Almond By-Products: A Comprehensive Review of Composition, Bioactivities, and Influencing Factors
Abstract
:1. Introduction
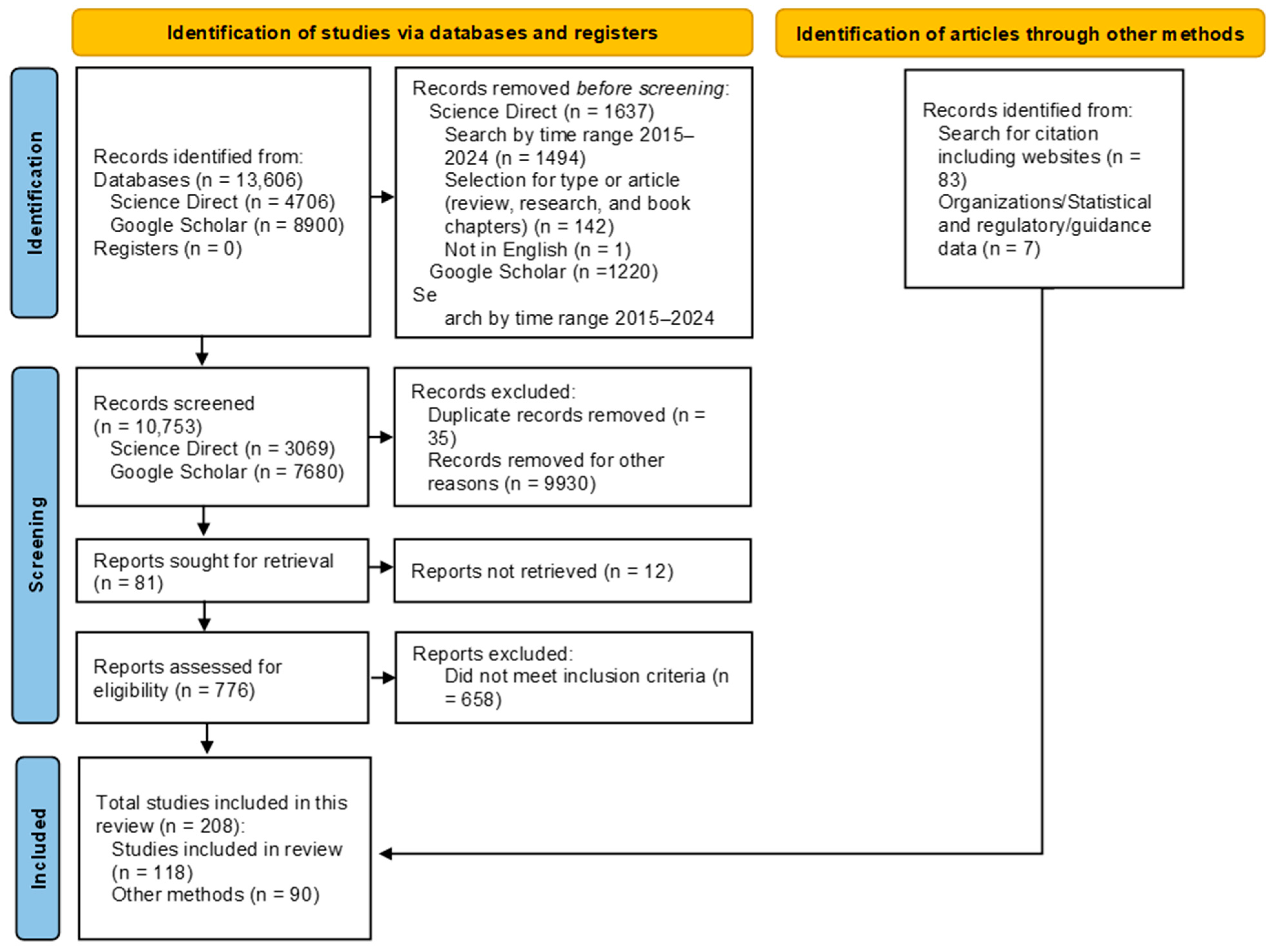
2. Methodology
2.1. Search Strategy and Data Sources
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Study Selection and Data Extraction
