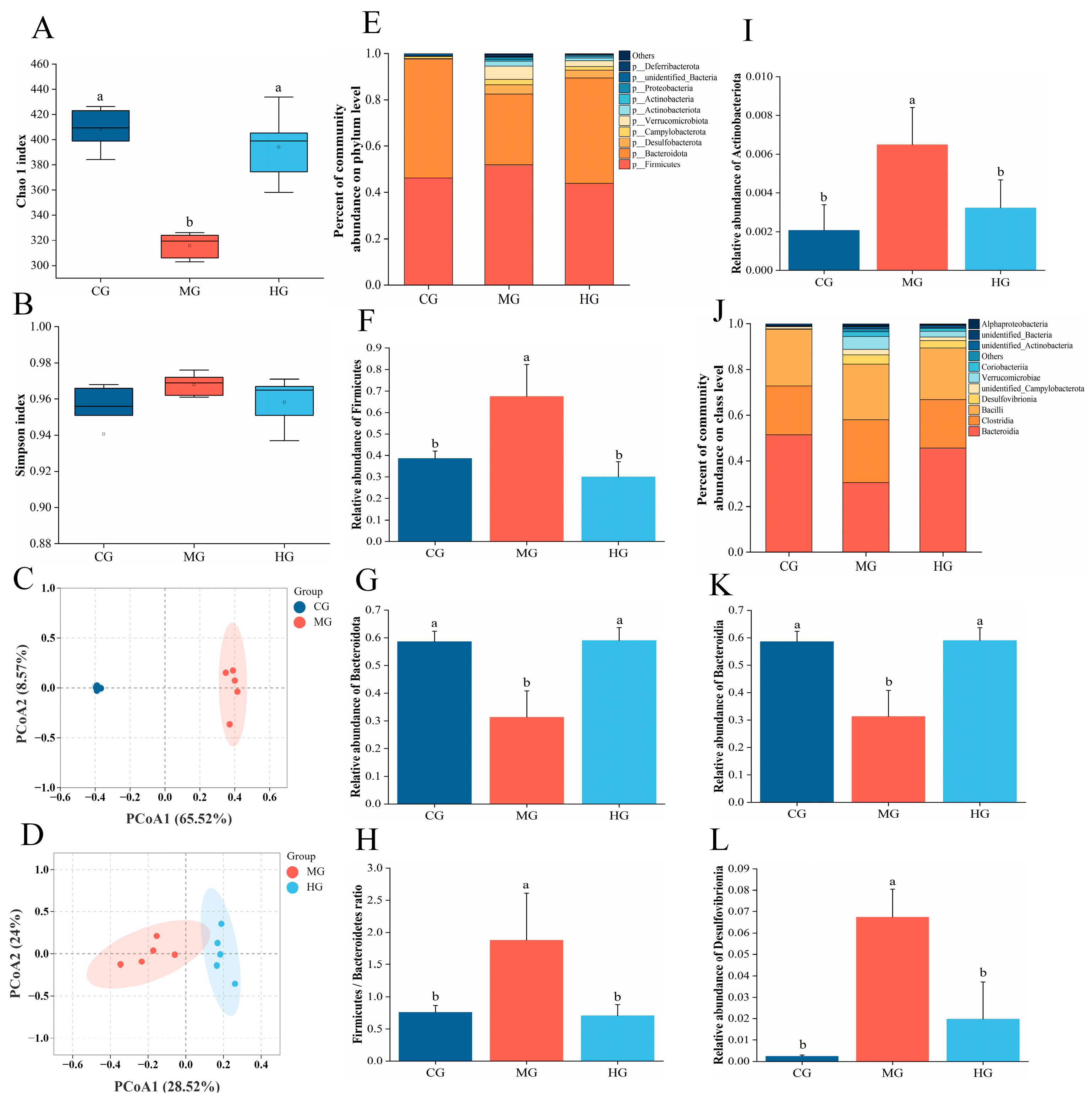
3.11. The Impact of PAE on Gut Microbiota Dysbiosis in NAFLD Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunt, E.M.; Kleiner, D.E.; Carpenter, D.H.; Rinella, M.; Harrison, S.A.; Loomba, R.; Younossi, Z.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; American Association for the Study of Liver Diseases NASH Task Force. NAFLD: Reporting Histologic Findings in Clinical Practice. Hepatology 2021, 73, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshioka, Y.; Kitakaze, T.; Yamashita, Y.; Ashida, H. Preventive effects of black soybean polyphenols on non-alcoholic fatty liver disease in three different mouse models. Food Funct. 2022, 13, 1000–1014. [Google Scholar] [CrossRef]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. ESPEN 2021, 44, 1–14. [Google Scholar] [CrossRef]
- Qin, Y.; Fan, R.Y.; Liu, Y.X.; Qiu, S.Y.; Wang, L. Exploring the potential mechanism of Rubus corchorifolius L. fruit polyphenol-rich extract in mitigating non-alcoholic fatty liver disease by integration of metabolomics and transcriptomics profiling. Food Funct. 2023, 14, 9295–9308. [Google Scholar] [CrossRef]
- Ran, X.; Hu, G.Q.; He, F.D.; Li, K.F.; Li, F.; Xu, D.W.; Liu, J.X.; Fu, S.P. Phytic Acid Improves Hepatic Steatosis, Inflammation, and Oxidative Stress in High-Fat Diet (HFD)-Fed Mice by Modulating the Gut-Liver Axis. J. Agric. Food Chem. 2022, 70, 11401–11411. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.X.; Li, Y.; Chen, H.W.; Wang, C.Y.; Wong, V.K.W.; Jiang, Z.H.; Zhang, W. Phytotherapy using blueberry leaf polyphenols to alleviate non-alcoholic fatty liver disease through improving mitochondrial function and oxidative defense. Phytomedicine 2020, 69, 153209. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.H.; Wong, X.J.; Chong, T.F.; Muangkot, P.; Heng, A.T.; Tanee, T.; Lee, S.Y. The complete chloroplast genome of Pandanus amaryllifolius Roxb. ex Lindl. (Pandanaceae) and its phylogenetic relationship. Mitochondrial DNA B Resour. 2024, 9, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.K.; An, N.T.D.; Anh, P.K.; Truc, T.T. Microwave-assisted extraction of chlorophyll and polyphenol with antioxidant activity from Pandanus amaryllifolius Roxb. in Vietnam. In Proceedings of the International Conference on Materials Science and Manufacturing Technology (ICMSMT 2021), Coimbatore, India, 8–9 April 2021; p. 012039. [Google Scholar]
