Cyclodextrin-Based Pickering Emulsion Significantly Increases 6-Gingerol Loading Through Two Different Mechanisms: Cyclodextrin Cavity and Pickering Core
Abstract
:1. Introduction
2. Materials and Methods
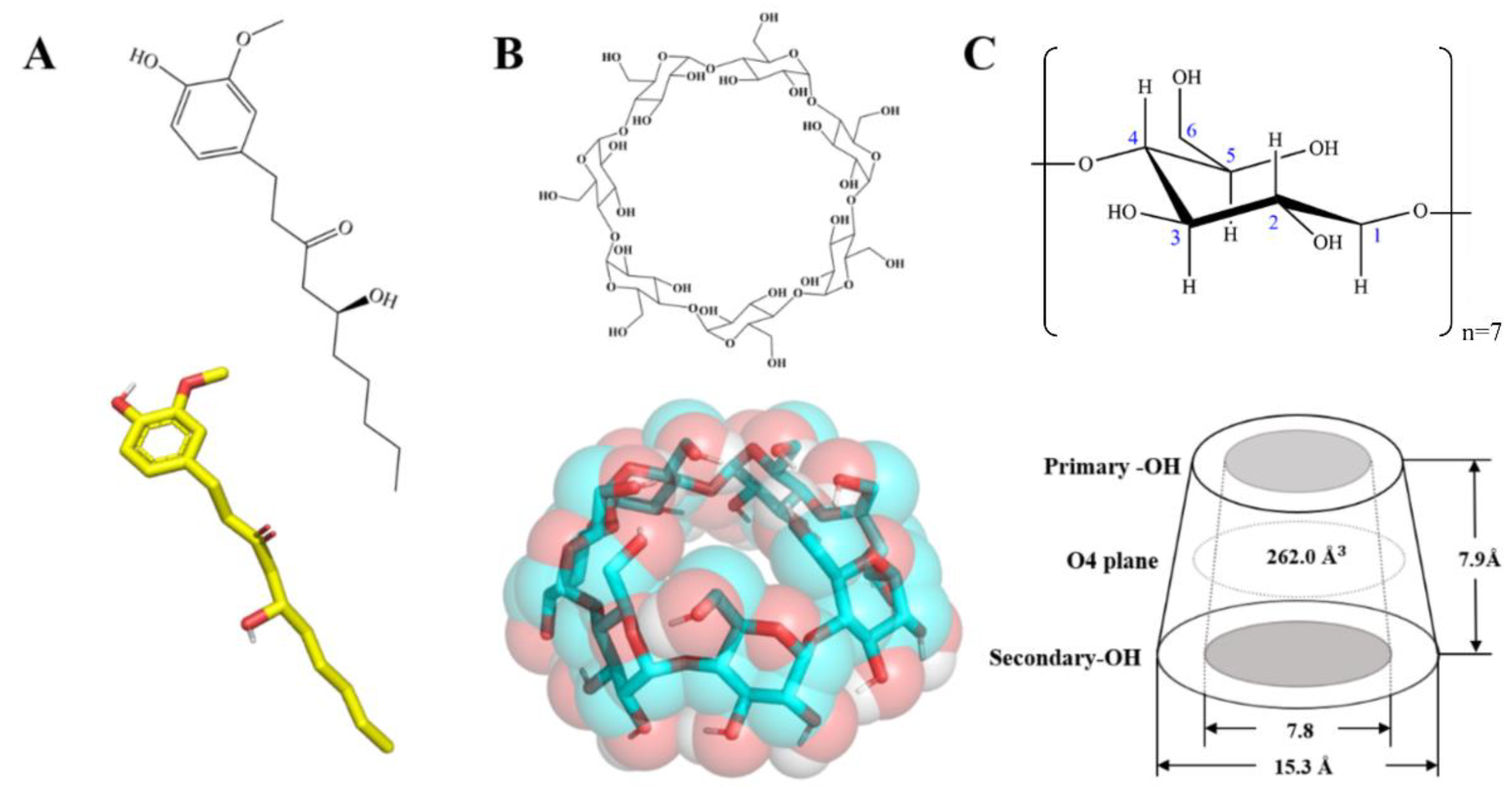
2.1. Materials and Chemicals
2.2. Sample Preparation
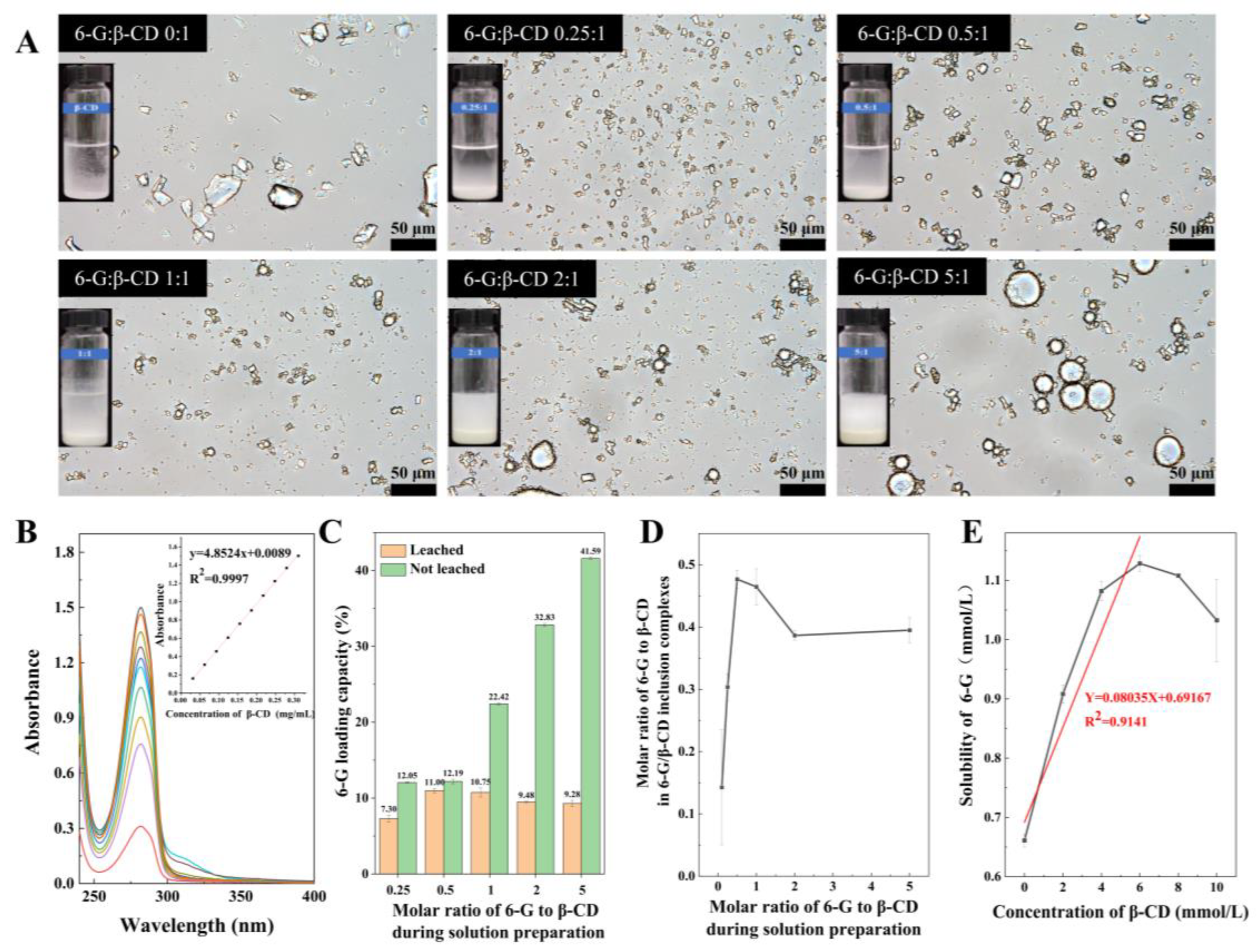
2.3. Microscopic Observation
2.4. Determination of 6-G Loading Capacity and 6G/β-CD Molar Ratio
2.5. Phase Solubility Measurement
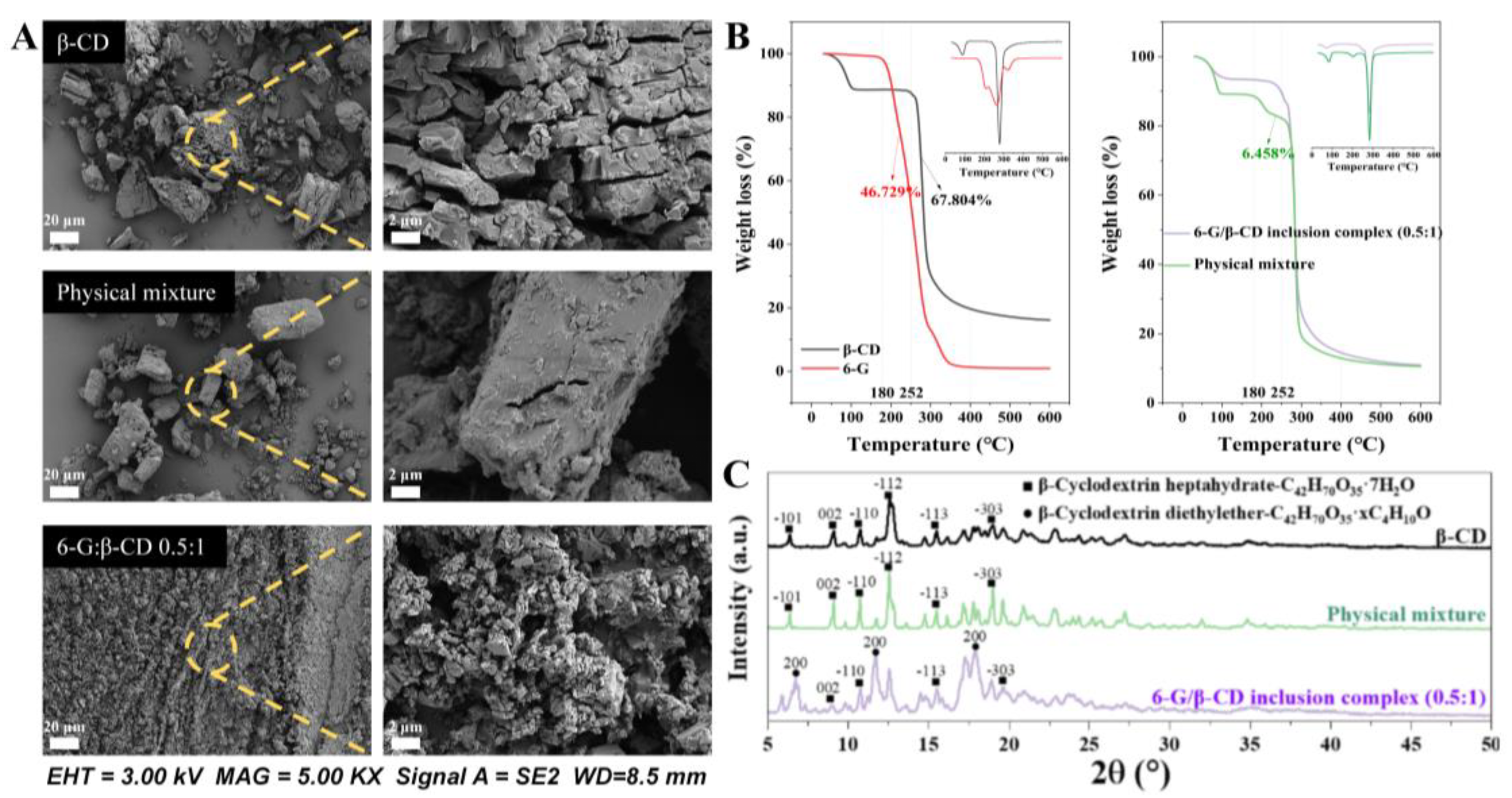
2.6. Thermogravimetric Analysis
2.7. Powder XRD
2.8. MD Simulation Setup
2.8.1. Model Construction
2.8.2. Simulation Parameters
2.8.3. Visualisation Method of Simulation Process
2.8.4. Weak Interaction Analysis
3. Results and Discussion
3.1. Sample Morphology
3.2. Loading Capacity of 6-G
3.2.1. 6-G Standard Curve
3.2.2. Loading Capacity of 6-G in Pickering Emulsion
3.2.3. Molar Ratio in Inclusion Complexes
3.3. Phase Solubility Analysis
3.4. Validation of 6-G/β-CD Inclusion Complex Formation
3.5. 6-G/β-CD Inclusion Complex Structure
3.6. MD Simulation
3.6.1. Visualisation of Simulation Process
3.6.2. Interaction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Promdam, N.; Panichayupakaranant, P. [6]-Gingerol: A narrative review of its beneficial effect on human health. Food Chem. Adv. 2022, 1, 100043. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Badria, F.A.; ElNaggar, M.H.; El-Masry, S.M.; Helmy, S.A. Development of Cyclodextrin-Functionalized Transethoniosomes of 6-Gingerol: Statistical Optimization, In Vitro Characterization and Assessment of Cytotoxic and Anti-Inflammatory Effects. Pharmaceutics 2022, 14, 1170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Feng, Y.; Firempong, C.K.; Zhu, Y.; Omari-Siaw, E.; Zheng, Y.; Pu, Z.; Xu, X.; Yu, J. Enhanced oral bioavailability of [6]-Gingerol-SMEDDS: Preparation, in vitro and in vivo evaluation. J. Funct. Foods 2016, 27, 703–710. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, R.; Wen, Q.