3. Almond By-Products
3.1. Physical Characterization of Almond By-Products
3.2. Chemical Characterization and Nutritional Content of Almond By-Products
3.2.1. Almond Hull (AH)
3.2.2. Almond Shell (AS)
3.2.3. Almond Skin (ASk)
4. Bioactive Compounds in Almond By-Products and Affecting Factors
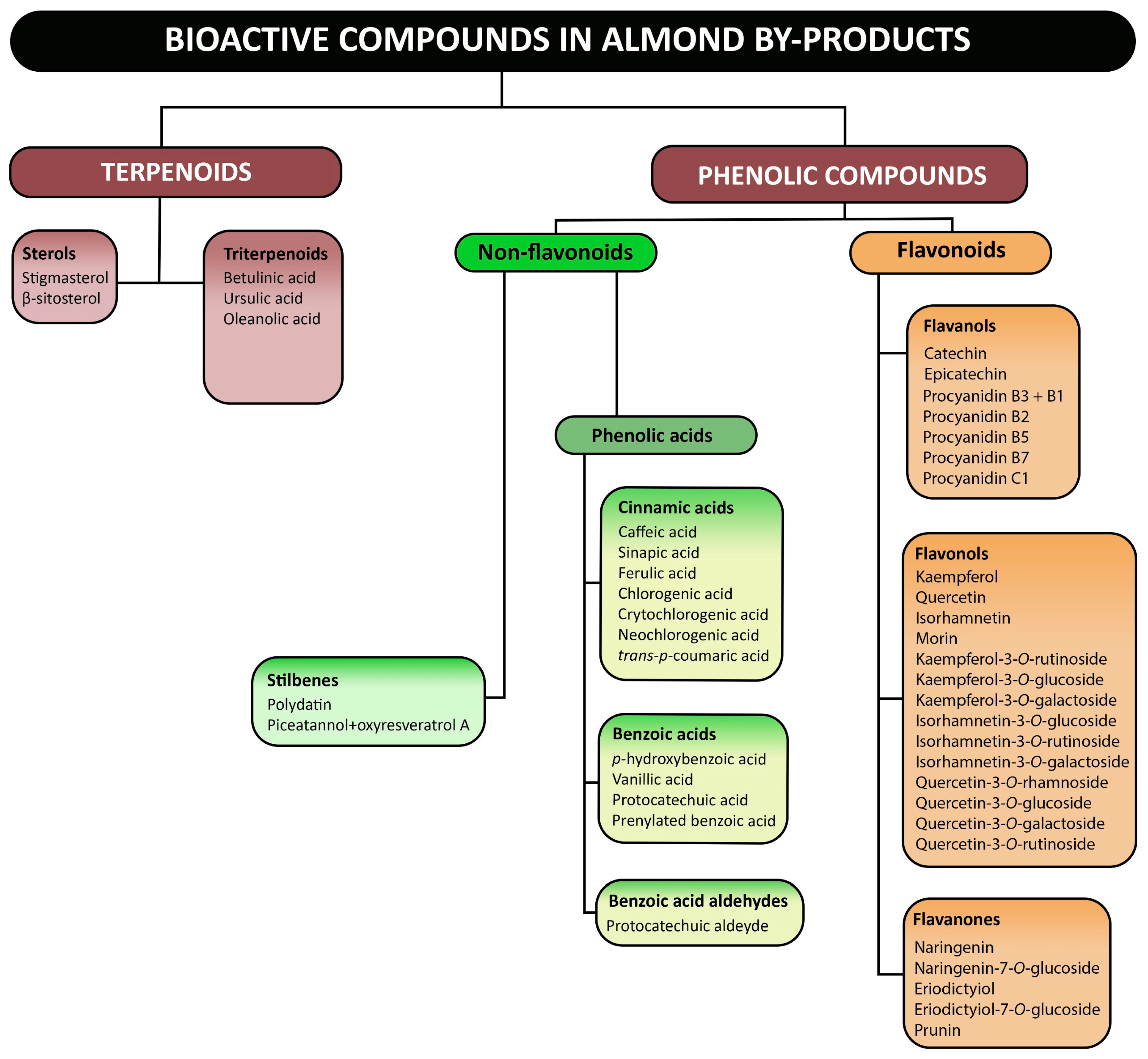
4.1. Overview of Bioactive Compounds
4.2. Bioactive Compounds from Almond By-Products
4.2.1. Factors Affecting Bioactive Compounds in Almond Hulls
4.2.2. Factors Affecting Bioactive Compounds in Almond Shell
4.2.3. Factors Affecting Bioactive Compounds in Almond Skin
4.2.4. Factors Affecting Bioactive Compounds in Almond Blanching Water
5. Biological Activities of Almond By-Products and Influencing Factors
5.1. Factors Affecting Antioxidant Capacity of Almond By-Products
5.2. Factors Affecting Antimicrobial Effect of Almond By-Products
5.3. Factors Affecting Anti-Inflammatory Effect of Almond By-Products
5.4. Factors Affecting Prebiotic Properties of Almond By-Products
5.5. Other Biological Activities of Almond By-Products and Influencing Factors
6. Conclusions
7. Challenges and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 20 August 2024).
- Lacivita, V.; Derossi, A.; Caporizzi, R.; Lamacchia, C.; Speranza, B.; Guerrieri, A.; Racioppo, A.; Corbo, M.R.; Sinigaglia, M.; Severini, C. Discover hidden value of almond by-products: Nutritional, sensory, technological and microbiological aspects. Future Foods 2024, 10, 100398. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food waste and byproducts: An opportunity to minimize malnutrition and hunger in developing countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Food Waste Index Report 2021. Nairobi. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 9 September 2024).
- Statista 2025. Consumer Goods & FMCG. Food & Nutrition. Food Waste per Capita in Portugal in 2022, by Sector. Eurostat: Statistics Portugal. 2022. Available online: https://www.statista.com/statistics/1394109/portugal-food-waste-per-capita-by-sector/ (accessed on 5 January 2025).
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle—Developing a circular economy in agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Barreira, J.C.M.; Ferreira, I.C.F.R.; Bento, A. Avaliação e Sistematização de Subprodutos–Frutos Secos: Uma Aproximação Quantitativa à Disponibilidade de Subprodutos, 1st ed.; CNCFS—Centro Nacional de Competências dos Frutos Secos: Bragança, Portugal, 2020; pp. 1–44. Available online: http://hdl.handle.net/10198/26874 (accessed on 20 August 2024).
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization challenges to almond residues: Phytochemical composition and functional application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.J.; Zhang, Y.-L.; Xu, X.-Q. Current situation, global potential distribution and evolution of six almond species in China. Front. Plant Sci. 2021, 12, 619883. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond by-products: Valorization for sustainability and competitiveness of the industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Nunes, M.A.; da Silva, B.V.; Pimentel, F.B.; Costa, A.S.G.; Alvarez-Ortí, M.; Pardo, J.E.; Oliveira, M.B.P.P. Almond cold-pressed oil by-product as ingredient for cookies with potential health benefits: Chemical and sensory evaluation. Food Sci. Hum. Wellness 2019, 8, 292–298. [Google Scholar] [CrossRef]
- Valverde, M.; Madrid, R.; García, A.L.; Del Amor, F.M.; Rincón, L.F. Use of almond shell and almond hull as substrates for sweet pepper cultivation. Effects on fruit yield and mineral content. Span. J. Agric. Res. 2013, 11, 164–172. [Google Scholar] [CrossRef]
- Berryman, C.E.; Preston, A.G.; Karmally, W.; Deckelbaum, R.J.; Kris-Etherton, P.M. Effects of almond consumption on the reduction of LDL-cholesterol: A discussion of potential mechanisms and future research directions. Nutr. Rev. 2011, 69, 171–185. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Rajoka, M.S.R.; Barros, L.; Mascoloti Sprea, R.; Amaral, J.S.; Prieto, M.A.; et al. Revalorization of almond by-products for the design of novel functional foods: An updated review. Foods 2021, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Vickers, Z.; Peck, A.; Labuza, T.; Huang, G. Impact of almond form and moisture content on texture attributes and acceptability. J. Food Sci. 2014, 79, S1399–S1406. [Google Scholar] [CrossRef]
- Cheely, A.N.; Pegg, R.B.; Kerr, W.L.; Swanson, R.B.; Huang, G.; Parrish, D.R.; Kerrihard, A.L. Modeling sensory and instrumental texture changes of dry roasted almonds under different storage conditions. LWT-Food Sci. Technol. 2018, 91, 498–504. [Google Scholar] [CrossRef]
- Franklin, L.M.; King, E.S.; Chapman, D.; Byrnes, N.; Huang, G.; Mitchell, A.E. Flavor and acceptance of roasted California almonds during accelerated storage. J. Agric. Food Chem. 2018, 66, 1222–1232. [Google Scholar] [CrossRef]
- Özcan, M.M. A review on some properties of almond: Impact of processing, fatty acids, polyphenols, nutrients, bioactive properties, and health aspects. J. Food Sci. Technol. 2023, 60, 1493–1504. [Google Scholar] [CrossRef]
- Sobhy, H.M.; El Abd, M.; Elsabie, W.; FathyForsan, H. Study of high nutritive value of almond milk beverage. Plant Archiv. 2021, 21, 2493–2496. [Google Scholar] [CrossRef]
- Tapsell, L.; Sabaté, J.; Martínez, R.; Llavanera, M.; Neale, E.; Salas-Huetos, A. Novel lines of research on the environmental and human health impacts of nut consumption. Nutrients 2023, 15, 955. [Google Scholar] [CrossRef]
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of nuts and seeds and health outcomes including cardiovascular disease, diabetes and metabolic disease, cancer, and mortality: An umbrella review. Adv. Nutr. 2022, 13, 2136–2148. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Elyaspour, Z. Effects of nut consumption on cardiovascular risk factors and coronary heart diseases. Funct. Foods Health Dis. 2022, 12, 639. [Google Scholar] [CrossRef]
- Gervasi, T.; Barreca, D.; Laganà, G.; Mandalari, G. Health benefits related to tree nut consumption and their bioactive compounds. Int. J. Mol. Sci. 2021, 22, 5960. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Economics, Statistics and Market Information System (ESMIS). Division: Foreign Agricultural Service. Category: Crops and Crop Products—Fruits. Tree Nuts: World Markets and Trade (Latest Release: Oct 24, 2024). USDA: Ithaca, NY 14853-4301, 2024. Available online: https://usda.library.cornell.edu (accessed on 11 December 2024).
- United States Department of Agriculture (USDA), Foreign Agricultural Services. Data and Analysis—Production—Almonds. USDA: Washington, DC 20250, 2024. Available online: https://fas.usda.gov/data/production/commodity/0577400 (accessed on 11 December 2024).
- Food and Agriculture Organization of the United Nations (FAO). Agriculture Data—Crops and Livestock Products—Almonds in Shell; FAOSTAT: Rome, Italy, 2024; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 January 2024).