- Thanebal, S.A.P.P.; Vun-Sang, S.; Iqbal, M. Hepatoprotective effects of Pandanus amaryllifolius against carbon tetrachloride (CCl4) induced toxicity: A biochemical and histopathological study. Arab. J. Chem. 2021, 14, 103390. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.; Han, M.; Gao, Z. Polyphenols synergistic drugs to ameliorate non-alcoholic fatty liver disease via signal pathway and gut microbiota: A review. J. Adv. Res. 2024, 68, 43–62. [Google Scholar] [CrossRef]
- Maciejewska, D.; Skonieczna-Zydecka, K.; Lukomska, A.; Gutowska, I.; Dec, K.; Kupnicka, P.; Palma, J.; Pilutin, A.; Marlicz, W.; Stachowska, E. The Short Chain Fatty Acids and Lipopolysaccharides Status in Sprague-Dawley Rats Fed with High-Fat and High-Cholesterol Diet. J. Physiol. Pharmacol. 2018, 69, 6. [Google Scholar] [CrossRef]
- Wang, R.M.; Wang, L.; Wu, H.B.; Zhang, L.; Hu, X.P.; Li, C.F.; Liu, S.X. Noni (Morinda citrifolia L.) fruit phenolic extract supplementation ameliorates NAFLD by modulating insulin resistance, oxidative stress, inflammation, liver metabolism and gut microbiota. Food Res. Int. 2022, 160, 111732. [Google Scholar] [CrossRef]
- Mu, H.N.; Zhou, Q.; Yang, R.Y.; Tang, W.Q.; Li, H.X.; Wang, S.M.; Li, J.; Chen, W.X.; Dong, J. Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice. Food Res. Int. 2021, 143, 110240. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wang, S.; Wang, J.; Su, C.; Zhang, L.; Li, C.; Liu, S. Phenolics from noni (Morinda citrifolia L.) fruit alleviate obesity in high fat diet-fed mice via modulating the gut microbiota and mitigating intestinal damage. Food Chem. 2022, 402, 134232. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Wang, R.M.; Hu, X.P.; Li, C.F.; Wang, L. Bio-affinity ultra-filtration combined with HPLC-ESI-qTOF-MS/MS for screening potential α-glucosidase inhibitors from (Bge.) Sok. leaf-tea and analysis. Food Chem. 2022, 373, 131528. [Google Scholar] [CrossRef]
- Hua, Y.J.; Lv, J.; Zhang, Y.; Ding, Y.J.; Chen, J.H. LC-MS-based serum metabolomics analysis and potential biomarkers for oxaliplatin induced neurotoxicity in colorectal cancer. J. Pharm. Biomed. 2025, 252, 116492. [Google Scholar] [CrossRef]
- Ren, F.; Chen, Q.P.; Meng, C.; Chen, H.M.; Zhou, Y.J.; Zhang, H.; Chen, W.J. Serum metabonomics revealed the mechanism of Ganoderma amboinense polysaccharides in preventing non-alcoholic fatty liver disease (NAFLD) induced by high-fat diet. J. Funct. Foods 2021, 82, 104496. [Google Scholar] [CrossRef]
- Guo, F.; Xiong, H.; Tsao, R.; Wen, X.; Liu, J.; Chen, D.; Jiang, L.; Sun, Y. Multi-omics reveals that green pea (Pisum sativum L.) hull supplementation ameliorates non-alcoholic fatty liver disease via the SHMT2/glycine/mTOR/PPAR-γ signaling pathway. Food Funct. 2023, 14, 7195–7208. [Google Scholar] [CrossRef]