-H.; Li, J.; Lin, J.-W.; Zeng, X.-A. The interaction between bovine serum albumin and [6]-, [8]- and [10]-gingerol: An effective strategy to improve the solubility and stability of gingerol. Food Chem. 2022, 372, 131280. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, Q.; Wang, Q.; Sun, C.; Zhu, Y.; Niu, Y.; Yu, J.; Xu, X. Formulation, Characterization, and Pharmacokinetic Studies of 6-Gingerol-Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2018, 19, 3661–3669. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; de Silva, N.; Bhandari, S.; Yap, Y.K.; Costha, N.P. pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38. [Google Scholar] [CrossRef]
- Zhen, L.; Wei, Q.; Wang, Q.; Zhang, H.; Adu-Frimpong, M.; Kesse Firempong, C.; Xu, X.; Yu, J. Preparation and in vitro/in vivo evaluation of 6-Gingerol TPGS/PEG-PCL polymeric micelles. Pharm Dev Technol 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Jug, M.; Yoon, B.K.; Jackman, J.A. Cyclodextrin-based Pickering emulsions: Functional properties and drug delivery applications. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 31–50. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Yuan, C.; Cheng, C.; Cui, B. Pickering Emulsions Stabilized by Cyclodextrin Nanoparticles: A Review. Starch-Stärke 2021, 73, 210007. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, Y.; Niu, Y.; Kou, X. Cyclodextrin supermolecules as excellent stabilizers for Pickering nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124367. [Google Scholar] [CrossRef]
- Cheng, C.; Yuan, C.; Cui, B.; Lu, L.; Li, J.; Sha, H. Interfacial behavior of cyclodextrins at the oil-water interface of Pickering emulsion. Food Hydrocoll. 2023, 134, 108104. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, X.; Ke, Q.; Meng, Q. Pickering emulsions stabilized by β-CD microcrystals: Construction and interfacial assembly mechanism. Front. Nutr. 2023, 10, 1161232. [Google Scholar] [CrossRef]