- Chen, P.; Cheng, Y.; Deng, S.; Lin, X.; Huang, G.; Ruan, R. Utilization of almond residues. Int. J. Agric. Biol. Eng. 2010, 3, 1–18. [Google Scholar] [CrossRef]
- Silva, V.; Oliveira, I.; Pereira, J.A.; Gonçalves, B. Almond by-products substrates as sustainable amendments for green bean cultivation. Plants 2024, 13, 540. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Santos, R.; Saavedra, M.J.; Aires, A.; Pascual-Seva, N.; Barros, A. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly)phenols. Ind. Crops Prod. 2019, 132, 186–196. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.-Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Abou-Zaid, M.M.; Shahidi, F. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem. 2006, 54, 312–318. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Amarowicz, R.; Shahidi, F. Antioxidant activity of almonds and their by-products in food model systems. J. Am. Oil Chem. Soc. 2006, 83, 223–230. [Google Scholar] [CrossRef]
- Fabroni, S.; Trovato, A.; Ballistreri, G.; Tortorelli, S.A.; Foti, P.; Romeo, F.V.; Rapisarda, P. Almond [Prunus dulcis (Mill.) DA Webb] processing residual hull as a new source of bioactive compounds: Phytochemical composition, radical Scavenging and antimicrobial activities of extracts from Italian cultivars (‘Tuono’, ‘Pizzuta’, ‘Romana’). Molecules 2023, 28, 605. [Google Scholar] [CrossRef]
- Najari, Z.; Khodaiyan, F.; Yarmand, M.S.; Hosseini, S.S. Almond hulls waste valorization towards sustainable agricultural development: Production of pectin, phenolics, pullulan, and single cell protein. J. Waste Manag. 2022, 141, 208–219. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Kacem, I.; Martinez-Saez, N.; Kallel, F.; Ben Jeddou, K.; Boisset Helbert, C.; Ellouze Chaabouni, S.; Del Castillo, M.D. Use of almond shell as food ingredient. Eur. Food Res. Technol. 2017, 243, 2115–2126. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of proanthocyanidins in almond blanch water by HPLC–ESI–QqQ–MS/MS and MALDI–TOF/TOF MS. Food Res. Int. 2012, 49, 798–806. [Google Scholar] [CrossRef]
- Gaglio, R.; Tesoriere, L.; Maggio, A.; Viola, E.; Attanzio, A.; Frazzitta, A.; Badalamenti, N.; Bruno, M.; Franciosi, E.; Moschetti, G.; et al. Reuse of almond by-products: Functionalization of traditional semolina sourdough bread with almond skin. Int. J. Food Microbiol. 2023, 395, 110194. [Google Scholar] [CrossRef]
- Massantini, R.; Frangipane, M.T. Progress in almond quality and sensory assessment: An overview. Agriculture 2022, 12, 710. [Google Scholar] [CrossRef]
- Tomishima, H.; Luo, K.; Mitchell, A.E. The almond (Prunus dulcis): Chemical properties, utilization, and valorization of coproducts. Annu. Rev. Food Sci. Technol. 2022, 13, 145–166. [Google Scholar] [CrossRef]
- Ollani, S.; Peano, C.; Sottile, F. Recent innovations on the reuse of almond and hazelnut by-products: A review. Sustainability 2024, 16, 2577. [Google Scholar] [CrossRef]
- Kernel Weight–USDA Incomings received by Almond Board of California. Shell & Hull Estimations—Varietal Coproduct Ratios and Production Volumes (ABC2020). Available online: https://www.almonds.com/sites/default/files/2023-08/2023.07_PosRpt8883.pdf (accessed on 11 December 2024).
- Toles, C.A.; Marshall, W.E.; Johns, M.M.; Wartelle, L.H.; McAloon, A. Acid-activated carbons from almond shells: Physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 71, 87–92. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. An efficient multiphase bioprocess for enhancing the renewable energy production from almond shells. Energy Convers. Manag. 2020, 203, 112235. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Martínez, G.A.; del Carmen Salas, M. Almond shell waste: Possible local rockwool substitute in soilless crop culture. Sci. Hortic. 2005, 103, 453–460. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Gradziel, T.M. Almond (Prunus dulcis) breeding. In Breeding Plantation Tree Crops: Temperate Species, 1st ed.; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; Volume 1, pp. 1–31. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of almond shell characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, C.A. Shell cracking strength in almond (Prunus dulcis [Mill.] D.A. Webb.) and its implication in uses as a value-added product. Bioresour. Technol. 2008, 99, 5567–5573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Ma, Q.-H. Lignin biosynthesis and its diversified roles in disease resistance. Genes 2024, 15, 295. [Google Scholar] [CrossRef]
- DePeters, E.J.; Swanson, K.L.; Bill, H.M.; Asmus, J.; Heguy, J.M. Nutritional composition of almond hulls. Appl. Anim. Sci. 2020, 36, 761–770. [Google Scholar] [CrossRef]
- Recalde, A.; Evan, T.; Fernández, C.; Roldán, R.A.; López-Feria, S.; Carro, M.D. Chemical composition and nutritive value of almond hulls from two almond varieties and influence of including almond hulls in the diet on in vitro ruminal fermentation and methane production. Vet. Sci. 2024, 11, 242. [Google Scholar] [CrossRef]
- Elahi, M.Y.; Kargar, H.; Dindarlou, M.S.; Kholif, A.E.; Elghandour, M.M.Y.; Rojas-Hernández, S.; Odongo, N.E.; Salem, A.Z.M. The chemical composition and in vitro digestibility evaluation of almond tree (Prunus dulcis D. A. Webb syn. Prunus amygdalus; var. Shokoufeh) leaves versus hulls and green versus dry leaves as feed for ruminants. Agrofor. Syst. 2017, 91, 773–780. [Google Scholar] [CrossRef]
- Feedipedia—Animal Feed Resources Information System—INRAE CIRAD AFZ and FAO: Almond Hulls and Almond By-Products 2020. Available online: https://www.feedipedia.org/node/27 (accessed on 21 February 2024).
- Wang, J.; Singh, A.K.; Kong, F.; Kim, W.K. Effect of almond hulls as an alternative ingredient on broiler performance, nutrient digestibility, and cecal microbiota diversity. Poult. Sci. 2021, 100, 100853. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Bertolino, M.; Barbosa-Pereira, L.; Ben Haj Kbaier, H.; Bouzouita, N.; Zeppa, G. Almond hull as a functional ingredient of bread: Effects on physico-chemical, nutritional, and consumer acceptability properties. Foods 2022, 11, 777. [Google Scholar] [CrossRef]
- Jafari, S.; Alizadeh, A.; Imani, A. Nutrition value of different varieties of almond (Prunus dulcis) hulls. Res. Opin. Anim. Vet. Sci. 2011, 11, 734–738. Available online: https://www.roavs.com/archive/vol-1-issue-11-2011.htm (accessed on 21 February 2024).
- Aguilar, A.A.; Smith, N.E.; Baldwin, R.L. Nutritional value of almond hulls for dairy cows. J. Dairy Sci. 1984, 67, 97–103. [Google Scholar] [CrossRef]
- Yalchi, T.; Kargar, S. Chemical composition and in situ ruminal degradability of dry matter and neutral detergent fiber from almond hulls. J. Food Agric. Environ. 2010, 8, 781–784. [Google Scholar] [CrossRef]
- Yalchi, T. Determination of digestibility of almond hull in sheep. Afr. J. Biotechnol. 2011, 10, 3022–3026. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Barba, F.J.; Moreno, A. Almond hull biomass: Preliminary characterization and development of two alternative valorization routes by applying innovative and sustainable technologies. Ind. Crop. Prod. 2022, 179, 114697. [Google Scholar] [CrossRef]
- Holtman, K.M.; Offeman, R.O.; Franqui-Villanueva, D.; Bayatii, A.K.; Orts, W.J. Countercurrent extraction of soluble sugars from almond hulls and assessment of the bioenergy potential. J. Agric. Food Chem. 2015, 63, 2490–2498. [Google Scholar] [CrossRef]
- Jafari, S.; Alizadeh, A.; Imni, A.; Meng, G.; Rajion, M.A.; Ebrahimi, M. In situ degradation of almond (Prunus dulcis L.) hulls, a potential feed material for ruminants. Turk. J. Vet. Anim. Sci. 2015, 39, 676–681. [Google Scholar] [CrossRef]
- DePeters, E.J.; Fadel, J.G.; Arana, M.J.; Ohanesian, N.; Etchebarne, M.A.; Hamilton, C.A.; Hinders, R.G.; Maloney, M.D.; Old, C.A.; Riordan, T.J.; et al. Variability in the chemical composition of seventeen selected by-product feedstuffs used by the California dairy industry. Prof. Anim. Sci. 2000, 16, 69–99. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Cañellas, J.; García-Raso, J. Contents of detergent-extracted dietary fibers and composition of hulls, shells, and teguments of almonds (Prunus amygdalus). J. Agric. Food Chem. 1983, 31, 1255–1259. [Google Scholar] [CrossRef]
- Vonghia, G.; Ciruzzi, B.; Vicenti, A.; Pinto, F. Performances of fattening lambs fed on mixed feeds containing two different levels of almond hulls. Asian-Austr. J. Anim. Sci. 1989, 2, 535–536. [Google Scholar] [CrossRef]
- Norollahi, H.; Kamalzadeh, A.; Karimi, A. Determination of chemical composition and digestibility of almond hull. Acta Hortic. 2006, 726, 591–594. [Google Scholar] [CrossRef]
- Arosemena, A.; DePeters, E.J.; Fadel, J.G. Extent of variability in nutrient composition within selected by-product feedstuffs. Anim. Feed Sci. Technol. 1995, 54, 103–120. [Google Scholar] [CrossRef]
- Vidal, R.V.; García, R.V.; Martínez, A.F.; Estébanez, F.J.d.l.S. Activated carbon from almond shells for water treatment: A mini review. Agric. Rev. 2019, 40, 271–280. [Google Scholar] [CrossRef]
- Fukuda, J.; Hsieh, Y.-L. Almond shell nanocellulose: Characterization and self-assembling into fibers, films, and aerogels. Ind. Crop. Prod. 2022, 186, 115188. [Google Scholar] [CrossRef]
- Maaloul, N.; Arfi, R.B.; Rendueles, M.; Ghorbal, A.; Diaz, M. Dialysis-free extraction and characterization of cellulose crystals from almond (Prunus dulcis) shells. J. Mater. Environ. Sci. 2017, 8, 4171–4181. Available online: https://www.jmaterenvironsci.com/Journal/vol8-11.html (accessed on 21 February 2024).