- Yang, H.B.; Song, J.Y.; Xu, C.; Li, J.; Zhang, C.; Xie, S.; Teng, C.L. Interventional effects of Pueraria oral liquid on T2DM rats and metabolomics analysis. Biomed. Pharmacother. 2024, 175, 116780. [Google Scholar] [CrossRef]
- Guo, F.H.; Tsao, R.; Li, C.Y.; Wang, X.Y.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol Content of Green Pea (Pisum sativum L.) Hull under In Vitro Digestion and Effects of Digestive Products on Anti-Inflammatory Activity and Intestinal Barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Shih, Y.Y.; Yeh, Y.T.; Huang, C.H.; Liao, C.A.; Hu, C.Y.; Nagabhushanam, K.; Ho, C.T.; Chen, Y.K. Pterostilbene and Its Derivative 3′-Hydroxypterostilbene Ameliorated Nonalcoholic Fatty Liver Disease Through Synergistic Modulation of the Gut Microbiota and SIRT1/AMPK Signaling Pathway. J. Agr. Food Chem. 2022, 70, 4966–4980. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Yang, C.; Xia, J.; Tan, Y.; Peng, X.Y.; Xiong, W.; Li, N. Novel insights into the role of quercetin and kaempferol from Carthamus tinctorius L. in the management of nonalcoholic fatty liver disease via NR1H4-mediated pathways. Int. Immunopharmacol. 2024, 143, 113035. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, A.; Lang, D.K.; Arora, R.; Kumar, N.; Diwan, V.; Saini, B. Natural Products as Sources of Multitarget Compounds: Advances in the Development of Ferulic Acid as Multitarget Therapeutic. Curr. Top. Med. Chem. 2022, 22, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Ouyang, H.; Gu, X.N.; Dong, S.Y.; Lu, B.; Huang, Z.L.; Li, J.; Ji, L.L. Caffeic acid ameliorates metabolic dysfunction-associated steatotic liver disease via alleviating oxidative damage and lipid accumulation in hepatocytes through activating Nrf2 via targeting Keap1. Free Radic. Bio Med. 2024, 224, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.J.; Zhang, C.; Lei, H.H.; Chen, C.; Cao, Z.; Song, Y.C.; Chen, G.; Wu, F.; Zhou, J.L.; Lu, Y.J.; et al. Structural Insights into Amelioration Effects of Quercetin and Its Glycoside Derivatives on NAFLD in Mice by Modulating the Gut Microbiota and Host Metabolism. J. Agric. Food Chem. 2022, 70, 14732–14743. [Google Scholar] [CrossRef]
- Dong, M.Y.; Cui, Q.; Li, Y.N.; Li, Y.J.; Chang, Q.Y.; Bai, R.X.; Wei, M.J.; Zhao, L.; Chen, Q.L. Ursolic acid suppresses fatty liver-associated hepatocellular carcinoma by regulating lipid metabolism. Food Biosci. 2024, 60, 104460. [Google Scholar] [CrossRef]
- Peverill, W.; Powell, L.W.; Skoien, R. Evolving Concepts in the Pathogenesis of NASH: Beyond Steatosis and Inflammation. Int. J. Mol. Sci. 2014, 15, 8591–8638. [Google Scholar] [CrossRef]
- Arner, P. The adipocyte in insulin resistance: Key molecules and the impact of the thiazolidinediones. Trends Endocrinol. Metab. 2003, 14, 137–145. [Google Scholar] [CrossRef]