- Oriani, V.B.; Alvim, I.D.; Consoli, L.; Molina, G.; Pastore, G.M.; Hubinger, M.D. Solid lipid microparticles produced by spray chilling technique to deliver ginger oleoresin: Structure and compound retention. Food Res. Int. 2016, 80, 41–49. [Google Scholar] [CrossRef]
- Shukla, A.; Das, C.; Goud, V.V. Design of a carrier system for gingerols enriched oleoresin tailored for food applications. Food Bioprod. Process. 2020, 124, 296–306. [Google Scholar] [CrossRef]
- da Silva, J.A.; Sampaio, P.A.; Dulcey, L.J.L.; Cominetti, M.R.; Rabello, M.M.; Rolim, L.A. Preparation and characterization of [6]-gingerol/β-cyclodextrin inclusion complexes. J. Drug Deliv. Sci. Technol. 2021, 61, 102103. [Google Scholar] [CrossRef]
- Nuchuchua, O.; Saesoo, S.; Sramala, I.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Physicochemical investigation and molecular modeling of cyclodextrin complexation mechanism with eugenol. Food Res. Int. 2009, 42, 1178–1185. [Google Scholar] [CrossRef]
- Cedillo-Flores, O.E.; Rodríguez-Laguna, N.; Hipólito-Nájera, A.R.; Nivón-Ramírez, D.; Gómez-Balderas, R.; Moya-Hernández, R. Effect of the pH on the thermodynamic stability of inclusion complexes of thymol and carvacrol in β-cyclodextrin in water. Food Hydrocoll. 2022, 124, 107307. [Google Scholar] [CrossRef]
- Fatimah, S.; Ragadhita, R.; Al Husaeni, D.F.; Nandiyanto, A.B.D. How to calculate crystallite size from x-ray diffraction (XRD) using Scherrer method. ASEAN J. Sci. Eng. 2022, 2, 65–76. [Google Scholar]
- Suárez, D.; Díaz, N. Amphiphilic cyclodextrins: Dimerization and diazepam binding explored by molecular dynamics simulations. J. Mol. Liq. 2022, 349, 118457. [Google Scholar] [CrossRef]
- Fourtaka, K.; Christoforides, E.; Tzamalis, P.; Bethanis, K. Inclusion of citral isomers in native and methylated cyclodextrins: Structural insights by X-ray crystallography and molecular dynamics simulation analysis. J. Mol. Struct. 2021, 1234, 130169. [Google Scholar] [CrossRef]