- Gil-Guillén, I.; Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Obtaining cellulose fibers from almond shell by combining subcritical water extraction and bleaching with hydrogen peroxide. Molecules 2024, 29, 3284. [Google Scholar] [CrossRef]
- Boulika, H.; El Hajam, M.; Nabih, M.H.; Kandri, N.I.; Zerouale, A. Physico-chemical properties and valorization perspectives of almond residues (shells & hulls) in the northern Morocco: A comparative study. Biomass Conv. Bioref. 2024, 2024, 1–10. [Google Scholar] [CrossRef]
- Queirós, C.S.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Conv. Bioref. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Ibáñez García, A.; Martínez García, A.; Ferrándiz Bou, S. Study of the influence of the almond shell variety on the mechanical properties of starch-based polymer biocomposites. Polymers 2020, 12, 2049. [Google Scholar] [CrossRef]
- Ledesma, B.; Olivares-Marín, M.; Álvarez-Murillo, A.; Roman, S.; Nabais, J.M.V. Method for promoting in-situ hydrochar porosity in hydrothermal carbonization of almond shells with air activation. J. Supercrit. Fluids 2018, 138, 187–192. [Google Scholar] [CrossRef]
- Hosseini, S.; Soltani, S.M.; Jahangirian, H.; Babadi, F.E.; Choong, T.S.Y.; Khodapanah, N. Fabrication and characterization porous carbon rod-shaped from almond natural fibers for environmental applications. J. Environ. Chem. Eng. 2015, 3, 2273–2280. [Google Scholar] [CrossRef]
- Deniz, F. Dye removal by almond shell residues: Studies on biosorption performance and process design. Mater. Sci. Eng. C. 2013, 33, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, A.; Boussetta, A.; Yu, J.; Halouani, K.; Li, Y. Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: Part I. Physico-chemical characterization of the biochar fuel and cell performance examination. Int. J. Hydrogen Energy 2013, 38, 16590–16604. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Nanna, F.; Tarquini, P.; Braccio, G. Semi-continuous biomass gasification with water under sub critical conditions. Fuel 2013, 112, 249–253. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A. Using almond (Prunus amygdalus L.) shell as a bio-waste resource in wood based composite. Compos. B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. Invitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.L.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.L.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Boukid, F.; Angelis, D.; Summo, C. Use of almond skins to improve nutritional and functional properties of biscuits: An example of upcycling. Foods 2020, 9, 1705. [Google Scholar] [CrossRef]
- Ingegneri, M.; Smeriglio, A.; Rando, R.; Gervasi, T.; Tamburello, M.P.; Ginestra, G.; La Camera, E.; Pennisi, R.; Sciortino, M.T.; Mandalari, G.; et al. Composition and biological properties of blanched skin and blanch water belonging to three sicilian almond cultivars. Nutrients 2023, 15, 1545. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Freije, A.; Abdulhussain, H.; Khonji, A.; Hasan, M.; Ferraris, C.; Gasparri, C.; Aziz Aljar, M.A.; Ali Redha, A.; Giacosa, A.; et al. Analysis of the antioxidant activity, lipid profile, and minerals of the skin and seed of hazelnuts (Corylus avellana L.), pistachios (Pistacia vera) and almonds (Prunus dulcis)—A comparative analysis. Applied Chem. 2023, 3, 110–118. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Dimitrova-Dimova, M.; Popova, A. Dietary phenolic compounds—Wellbeing and perspective applications. Int. J. Mol. Sci. 2024, 25, 4769. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Puangpraphant, S.; Cuevas-Rodríguez, E.-O.; Oseguera-Toledo, M. Anti-inflammatory and antioxidant phenolic compounds. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress, 1st ed.; Hernández-Ledesma, B., Martínez-Villaluenga, C., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 1, pp. 165–180. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B.; Yildiz, F. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent. Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Ullah, H.; Hussain, Y.; Santarcangelo, C.; Baldi, A.; Di Minno, A.; Khan, H.; Xiao, J.; Daglia, M. Natural polyphenols for the preservation of meat and dairy products. Molecules 2022, 27, 1906. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.H. New prenilated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T. Antioxidant constituents of almond (Prunus dulcis (Mill) D.A. Webb) hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.Y.O. Quantification of almond skin polyphenols by liquid chromatography-mass spectrometry. J. Food Sci. 2009, 74, C326–C332. [Google Scholar] [CrossRef]
- Arráez-Román, D.; Fu, S.; Sawalha, S.M.S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC/CE-ESI-TOF-MS methods for the characterization of polyphenols in almond-skin extracts. Electrophoresis 2010, 31, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Bartolome, B.; Gómez-Cordovés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) skins as a potential source of bioactive polyphenols. J. Agric. Food Chem. 2007, 55, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, B.; Monagas, M.; Garrido, I.; Gómez-Cordovés, C.; Martín-Álvarez, P.J.; Lebrón-Aguilar, R.; Urpí-Sardà, M.; Llorach, R.; Andrés-Lacueva, C. Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: From chemical characterization to targeted analysis of phenolic metabolites in humans. Arch. Biochem. Biophys. 2010, 501, 124–133. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crop. Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Hughey, C.A.; Janusziewicz, R.; Minardi, C.S.; Phung, J.; Huffman, B.A.; Reyes, L.; Wilcox, B.E.; Prakash, A. Distribution of almond polyphenols in blanch water and skins as a function of blanching time and temperature. Food Chem. 2012, 131, 1165–1173. [Google Scholar] [CrossRef]
- Sang, S.; Cheng, X.; Fu, H.-Y.; Shieh, D.-E.; Bai, N.; Lapsley, K.; Stark, R.E.; Rosen, R.T.; Ho, C.-T. New type sesquiterpene lactone from almond hulls (Prunus amygdalus Batsch). TETL 2002, 43, 2547–2549. [Google Scholar] [CrossRef]
- Valdés, A.; Garrigós, M.C.; Jiménez, A. Extraction and characterization of antioxidant compounds in almond (Prunus amygdalus) shell residues for food packaging applications. Membranes 2022, 12, 806. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I. A box-behnken design for optimal extraction of phenolics from almond by-products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of polyphenolic compounds extracted from different varieties of almond hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Sfahlan, A.J.; Mahmoodzadeh, A.; Hasanzadeh, A.; Heidari, R.; Jamei, R. Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chem. 2009, 115, 529–533. [Google Scholar] [CrossRef]