- JParada, J. Aguilera, Starch matrices and the glycemic response. Food Sci. Technol. Int. 2011, 17, 187–204. [Google Scholar] [CrossRef]
- Ajmera, V.; Loomba, R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol. Metab. 2020, 50, 101167. [Google Scholar] [CrossRef]
- Fan, C.W.; Ling-Hu, A.L.; Sun, D.L.; Gao, W.M.; Zhang, C.F.; Duan, X.Q.; Li, H.Y.; Tian, W.Y.; Yu, Q.; Ke, Z.L. Nobiletin Ameliorates Hepatic Lipid Deposition, Oxidative Stress, and Inflammation by Mechanisms That Involve the Nrf2/NF-κB Axis in Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2023, 71, 20105–20117. [Google Scholar] [CrossRef] [PubMed]
- Darvin, S.S.; Toppo, E.; Esakkimuthu, S.; Krishna, T.P.A.; Ceasar, S.A.; Stalin, A.; Balakrishna, K.; Muniappan, N.; Pazhanivel, N.; Mahaprabhu, R.; et al. Hepatoprotective effect of bisbenzylisoquinoline alkaloid tiliamosine from in high-fat diet/diethylnitrosamine-induced non-alcoholic steatohepatitis. Biomed. Pharmacother. 2018, 108, 963–973. [Google Scholar] [CrossRef]
- Kim, S.-J.; Cho, Y.-C. The Association of AUDIT Levels with Obesity Indices, Liver Function Tests, and Serum Lipid Levels in Male Health Checkup Examinees. J. Korea Acad. Ind. Coop. Soc. 2015, 16, 3230–3242. [Google Scholar] [CrossRef]
- Kotani, K.; Satoh, N.; Kato, Y.; Araki, R.; Koyama, K.; Okajima, T.; Tanabe, M.; Oishi, M.; Yamakage, H.; Yamada, K.; et al. A novel oxidized low-density lipoprotein marker, serum amyloid A-LDL, is associated with obesity and the metabolic syndrome. Atherosclerosis 2009, 204, 526–531. [Google Scholar] [CrossRef]
- Rader, D.J. Spotlight on HDL biology: New insights in metabolism, function, and translation. Cardiovasc. Res. 2014, 103, 337–340. [Google Scholar] [CrossRef]
- Wang, R.; Yao, L.; Lin, X.; Hu, X.; Wang, L. Exploring the potential mechanism of Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic rich extract on ameliorating nonalcoholic fatty liver disease by integration of transcriptomics and metabolomics profiling. Food Res. Int. 2022, 151, 110824. [Google Scholar] [CrossRef]
- Albi, E.; Cataldi, S.; Rossi, G.; Magni, M.V. A possible role of cholesterol-sphingomyelin/phosphatidylcholine in nuclear matrix during rat liver regeneration. J. Hepatol. 2003, 38, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and their anti-obesity role mediated by the gut microbiota: A comprehensive review. Rev. Endocr. Metab. Dis. 2021, 22, 367–388. [Google Scholar] [CrossRef]
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quinones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendes, A.; et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004, 106, 261–268. [Google Scholar] [CrossRef]