- Christoforides, E.; Andreou, A.; Papaioannou, A.; Bethanis, K. Structural Studies of Piperine Inclusion Complexes in Native and Derivative β-Cyclodextrins. Biomolecules 2022, 12, 1762. [Google Scholar] [CrossRef] [PubMed]
- Christoforides, E.; Fourtaka, K.; Andreou, A.; Bethanis, K. X-ray crystallography and molecular dynamics studies of the inclusion complexes of geraniol in β-cyclodextrin, heptakis (2,6-di-O-methyl)-β-cyclodextrin and heptakis (2,3,6-tri-O-methyl)-β-cyclodextrin. J. Mol. Struct. 2020, 1202, 127350. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.C.S.; Hu, Z. Using PyMOL as a platform for computational drug design. WIREs Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Rajendran, P.; Rathinasabapathy, R.; Chandra Kishore, S.; Bellucci, S. Computational-Simulation-Based Behavioral Analysis of Chemical Compounds. J. Compos. Sci. 2023, 7, 196. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Deng, C.; Cao, C.; Zhang, Y.; Hu, J.; Gong, Y.; Zheng, M.; Zhou, Y. Formation and stabilization mechanism of β-cyclodextrin inclusion complex with C10 aroma molecules. Food Hydrocoll. 2022, 123, 107013. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: AnN⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, Y.; Su, D.; Wang, H.; Huang, X.; Niu, Y.; Ke, Q.; Xiao, Z.; Meng, Q. Study on host-guest interaction of aroma compounds/γ-cyclodextrin inclusion complexes. LWT 2023, 178, 114589. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Henon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar]
- Wang, H.; Zhang, Y.; Tian, Z.; Ma, J.; Kang, M.; Ding, C.; Ming, D. Preparation of β-CD-Ellagic Acid Microspheres and Their Effects on HepG2 Cell Proliferation. Molecules 2017, 22, 2175. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Zhao, S.; Guo, J.; Wang, Y.; Feng, L.; Shan, Y.; Zheng, J. The sustained-release mechanism of citrus essential oil from cyclodextrin/cellulose-based Pickering emulsions. Food Hydrocoll. 2023, 144, 109023. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef]