- Siriwardhana, S.S.K.W.; Shahidi, F. Antiradical activity of extracts of almond and its by-products. J. Amer. Oil Chem. Soc. 2002, 79, 903–908. [Google Scholar] [CrossRef]
- Moure, A.; Pazos, M.; Medina, I.; Domínguez, H.; Parajó, J.C. Antioxidant activity of extracts produced by solvent extraction of almond shells acid hydrolysates. Food Chem. 2007, 101, 193–201. [Google Scholar] [CrossRef]
- Bolling, B.W.; Blumberg, J.B.; Chen, C.Y.O. The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chem. 2010, 123, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, 106–115. [Google Scholar] [CrossRef]
- Mandalari, G.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Rizza, L.; Bonina, F.P.; Trombetta, D.; Tomaino, A. Antioxidant and photoprotective effects of blanch water, a byproduct of the almond processing industry. Molecules 2013, 18, 12426–12440. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, J.; Liang, Y.; Ma, Y.; Chen, C.; Cheng, Y.; Peng, P.; Zhou, N.; Zhang, R.; Addy, M.; et al. Characterization, bioavailability and protective effects of phenolic-rich extracts from almond hulls against pro-oxidant induced toxicity in Caco-2 cells. Food Chem. 2020, 322, 126742. [Google Scholar] [CrossRef]
- Takeoka, G.; Dao, L.; Teranishi, R.; Wong, R.; Flessa, S.; Harden, L.; Edwards, R. Identification of three triterpenoids in almond hulls. J. Agric. Food Chem. 2000, 48, 3437–3439. [Google Scholar] [CrossRef]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS Identification of Phenolic Antioxidants from Agricultural Residues: Almond Hulls and Grape Pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Montané, D.; Kardošová, A.; Bekešová, S.; Hříbalová, V.; Ebringerová, A. Almond shell xylo-oligosaccharides exhibiting immunostimulatory activity. Carbohydr. Res. 2007, 342, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Peano, C.; Sottile, F. Post-harvest industrial processes of almond (Prunus dulcis L. Mill) in Sicily influence the nutraceutical properties of by-products at harvest and during storage. Front. Nutr. 2021, 8, 659378. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Leporini, M.; D’Urso, G.; Gagliano Candela, R.; Falco, T.; Piacente, S.; Bruno, M.; Sottile, F. Almond (Prunus dulcis cv. Casteltermini) skin confectionery by-products: New opportunity for the development of a functional blackberry (Rubus ulmifolius Schott) jam. Antioxidants 2021, 10, 1218. [Google Scholar] [CrossRef]
- Picerno, P.; Crascì, L.; Iannece, P.; Esposito, T.; Franceschelli, S.; Pecoraro, M.; Giannone, V.; Panico, A.M.; Aquino, R.P.; Lauro, M.R. A green bioactive by-product almond skin functional extract for developing nutraceutical formulations with potential antimetabolic activity. Molecules 2023, 28, 7913. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite profiling and antioxidant activity of the polar fraction of Italian almonds (Toritto and Avola): Analysis of seeds, skins, and blanching water. J. Pharm. Biomed. Anal. 2020, 190, 113518. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Ghoul, M. Biological activities and effects of food processing on flavonoids as phenolic antioxidants. In Advances in Applied Biotechnology, 1st ed.; Petre, M., Ed.; InTech: Rijeka, Croatia, 2012; Volume 1, pp. 101–124. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; Del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Martín-Pérez, L.; Gil-Guillén, I.; González-Martínez, C.; Chiralt, A. Subcritical water extraction for valorisation of almond skin from almond industrial processing. Foods 2023, 12, 3759. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, S.C.; Hu, C.C.; Shyu, Y.S.; Hsu, C.Y.; Yang, D.J. Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef]
- Tabib, M.; Tao, Y.; Ginies, C.; Bornard, I.; Rakotomanomana, N.; Remmal, A.; Chemat, F. A one-pot ultrasound-assisted almond skin separation/polyphenols extraction and its effects on structure, polyphenols, lipids, and proteins quality. Appl. Sci. 2020, 10, 3628. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Jeong, W.S.; Lachance, P.A.; Ho, C.T.; Rosen, R.T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef]
- Frison-Norrie, S.; Sporns, P. Variation in the flavonol glycoside composition of almond seedcoats as determined by MALDI-TOF mass spectrometry. J. Agric. Food Chem. 2002, 50, 6818–6822. [Google Scholar] [CrossRef] [PubMed]
- Frison-Norrie, S.; Sporns, P. Identification and quantification of flavonol glycosides in almond seedcoats using MALDI-TOF MS. J. Agric. Food Chem. 2002, 50, 2782–2787. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszyńska, A.; Shahidi, F. Antioxidant activity of almond seed extract and its fractions. J. Food Lipids 2005, 12, 344–358. [Google Scholar] [CrossRef]
- Xie, L.; Bolling, B.W. Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC–MS. Food Chem. 2014, 148, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Tabib, M.; Ginies, C.; Rakotomanomana, N.; Remmal, A. Adsorption of polyphenols from almond blanching water by macroporous resin. Int. J. Food Sci. 2002, 2002, 7847276. [Google Scholar] [CrossRef]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [CrossRef]
- Monagas, M.; Garrido, I.; Lebrón-Aguilar, R.; Carmen Gómez-Cordovés, M.; Rybarczyk, A.; Amarowicz, R.; Bartolomé, B. Comparative flavan-3-ol profile and antioxidant capacity of roasted peanut, hazelnut, and almond skins. J. Agric. Food Chem. 2009, 57, 10590–10599. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S.J. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Genovese, T.; Mazzon, E.; Wickham, M.S.J.; Paterniti, I.; Cuzzocrea, S. Natural almond skin reduced oxidative stress and inflammation in an experimental model of inflammatory bowel disease. Int. Immunopharmacol. 2011, 11, 915–924. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): Identification and structure-activity relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.K.; Katyal, T. Abatement of detrimental effects of photoaging by Prunus amygdalus skin extract. Int. J. Curr. Pharm. Res. 2011, 3, 57–59. Available online: https://innovareacademics.in/journal/ijcpr/Issues/Vol3Issue1/266.pdf (accessed on 19 May 2024).