- Choi, C.-S.; Chung, H.-K.; Choi, M.-K.; Kang, M.-H. Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Nutr. Res. Pract. 2010, 4, 114–120. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Radwan, N.M.; Aboul Ezz, H.S.; Salama, N.A. The antioxidant effect of Green Tea Mega EGCG against electromagnetic radiation-induced oxidative stress in the hippocampus and striatum of rats. Electromagn. Biol. Med. 2016, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Nishiumi, S.; Saito, M.; Yano, Y.; Azuma, T.; Yoshida, M. Identification of Lipid Species Linked to the Progression of Non-Alcoholic Fatty Liver Disease. Curr. Drug Targets 2015, 16, 1293–1300. [Google Scholar] [CrossRef]
- Régnier, M.; Polizzi, A.; Guillou, H.; Loiseau, N. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 2019, 159, 9–22. [Google Scholar] [CrossRef]
- Kihara, A. Sphingosine 1-phosphate is a key metabolite linking sphingolipids to glycerophospholipids. Biochim. Biophys. Acta Biomembr. 2014, 1841, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Xu, J.Y.; Jia, W.J.; Zhang, G.Y.; Liu, L.Y.; Wang, L.Y.; Wu, D.; Tao, J.H.; Yue, H.L.; Zhang, D.J.; Zhao, X.H. Extract of Silphium perfoliatum L. improve lipid accumulation in NAFLD mice by regulating AMPK/FXR signaling pathway. J. Ethnopharmacol. 2024, 327, 118054. [Google Scholar] [CrossRef]
- Thakuri, L.S.; Park, C.M.; Kim, H.-A.; Kim, H.J.; Park, J.W.; Park, J.C.; Rhyu, D.Y. Gracilaria chorda subcritical water ameliorates hepatic lipid accumulation and regulates glucose homeostasis in a hepatic steatosis cell model and obese C57BL/6J mice. J. Ethnopharmacol. 2024, 320, 117395. [Google Scholar] [CrossRef]
- Dai, C.S.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.S.; Hoyer, D.; Schneider, E.K.; Velkov, T.; Xiao, X.L. Rapamycin Confers Neuroprotection against Colistin-Induced Oxidative Stress, Mitochondria Dysfunction, and Apoptosis through the Activation of Autophagy and mTOR/Akt/CREB Signaling Pathways. ACS Chem. Neurosci. 2018, 9, 824–837. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Khambu, B.; Zhang, H.; Yin, X.M. Autophagy in alcoholic liver disease, self-eating triggered by drinking. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Suh, H.R.; Yoon, Y.; Lee, K.J.; Kim, D.G.; Kim, S.; Lee, B.H. Protective effect of resveratrol derivatives on high-fat diet induced fatty liver by activating AMP-activated protein kinase. Arch. Pharm. Res. 2014, 37, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, A.S.; El-Lakkany, N.M.; Mantawy, E.M.; Hammam, O.A.; Botros, S.S.; El-Demerdash, E. Vildagliptin alleviates liver fibrosis in NASH diabetic rats via modulation of insulin resistance, oxidative stress, and inflammatory cascades. Life Sci. 2022, 304, 120695. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Blancher, F.; Berger, B.; Moine, D.; Krause, L.; Bibiloni, R.; Bruneau, A.; Gérard, P.; Siddharth, J.; et al. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci. Rep. 2016, 6, 32484. [Google Scholar] [CrossRef]
- Dong, Y.J.; Lin, M.Q.; Fang, X.; Xie, Z.Y.; Luo, R.; Teng, X.; Li, B.; Li, B.; Li, L.Z.; Jin, H.Y.; et al. Modulating effects of a functional food containing on immune response and gut microbiota in mice treated with cyclophosphamide. J. Funct. Foods 2022, 94, 105102. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.C.; Li, J.E.; Lin, L.Z.; Zheng, G.D. A flavonoid-rich Smilax china L. extract prevents obesity by upregulating the adiponectin-receptor/AMPK signalling pathway and modulating the gut microbiota in mice. Food Funct. 2021, 12, 5862–5875. [Google Scholar] [CrossRef]


| No. | TR (min) | Formula | Adduct | Mw (Da) | Compounds |
|---|---|---|---|---|---|
| 1 | 3.88 | C9H12O2 | [2M+NH4]+ | 152.08 | 4-Ethyl-2-methoxyphenol |
| 2 | 3.18 | C9H8O3 | [M−H]− | 164.05 | 2-Hydroxycinnamic acid |
| 3 | 4.57 | C27H30O15 | [M−H]− | 594.16 | Kaempferol 3-O-beta-D-glucopyranosyl-7-O-alpha-L-rhamnopyranoside |
| 4 | 4.43 | C21H20O12 | [M−H]− | 464.10 | Hyperoside |
| 5 | 4.69 | C25H24O12 | [2M−H]− | 516.13 | CID 153946 |
| 6 | 3.58 | C16H18O9 | [M−H]− | 354.10 | Chlorogenic Acid |
| 7 | 4.28 | C27H30O14 | [M−H]− | 578.16 | Vitexin 2″-O-rhamnoside |
| 8 | 3.74 | C26H28O14 | [2M−H]− | 564.15 | Neoschaftoside |
| 9 | 4.83 | C32H22O10 | [M−H]− | 566.12 | Isoginkgetin |
| 10 | 4.04 | C33H40O19 | [M−H]− | 740.22 | Kaempferol3-rhamninoside |
| 11 | 3.26 | C27H30O16 | [M−H]− | 610.15 | Quercetin 3-(2-glucosylrhamnoside) |
| 12 | 3.78 | C25H24O12 | [M−H2O−H]− | 516.13 | Isochlorogenic acid A |
| 13 | 2.21 | C9H12O3 | [2M+NH4]+ | 168.08 | (4-Hydroxy-3-methoxyphenyl) ethanol |
| 14 | 3.78 | C17H14O5 | [2M−H]− | 298.08 | 5,4′-DIMETHOXY-7-HYDROXYFLAVONE |
| 15 | 3.52 | C27H32O15 | [M−H]− | 596.17 | Butrin |
| 16 | 1.69 | C9H10O2 | [M+H]+ | 150.07 | 4′-Methoxyacetophenone |
| 17 | 4.57 | C27H30O15 | [2M−H]− | 594.16 | 3″-O-L-Rhamnopyranosylastragalin |
| 18 | 4.49 | C10H10O4 | [M−H]− | 194.06 | Ferulic acid |
| 19 | 3.58 | C9H8O4 | [M−H]− | 180.04 | Caffeic acid |
| 20 | 6.32 | C23H27NO8 | [M−H]− | 445.17 | Narceine |
| No. | Metabolite | RT (min) | Molecular Formula | Pub Chem CID | MG vs. CG | HG vs. MG | ||
|---|---|---|---|---|---|---|---|---|
| FC a | Trend | FC a | Trend | |||||