- Das, B.; Baidya, A.T.K.; Mathew, A.T.; Yadav, A.K.; Kumar, R. Structural modification aimed for improving solubility of lead compounds in early phase drug discovery. Bioorganic Med. Chem. 2022, 56, 116614. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Vandecruys, R.; Peeters, J.; Neeskens, P.; Verreck, G.; Loftsson, T. Comparative interaction of 2-hydroxypropyl-β-cyclodextrin and sulfobutylether-β-cyclodextrin with itraconazole: Phase-solubility behavior and stabilization of supersaturated drug solutions. Eur. J. Pharm. Sci. 2008, 34, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Hou, W.; Kang, Y.; Niu, Y.; Kou, X. Encapsulation and sustained release properties of watermelon flavor and its characteristic aroma compounds from γ-cyclodextrin inclusion complexes. Food Hydrocoll. 2019, 97, 105202. [Google Scholar] [CrossRef]
- He, Y.; Fu, P.; Shen, X.; Gao, H. Cyclodextrin-based aggregates and characterization by microscopy. Micron 2008, 39, 495–516. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; Hădărugă, D.I. Thermal Analyses of Cyclodextrin Complexes. In Cyclodextrin Fundamentals, Reactivity and Analysis; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 155–221. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhou, R.; Liu, J.; Zhu, G. Encapsulation of hydroxycitronellal in β-cyclodextrin and the characteristics of the inclusion complex. Pol. J. Chem. Technol. 2023, 25, 20–27. [Google Scholar] [CrossRef]
- Papaioannou, A.; Christoforides, E.; Bethanis, K. Inclusion Complexes of Naringenin in Dimethylated and Permethylated β-Cyclodextrins: Crystal Structures and Molecular Dynamics Studies. Crystals 2020, 10, 10. [Google Scholar] [CrossRef]
- ICDD/PDF No. 00-054-1848; β-CD·7H₂O (Beta-Cyclodextrin Heptahydrate). International Centre for Diffraction Data: Newtown Square, PA, USA.
- Liang, B.; Hao, J.; Zhu, N.; Han, L.; Song, L.; Hong, H. Formulation of nitrendipine/hydroxypropyl-β-cyclodextrin inclusion complex as a drug delivery system to enhance the solubility and bioavailability by supercritical fluid technology. Eur. Polym. J. 2023, 187, 111880. [Google Scholar] [CrossRef]