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.D. Pharmacological activities of flavonoids: A review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Salleh, S.N.; Fairus, A.A.H.; Zahary, M.N.; Bhaskar Raj, N.; Mhd Jalil, A.M. Unravelling the effects of soluble dietary fibre supplementation on energy intake and perceived satiety in healthy adults: Evidence from systematic review and meta-analysis of randomised-controlled trials. Foods 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Arangia, A.; Ragno, A.; Cordaro, M.; D’Amico, R.; Siracusa, R.; Fusco, R.; Marino Merlo, F.; Smeriglio, A.; Impellizzeri, D.; Cuzzocrea, S.; et al. Antioxidant activity of a Sicilian almond skin extract using in vitro and in vivo models. Int. J. Mol. Sci. 2023, 24, 12115. [Google Scholar] [CrossRef]
- Oliveira, I.; Marinho, B.; Szymanowska, U.; Karas, M.; Vilela, A. Chemical and sensory properties of waffles supplemented with almond skins. Molecules 2023, 28, 5674. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Chen, C.Y.O.; Blumberg, J.B. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. J. Agric. Food Chem. 2008, 56, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Bottone, A.; Masullo, M.; Montoro, P.; Pizza, C.; Piacente, S. HR-LC-ESI-Orbitrap-MS based metabolite profiling of Prunus dulcis Mill. (Italian cultivars Toritto and Avola) husks and evaluation of antioxidant activity. Phytochem. Anal. 2019, 30, 415–423. [Google Scholar] [CrossRef]
- Truong, V.L.; Bak, M.J.; Jun, M.; Kong, A.N.T.; Ho, C.T.; Jeong, W.S. Antioxidant defense and hepatoprotection by procyanidins from almond (Prunus amygdalus) skins. J. Agric. Food Chem. 2014, 62, 8668–8678. [Google Scholar] [CrossRef]
- Meshkini, A. Acetone extract of almond hulls provides protection against oxidative damage and membrane protein degradation. J. Acupunct. Meridian Stud. 2016, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in almond skins after blanching modulate plasma biomarkers of oxidative stress in healthy humans. Antioxidants 2019, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jia, X.; Chen, C.Y.O.; Blumberg, J.B.; Song, Y.; Zhang, W.; Zhang, X.; Ma, G.; Chen, J. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J. Nutr. 2007, 137, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Timón, M.; Andrés, A.I.; Sorrentino, L.; Cardenia, V.; Petrón, M.J. Effect of phenolic compounds from almond skins obtained by water extraction on pork patty shelf life. Antioxidants 2022, 11, 2175. [Google Scholar] [CrossRef]
- Karimi, Z.; Firouzi, M.; Dadmehr, M.; Javad-Mousavi, S.A.; Bagheriani, N.; Sadeghpour, O. Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: A narrative review. Phytother. Res. 2021, 35, 2997–3012. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Loizzo, M.R.; Puccio, V.; Gaglio, R.; Francesca, N.; Settanni, L.; Sottile, F. Antibacterial activity and chemical characterization of almond (Prunus dulcis L.) peel extract. Nat. Prod. Res. 2022, 37, 1680–1686. [Google Scholar] [CrossRef]
- Bisignano, C.; Filocamo, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and -negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. [Google Scholar] [CrossRef]
- Arena, A.; Bisignano, C.; Stassi, G.; Mandalari, G.; Wickham, M.S.J.; Bisignano, G. Immunomodulatory and antiviral activity of almond skins. Immunol. Lett. 2010, 132, 18–23. [Google Scholar] [CrossRef]
- Khan, N.; Ahmad, I.; Sadiq, M.B. Optimization of ultrasonic assisted extraction of bioactive compounds from the almond hull. Sarhad J. Agric. 2022, 38, 676–684. [Google Scholar] [CrossRef]
- D’Arcangelo, S.; Santonocito, D.; Messina, L.; Greco, V.; Giuffrida, A.; Puglia, C.; Di Giulio, M.; Inturri, R.; Vaccaro, S. Almond Hull Extract Valorization: From Waste to Food Recovery to Counteract Staphylococcus aureus and Escherichia coli in Formation and Mature Biofilm. Foods 2024, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Marzocco, S.; Rapa, S.F.; Musumeci, T.; Giannone, V.; Picerno, P.; Aquino, R.P.; Puglisi, G. Recycling of almond by-products for intestinal inflammation: Improvement of physical-chemical, technological and biological characteristics of a dried almond skins extract. Pharmaceutics 2020, 12, 884. [Google Scholar] [CrossRef]
- Zorrilla, P.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, N.; Olivares, M.; Rondón, D.; Zarzuelo, A.; Utrilla, M.P.; Galvez, J.; Rodriguez-Cabezas, M.E. Intestinal anti-inflammatory activity of the polyphenolic-enriched extract Amanda® in the trinitrobenzenesulphonic acid model of rat colitis. J. Funct. Foods 2014, 11, 449–459. [Google Scholar] [CrossRef]
- Kumar, V.; Sachan, R.; Rahman, M.; Sharma, K.; Al-Abbasi, F.A.; Anwar, F. Preclinical renal chemo-protective potential of Prunus amygdalus Batsch seed coat via alteration of multiple molecular pathways. Arch. Physiol. Biochem. 2017, 124, 88–96. [Google Scholar] [CrossRef]
- Martins, I.M.; Chen, Q.; Oliver Chen, C.Y. Emerging functional foods derived from almonds. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications, 1st ed.; Ferreira, I.C.F.R., Morales, P., Barrosn, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 1, pp. 445–469. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Bäumler, A.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: A randomized controlled trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef]
- Rocchetti, G.; Bhumireddy, S.R.; Giuberti, G.; Mandal, R.; Lucini, L.; Wishart, D.S. Edible nuts deliver polyphenols and their transformation products to the large intestine: An in vitro fermentation model combining targeted/untargeted metabolomics. Food Res. Int. 2019, 116, 786–794. [Google Scholar] [CrossRef]
- Rad, M.I.; Rouzbehan, Y.; Rezaei, J. Effect of dietary replacement of alfalfa with urea-treated almond hulls on intake, growth, digestibility, microbial nitrogen, nitrogen retention, ruminal fermentation, and blood parameters in fattening lambs. J. Anim. Sci. 2016, 94, 349–358. [Google Scholar] [CrossRef]
- Kilama, J.; Yakir, Y.; Shaani, Y.; Adin, G.; Kaadan, S.; Wagali, P.; Sabastian, C.; Ngomuo, G.; Mabjeesh, S.J. Chemical composition, in vitro digestibility, and storability of selected agro-industrial by-products: Alternative ruminant feed ingredients in Israel. Heliyon 2023, 9, e14581. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, G.S.; Lim, C.B.; Kim, I.H. Effect of dietary almond hull on growth performance, nutrient digestibility, fecal microbial, fecal score, and noxious gas emission in growing pigs. Can. J. Anim. Sci. 2024, 104, 214–220. [Google Scholar] [CrossRef]