| 1 | PC (15:0/20:5 (5Z,8Z,11Z,14Z,17Z)) | 8.70 | C43H76NO8P | 52922332 | 0.66 | ↓ | 1.78 | ↑ |
| 2 | PC (18:3(6Z,9Z,12Z)/20:5 (5Z,8Z,11Z,14Z,17Z)) | 7.37 | C46H76NO8P | 52922807 | 0.54 | ↓ | 4.42 | ↑ |
| 3 | PC (P-18:0/20:4 (5Z,8Z,11Z,14Z)) | 7.51 | C46H84NO7P | 24779390 | 0.37 | ↓ | 1.97 | ↑ |
| 4 | L-Threonine | 0.82 | C4H9NO3 | 6288 | 1.36 | ↑ | 0.89 | ↓ |
| 5 | Paeoniflorin | 2.82 | C23H28O11 | 442534 | 2.17 | ↑ | 0.50 | ↓ |
| 6 | L-Aspartate-semialdehyde | 0.80 | C4H7NO3 | 439235 | 1.09 | ↑ | 0.88 | ↓ |
| 7 | 1-Tetradecanoyl-2-docosanoyl-sn-glycero-3-phosphocholine | 8.70 | C44H88NO8P | 24778638 | 1.66 | ↑ | 0.09 | ↓ |
| 8 | TG (10:0/16:0/i-20:0) | 6.58 | C49H94O6 | 131777989 | 1.73 | ↑ | 0.71 | ↓ |
| 9 | Isodeoxycholic acid | 6.13 | C24H40O4 | 164672 | 8.54 | ↑ | 0.20 | ↓ |
| 10 | Asiatic acid | 5.47 | C30H48O5 | 119034 | 2.47 | ↑ | 0.39 | ↓ |
| 11 | Dihydrophaseic acid | 4.81 | C15H22O5 | 11988272 | 3.54 | ↑ | 0.69 | ↓ |
| 12 | Ursodeoxycholic acid | 6.13 | C24H40O4 | 31401 | 3.32 | ↑ | 0.49 | ↓ |
| 13 | N-acetyl-alpha-D-glucosamine 1-phosphate | 0.85 | C8H16NO9P | 440364 | 1.18 | ↑ | 0.80 | ↓ |
| 14 | 2-Aminoethyl dihydrogen phosphate | 0.85 | C2H8NO4P | 1015 | 1.26 | ↑ | 0.82 | ↓ |
| 15 | 1-pentadecanoyl-2-(11Z-eicosenoyl)-glycero-3-phosphocholine | 6.47 | C43H84NO8P | 52922324 | 2.27 | ↑ | 0.21 | ↓ |
| 16 | LPS (18:3) | 8.79 | C24H42NO9P | 52926285 | 2.40 | ↑ | 0.47 | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Ren, F.; Zhu, M.; Hu, Y.; Zhao, Z.; Pei, J.; Chen, H.; Chen, W.; Zhong, Q.; Lyu, Y.; et al. Pandanus Amaryllifolius Roxb. Polyphenol Extract Alleviates NAFLD via Regulating Gut Microbiota and AMPK/AKT/mTOR Signaling Pathway. Foods 2025, 14, 1000. https://doi.org/10.3390/foods14061000
Lin J, Ren F, Zhu M, Hu Y, Zhao Z, Pei J, Chen H, Chen W, Zhong Q, Lyu Y, et al. Pandanus Amaryllifolius Roxb. Polyphenol Extract Alleviates NAFLD via Regulating Gut Microbiota and AMPK/AKT/mTOR Signaling Pathway. Foods. 2025; 14(6):1000. https://doi.org/10.3390/foods14061000
Chicago/Turabian StyleLin, Jinji, Fei Ren, Mengxu Zhu, Yibo Hu, Zhiao Zhao, Jianfei Pei, Haiming Chen, Weijun Chen, Qiuping Zhong, Ying Lyu, and et al. 2025. "Pandanus Amaryllifolius Roxb. Polyphenol Extract Alleviates NAFLD via Regulating Gut Microbiota and AMPK/AKT/mTOR Signaling Pathway" Foods 14, no. 6: 1000. https://doi.org/10.3390/foods14061000
APA StyleLin, J., Ren, F., Zhu, M., Hu, Y., Zhao, Z., Pei, J., Chen, H., Chen, W., Zhong, Q., Lyu, Y., He, R., & Chen, W. (2025). Pandanus Amaryllifolius Roxb. Polyphenol Extract Alleviates NAFLD via Regulating Gut Microbiota and AMPK/AKT/mTOR Signaling Pathway. Foods, 14(6), 1000. https://doi.org/10.3390/foods14061000