- Niu, H.; Chen, W.; Chen, W.; Yun, Y.; Zhong, Q.; Fu, X.; Chen, H.; Liu, G. Preparation and Characterization of a Modified-β-Cyclodextrin/β-Carotene Inclusion Complex and Its Application in Pickering Emulsions. J. Agric. Food Chem. 2019, 67, 12875–12884. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.A.B.d.; Costa, C.H.S.; da Costa, K.S.; da Paz, S.P.A.; Silva, J.R.A.; Alves, C.N.; Lameira, J. Assessment of host–guest molecular encapsulation of eugenol using β-cyclodextrin. Front. Chem. 2023, 10, 1061624. [Google Scholar] [CrossRef]
- Chabalala, M.B.; Zikalala, S.A.; Ndlovu, L.; Mamba, G.; Mamba, B.B.; Nxumalo, E.N. A green synthetic approach for the morphological control of ZnO-Ag using β-cyclodextrin and honey for photocatalytic degradation of bromophenol blue. Chem. Eng. Res. Des. 2023, 197, 307–322. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Du, M.; Xie, C.; Xie, X.; Zhang, H.; Meng, X.; Li, A.; Deng, T. Encapsulation of catechin into nano-cyclodextrin-metal-organic frameworks: Preparation, characterization, and evaluation of storage stability and bioavailability. Food Chem. 2022, 394, 133553. [Google Scholar] [CrossRef]
- Casas, E.A. A Comparison of Sensitization Kinetics in 304 and 316 Stainless Steels; The University of Texas at El Paso: El Paso, TX, USA, 1999. [Google Scholar]
- Pan, F.; Li, X.; Tuersuntuoheti, T.; Zhao, L.; Liu, M.; Fang, X.; Peng, W.; Tian, W. Self-assembled condensed tannins supramolecular system can adsorb cholesterol micelles to promote cholesterol excretion. Int. J. Biol. Macromol. 2023, 253, 126549. [Google Scholar] [CrossRef]
- Shimizu, S.; Wada-Hirai, A.; Li, Y.; Shimada, Y.; Otsuka, Y.; Goto, S. Relationship Between Phase Solubility Diagrams and Crystalline Structures During Dissolution of Cimetidine/Cyclodextrin Complex Crystals. J. Pharm. Sci. 2020, 109, 2206–2212. [Google Scholar] [CrossRef]