- Ahammad, G.S.; Lim, C.B.; Kim, I.H. Effect of dietary almond hull on growth performance, nutrient digestibility, organ weight, caecum microbial counts, and noxious gas emission in broilers. Braz. J. Poult. Sci. 2024, 26, 2024. [Google Scholar] [CrossRef]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food matrix effects of polyphenol bioaccessibility from almond skin during simulated human digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Huang, G.; Zhang, W.; Ni, L. In vitro and in vivo evaluation of the prebiotic effect of raw and roasted almonds (Prunus amygdalus). J. Sci. Food Agric. 2016, 96, 1836–1843. [Google Scholar] [CrossRef]
- Dhingra, N.; Kar, A.; Sharma, R.; Bhasin, S. In-vitro antioxidative potential of different fractions from Prunus dulcis seeds: Vis a vis antiproliferative and antibacterial activities of active compounds. S. Afr. J. Bot. 2017, 108, 184–192. [Google Scholar] [CrossRef]
- Lim, T.K. Prunus dulcis. In Edible Medicinal and Non-Medicinal Plants, 1st ed.; Lim, T.K., Ed.; Springer Dordrecht: Berlin, Germany, 2012; Volume 4, Fruits; pp. 480–491. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayi, H.; Hanoglu, A.; Hanoglu, D.Y.; Yavuz, D.O.; Ozek, T.; Vatansever, S. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef]
- Khani, A.; Meshkini, A. Anti-proliferative activity and mitochondria-dependent apoptosis induced by almond and walnut by-product in bone tumor cells. Waste Biomass Valoriz. 2021, 12, 1405–1416. [Google Scholar] [CrossRef]
- Dammak, M.I.; Chakroun, I.; Mzoughi, Z.; Amamou, S.; Mansour, H.B.; Le Cerf, D.; Majdoub, H. Characterization of polysaccharides from Prunus amygdalus peels: Antioxidant and antiproliferative activities. Int. J. Biol. Macromol. 2018, 119, 198–206. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, H.; Qi, J.; Hu, A.; Jiang, Q.; Hou, Y.; Feng, Q.; Ojo, O.; Wang, X. An almond-based low carbohydrate diet improves depression and glycometabolism in patients with type 2 diabetes through modulating gut microbiota and GLP-1: A randomized controlled trial. Nutrients 2020, 12, 3036. [Google Scholar] [CrossRef]
- Li, S.C.; Liu, Y.H.; Liu, J.F.; Chang, W.H.; Chen, C.M.; Chen, C.Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.; Khandelwal, S.; Madan, J.; Pandya, H.; Sesikeran, B.; Krishnaswamy, K. Almonds and cardiovascular health: A review. Nutrients 2018, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Eslampour, E.; Asbaghi, O.; Hadi, A.; Abedi, S.; Ghaedi, E.; Lazaridi, A.V.; Miraghajani, M. The effect of almond intake on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 50, 102399. [Google Scholar] [CrossRef] [PubMed]
- Meadows, R. Almond waste is a growing challenge. ACS Cent. Sci. 2023, 9, 2171–2174. [Google Scholar] [CrossRef]


| Parameter | Content | References |
|---|---|---|
| Routine Analyses | ||
| Dry matter (DM) | 60.60–97.12 a | [44,56,57,60] |
| Organic matter (OM) | 86.87–93.90 a | [56,58,66] |
| Volatile matter | 71.20–75.73 a | [30,66] |
| Moisture | 0.65–11.30 a | [30,66] |
| 6.95–9.01 (GH) a | [61] | |
| 6.01–9.80 (MH) a | [61] | |
| Crude protein (CP) | 1.60–26.50 a | [44,57,59,60,61] |
| Soluble crude protein (SCP) | 1.40–57.00 a | [56,69] |
| Sugar | 15.90–34.30 a | [44,57,58,59,60] |
| Ash | 1.70–12.83 a | [44,57,59,60,61,66,68] |
| Lipids | 1.15–2.65 (GH) a | [61] |
| 2.45–2.71 (MH) a | [61] | |
| Pectins | 4.00 a | [70] |
| Crude fiber (CF) | 10.40–35.77 a | [44,57,59,60,61] |
| Neutral detergent fiber (NDF) | 18.00–61.98 a | [56,57,58,59] |
| Acid detergent fiber (ADF) | 12.60–34.60 a | [56,57,58,59,62] |
| Soluble fiber | 1.89–6.12 (GH) a | [61] |
| 1.35–2.07 (MH) a | [61] | |
| Insoluble fiber | 18.47–33.04 (GH) a | [61] |
| 22.32–33.70 (MH) a | [61] | |
| Lignin | 5.00–24.80 a | [44,56,57,59,66] |
| Lignin/NDF | 28.20–36.90 b | [57] |
| Acid detergent lignin (ADL) | 9.24–14.31 a | [62,64,65,68] |
| Water Soluble carbohydrate (WSC) | 12.63–14.13 a | [68] |
| Nonfibrous carbohydrate (NFC) | 5.04–70.96 a | [56,62,64,65,68] |
| Nitrogen-free extract (NFE) | 48.90–61.18 a | [60,71,72] |
| Non-Structural Carbohydrates (NSC) | 23.50–40.40 a | [56] |
| Holocellulose | 16.43 a | [66] |
| Hemicellulose | 6.00–12.86 a | [44,65,66,67] |
| Cellulose | 6.60–20.70 a | [44,63,64,65,66] |
| Eter extract (EE) | 0.40–8.20 a | [57,58,59,60,65] |
| Ethanol-soluble carbohydrates | 23.31–39.88 a | [56] |
| N-free extract | 61.18 a | [71] |
| Starch | 0.00–10.00 a | [56,59] |
| Gross energy (GE) | 15.10–19.70 c | [59] |
| Carbon (C) | 42.92–43.00 a | [30,66] |
| Hydrogen (H) | 5.70–5.80 a | [30,66] |
| Minerals | ||
| Calcium (Ca) | 0.03–1.00 a | [44,56,57,59,68] |
| 2.30–2.70 e | [60] | |
| Phosphorus (P) | 0.00–2.00 a | [56,57,59,60,68] |
| Magnesium (Mg) | 0.07–0.40 a | [56,57,59,69] |
| 0.09–0.12 e | [60] | |
| Potassium (K) | 2.02–4.57 a | [44,56,57,59,69] |
| 27.60–36.30 e | [60] | |
| Sodium (Na) | 0.01–0.40 a | [56,57,59,60] |
| Copper (Cu) | 1.00–19.00 e | [56,57,59,60,73] |
| Manganese (Mn) | 5.00–69.00 e | [56,59,60,73] |
| Iron (Fe) | 0.08–0.71 d | [56,59,60,73] |
| Aluminum (Al) | 6.00–17.00 e | [60] |
| Zinc (Zn) | 6.00–63.00 e | [56,59,60,73] |
| Nitrogen (N) | 0.73–3.28 a | [30,66] |
| Chlorine (Cl) | 0.01–0.07 a | [30,59,69] |
| Sulfur (S) | 0.01–0.03 a | [30,59,69] |
| Selenium (Se) | 0.04–0.10 e | [59,73] |
| Molybdenum (Mo) | 0.00–12.1 e | [69] |
| Essential amino acids | ||
| Arginine | 0.12–0.13 a | [60] |
| Histidine | 0.07 a | [60] |
| Isoleucine | 0.10–0.12 a | [60] |
| Leucine | 0.17–0.20 a | [60] |
| Lysine | 0.14–0.15 a | [60] |
| Methionine | 0.03–0.04 a | [60] |
| Phenylalanine | 0.12–0.13 a | [60] |
| Threonine | 0.11–0.13 a | [60] |
| Tryptophan | <0.02 a | [60] |
| Valine | 0.15–0.17 a | [60] |
| Parameter | Content | References |
|---|---|---|
| Routine Analyses | ||
| Dry matter (DM) | 84.80–93.20 b | [59] |
| Moisture | 3.30–11.20 a | [30,80,81,82,85] |
| Volatile matter | 73.00–81.20 a | [30,78,81,82] |
| Crude protein (CP) | 1.40–4.70 a | [59,77] |
| Ash | 0.55–8.70 a | [76,77,78,79,80] |
| Crude fiber (CF) | 51.80–62.00 a | [59] |
| Neutral detergent fiber (NDF) | 90.10 a | [59] |
| Acid detergent fiber (ADF) | 57.20–66.00 a | [59] |
| Lignin | 20.10–32.70 a | [44,59,74,76,77,79] |
| Holocellulose | 64.30 a | [86] |
| Hemicellulose | 19.70–35.20 a | [44,52,74,75,76,77,78] |
| Cellulose | 22.80–40.50 a | [44,74,75,76,77,78] |
| Eter extract (EE) | 0.20–1.10 a | [59] |
| Gross energy (GE) | 19.40 e | [59] |
| Polysaccharides | 56.10 a | [79] |
| Carbon (C) | 45.60–50.50 a | [74,81,82,83] |
| Hydrogen (H) | 5.40–6.60 a | [74,81,82,83,84,85] |
| Oxygen (O) | 37.97–45.94 a | [74,81,82,83,84] |
| Minerals | ||
| Calcium (Ca) | 1.18–1.80 c | [44,59,78,79] |