- Miyamoto, H.; Rein, D.M.; Ueda, K.; Yamane, C.; Cohen, Y. Molecular dynamics simulation of cellulose-coated oil-in-water emulsions. Cellulose 2017, 24, 2699–2711. [Google Scholar] [CrossRef]
- Osaki, M.; Takashima, Y.; Kamitori, S.; Yamaguchi, H.; Harada, A. X-ray crystal structures of α-cyclodextrin–5-hydroxypentanoic acid, β-cyclodextrin–5-hydroxypentanoic acid, β-cyclodextrin–ε-caprolactone, and β-cyclodextrin–ε-caprolactam inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 93–99. [Google Scholar] [CrossRef]
- Ishimoto, A.; Sasako, H.; Omori, M.; Higashi, K.; Ueda, K.; Koyama, K.; Moribe, K. Drug-Loaded Nanocarriers Composed of Cholesteryl Oleate Crystal Cores and Multiple-Nanosheet Shells of γ-Cyclodextrin Inclusion Complex Crystals. Langmuir 2022, 38, 10454–10464. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, X.; Su, D.; Zhang, J.; Pan, F.; Zhu, J.; Meng, Q.; Ke, Q. Cyclodextrin-Based Pickering Emulsion Significantly Increases 6-Gingerol Loading Through Two Different Mechanisms: Cyclodextrin Cavity and Pickering Core. Foods 2025, 14, 1066. https://doi.org/10.3390/foods14061066
Kou X, Su D, Zhang J, Pan F, Zhu J, Meng Q, Ke Q. Cyclodextrin-Based Pickering Emulsion Significantly Increases 6-Gingerol Loading Through Two Different Mechanisms: Cyclodextrin Cavity and Pickering Core. Foods. 2025; 14(6):1066. https://doi.org/10.3390/foods14061066
Chicago/Turabian StyleKou, Xingran, Dongdong Su, Jingzhi Zhang, Fei Pan, Jiamin Zhu, Qingran Meng, and Qinfei Ke. 2025. "Cyclodextrin-Based Pickering Emulsion Significantly Increases 6-Gingerol Loading Through Two Different Mechanisms: Cyclodextrin Cavity and Pickering Core" Foods 14, no. 6: 1066. https://doi.org/10.3390/foods14061066
APA StyleKou, X., Su, D., Zhang, J., Pan, F., Zhu, J., Meng, Q., & Ke, Q. (2025). Cyclodextrin-Based Pickering Emulsion Significantly Increases 6-Gingerol Loading Through Two Different Mechanisms: Cyclodextrin Cavity and Pickering Core. Foods, 14(6), 1066. https://doi.org/10.3390/foods14061066