| Potassium (K) | 4.30–12.30 c | [44,59,78,79] |
| Phosphorus (P) | 0.20–0.65 c | [59,78,79] |
| Sodium (Na) | 0.17–0.60 c | [59,78,79] |
| Magnesium (Mg) | 0.14–0.50 c | [78,79] |
| Sulfur (S) | 0.01–0.03 a | [79,81,82,85] |
| Manganese (Mn) | 0.01–0.03 c | [78,79] |
| Zinc (Zn) | 0.01 c | [78,79] |
| Cooper (Cu) | 3.20–10.00 d | [78,79] |
| Iron (Fe) | 0.04–1.64 c | [59,78,79] |
| Molybdenum (Mo) | 3.30 d | [79] |
| Nitrogen (N) | 0.17–0.44 a | [74,81,82,85] |
| Boron (B) | 0.01–0.02c | [78,79] |
| Cloride (Cl-) | 0.02 a | [85] |
| Chlorine (Cl) | 0.05–0.04 a | [30,59] |
| Parameter | Content | References |
|---|---|---|
| Routine Analyses | ||
| Moisture | 6.43–18.39 (BS) a | [90,91] |
| Ash | 1.63–5.20 (BS) a | [88,91] |
| 4.80 (NS) a | [88] | |
| Protein | 10.30 (NS) a | [87,88] |
| 10.60–12.80 (BS) a | [87,88,90,91] | |
| Sugar | 4.14–5.65 (BS) a | [90,91] |
| Total Dietary Fiber (TDF) | 47.50 (NS) a | [87,89] |
| 45.10–60.25 (BS) a | [87,88,89,90,91] | |
| Soluble Dietary Fiber | 2.70 (NS)–3.80 (BS) a | [87,88] |
| Fat | 10.30–22.20 (NS) a | [87,88,89] |
| 9.50–24.20 (BS) a | [87,88,89] | |
| Minerals | ||
| Manganese (Mn) | 2.08 b | [92] |
| Zinc (Zn) | 2.96 b | [92] |
| Cooper (Cu) | 0.16 b | [92] |
| Iron (Fe) | 3.72 b | [92] |
| Selenium (Se) | 0.46 b | [92] |
| Vitamins | ||
| Vitamin E (α-Tocopherol) | 13.00 (BS)–14.00 (NS) a | [88] |
| Fatty acids | ||
| Palmitic acid, 16:0 | 8.01–10.30 c | [88,91,92] |
| Palmitoleic acid, 16:1 | 0.63–1.11 c | [88,91,92] |
| Stearic acid, 18:1 | 1.37–2.39 c | [88,91,92] |
| Oleic acid, 18:1 | 43.08–56.69 c | [88,91,92] |
| Linoleic acid, 18:2 | 31.36–36.98 c | [88,91,92] |
| α-Linolenic acid, 18:3 | 0.27–5.65 c | [88,91,92] |
| MUFA | 55.24–57.66 c | [91] |
| PUFA | 31.63–33.38 c | [91] |
| SFA | 10.71–11.39 c | [91] |
| Almond By-Product | Total Phenolics | Flavonoids | Ortho-Diphenols | Condensed Tannins | Antioxidant Capacity | References |
|---|---|---|---|---|---|---|
| Hull | 18,307.26–22,593.33 i | – | – | – | 29,250.00–44,424.00 p | [36] |
| 103.44–184.53 d | – | – | – | 671.78–1159.83 l | [61] | |
| 3.08–210.49 d | 0.87–120.04 a | – | 0.09–123.54 a | 23.43–1938.07 j | [121] | |
| 7.90–32.66 d | 4.28–29.05 u | 8.28–24.53 d | – | 0.07–0.28 x | [32] | |
| 91.76–138.90 d | 36.99–125.35 a | 107.34–131.34 d | – | 0.85–1.54 x | [120] | |
| 32.00–35.70 h | – | – | 23.20–28.40 h | – | [62,68] | |
| 304.78–859.07 d | 70.48–284.61 u | – | – | 169.85–376.30 y | [122] | |
| 35.90–166.70 d | – | – | – | 29.70–98.70 q | [123] | |
| 71.00 b | – | – | – | – | [35] | |
| 71.10 u | – | – | – | 41.10 o | [124] | |
| Shell | 6.59 d | 1.42 a | – | – | 4.21–6.20 k | [119] |
| 188.60 d | 99.40 u | – | 34.60 u | 646.00 k | [79] | |
| 3.55–8.62 d | 1.74–6.05 a | 3.43–9.95 d | – | 0.03–0.10 x | [120] | |
| 18.40–62.70 d | – | – | – | 29.3–63.50 q | [123] | |
| 13.73–19.76 c | – | – | – | 27.90–82.70 y | [125] | |
| Natural Skin | 703.03 e | – | – | – | 6034.43–16,259.40 m | [116] |
| 3471.10 e | – | – | – | 0.20 y | [89] | |
| 27.60 d | – | – | – | 210.00 l | [126] | |
| Blanched Skin | 1.72–7.07 c | 0.52–1.20 v | – | – | – | [91] |
| – | – | – | – | 80.17 n | [92] | |
| 13.44–34.71 d | 11.14–34.43 u | 10.65–26.59 d | – | 0.06–0.18 x | [32] | |
| 88.00 b | – | – | – | – | [35] | |
| 7.62–25.17 d | 4.45–13.66 a | 6.95–23.32 d | – | 0.04–0.30 x | [120] | |
| 1110.00–1773.00 (ND) f | – | – | – | 40.40 l (ND) | [39] | |
| 253.60–857.00 f | – | – | – | 59.20–90.40 l | [39] | |
| 313.76 e | – | – | – | 2925.15–7363.06 m | [116] | |
| 165.00–370.00 f | – | – | [115] | |||
| 278.90 e | – | – | – | 6.50 y | [89] | |
| 242.00–413.00 f | – | – | – | 0.40–0.50 g | [114] | |
| 88.00 b | [35] | |||||
| 87.80 u | – | – | – | 52.90 o | [124] | |
| Roasted Skin | – | – | – | – | 0.80–1.08 g | [127] |
| 18.50 d | 119.00 l | [126] | ||||
| Skin (B + D) | – | – | – | – | 0.40–0.58 g | [127] |
| Skin (B + FD) | – | – | – | – | 0.33–0.45 g | [127] |
| Blanching Water | 392.16–505.95 r | 292.78–467.78 t | 224.21–318.07 r | – | 1.98– 3.64 w | [32] |
| 510.00–917.00 s | – | – | – | 17.40 l | [39] | |
| 0.56–3.36 c | 0.18–0.77 v | – | – | [91] | ||
| 73.86 e | – | – | – | 575.08–1049.95 m | [116] | |
| 90.28 d | – | – | – | 132.82 y | [128] | |
| 33.30 e | [89] | |||||
| 50.30–153.90 e | – | – | – | – | [33] |
| Almond By-Products | Bioativities | Main Compounds Responsible | References |
|---|---|---|---|
| Hull | Antioxidant | Polyphenols (such as chlorogenic acid) | [11,32,116,121,123,129,171] |
| Antimicrobial | Polyphenols (such as naringenin, catechin epicat-echin, protocatechuic acid, isorhamnetin) | [11,36,38,183,184] | |
| Antitumor/Anticancer | Polyphenols, acid-soluble polysaccharides, triterpenoids acidsand UFAs | [17,154,178,199,200,201,202,203] | |
| Shell | Antioxidant | Phenolic compounds | [123] |
| Skin (Natural and Blanched) | Antioxidant | Polyphenols (mainly flavonols and proanthocyanidins) | [11,32,38,39,50,113,114,137,172,175,176,203] |
| Antitumor/Anticancer | Polyphenols, acid-solubles polysacharides, triterpenoids acis and UFAs | [11,178,199,201,202,203] | |
| Antimicrobial | Polyphenols (such as naringenin, catechin epicatechin, protocatechuic acid, isorhamnetin) | [11,32,38,116,152,158,181,182] | |
| Photoprotective | Polyphenols | [128,156] | |
| Anti-inflammatory | Polyphenols, UFAs, protein hidrolysates | [11,153,185,187] | |
| Prebiotic | Dietary Fibers, XOS, polysaccharides, and hemicellulose | [11,155,178,188,198] | |
| Blanching Water | Antioxidant | Polyphenols | [11,32,137] |
| Antimicrobial | Polyphenols | [176,178] | |
| Anti-inflammatory | Polyphenols | [186] | |
| Photoprotective | Polyphenols | [128,156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Oliveira, I.; Pereira, J.A.; Gonçalves, B. Almond By-Products: A Comprehensive Review of Composition, Bioactivities, and Influencing Factors. Foods 2025, 14, 1042. https://doi.org/10.3390/foods14061042
Silva V, Oliveira I, Pereira JA, Gonçalves B. Almond By-Products: A Comprehensive Review of Composition, Bioactivities, and Influencing Factors. Foods. 2025; 14(6):1042. https://doi.org/10.3390/foods14061042
Chicago/Turabian StyleSilva, Vânia, Ivo Oliveira, José Alberto Pereira, and Berta Gonçalves. 2025. "Almond By-Products: A Comprehensive Review of Composition, Bioactivities, and Influencing Factors" Foods 14, no. 6: 1042. https://doi.org/10.3390/foods14061042
APA StyleSilva, V., Oliveira, I., Pereira, J. A., & Gonçalves, B. (2025). Almond By-Products: A Comprehensive Review of Composition, Bioactivities, and Influencing Factors. Foods, 14(6), 1042. https://doi.org/10.3390/foods14061042









