Effect of Baking Conditions on Mycotoxin Levels in Flatbreads Prepared from Artificially Contaminated Doughs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flatbread Preparation
2.3. Extraction of Mycotoxins from Flatbreads
2.4. Recovery Studies
2.5. LC-MS/MS Analysis
2.6. Mycotoxin Quantification in Flatbread and Data Expression
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction Procedure and LC-MS/MS Method Evaluation
3.2. Effect of Baking Conditions on Mycotoxin Levels
3.3. Principal Component Analysis and Hierarchical Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Aflatoxin |
| DON | Deoxynivalenol |
| OTA | Ochratoxin A |
| DOA | Deoxynivalenol |
| LOD | Limit of detection |
| LOQ | Limit ofquantification |
| QuEChERS | Quick, Easy, Cheap, Effective, Rugged, and Safe |
References
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Lin, C.; Feng, X.-l.; Liu, Y.; Li, Z.-c.; Li, X.-Z.; Qi, J. Bioinformatic Analysis of Secondary Metabolite Biosynthetic Potential in Pathogenic Fusarium. J. Fungi 2023, 9, 850. [Google Scholar] [CrossRef]
- EC. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Skrzydlewski, P.; Twarużek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef]
- Numanoglu, E.; Gökmen, V.; Uygun, U.; Koksel, H. Thermal degradation of deoxynivalenol during maize bread baking. Food Addit. Contam. Part A 2012, 29, 423–430. [Google Scholar] [CrossRef]
- Vidal, A.; Sanchis, V.; Ramos, A.J.; Marín, S. Thermal stability and kinetics of degradation of deoxynivalenol, deoxynivalenol conjugates and ochratoxin A during baking of wheat bakery products. Food Chem. 2015, 178, 276–286. [Google Scholar] [CrossRef]
- Generotti, S.; Cirlini, M.; Malachova, A.; Sulyok, M.; Berthiller, F.; Dall’Asta, C.; Suman, M. Deoxynivalenol & Deoxynivalenol-3-Glucoside Mitigation through Bakery Production Strategies: Effective Experimental Design within Industrial Rusk-Making Technology. Toxins 2015, 7, 2773–2790. [Google Scholar] [CrossRef]
- Samar, M.; Resnik, S.L.; González, H.H.L.; Pacin, A.M.; Castillo, M.D. Deoxynivalenol reduction during the frying process of turnover pie covers. Food Control 2007, 18, 1295–1299. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Pascale, M.; Visconti, A. Fate of deoxynivalenol, T-2 and HT-2 toxins and their glucoside conjugates from flour to bread: An investigation by high-performance liquid chromatography high-resolution mass spectrometry. Food Addit. Contam. Part A 2013, 30, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.A.; Banks, J.; MacDonald, S.J. Fate of ochratoxin A in the processing of whole wheat grains during milling and bread production. Food Addit. Contam. 2003, 20, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Morales, H.; Sanchis, V.; Ramos, A.J.; Marín, S. Stability of DON and OTA during the breadmaking process and determination of process and performance criteria. Food Control 2014, 40, 234–242. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Morales, H.; Ramos, A.J.; Sanchis, V. The fate of deoxynivalenol and ochratoxin A during the breadmaking process, effects of sourdough use and bran content. Food Chem. Toxicol. 2014, 68, 53–60. [Google Scholar] [CrossRef]
- Valle-Algarra, F.M.; Mateo, E.M.; Medina, Á.; Mateo, F.; Gimeno-Adelantado, J.V.; Jiménez, M. Changes in ochratoxin A and type B trichothecenes contained in wheat flour during dough fermentation and bread-baking. Food Addit. Contam. Part A 2009, 26, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Numanoglu, E.; Yener, S.; Gökmen, V.; Uygun, U.; Koksel, H. Modelling thermal degradation of zearalenone in maize bread during baking. Food Addit. Contam. Part A 2013, 30, 528–533. [Google Scholar] [CrossRef]
- El-Desouky, T. Effects of pan bread making on zearalenone levels in artificial contaminated wheat flour. Hung. J. Ind. Chem. 2012, 40, 107–111. [Google Scholar]
- Noroozi, R.; Kobarfard, F.; Rezaei, M.; Ayatollahi, S.A.; Paimard, G.; Eslamizad, S.; Razmjoo, F.; Sadeghi, E. Occurrence and exposure assessment of aflatoxin B1 in Iranian breads and wheat-based products considering effects of traditional processing. Food Control 2022, 138, 108985. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Ramadan, M.F.; El-Gohery, S.S.; Abol-Ela, M.F.; Azeke, M.A. Ability of selected microorganisms for removing aflatoxins in vitro and fate of aflatoxins in contaminated wheat during baladi bread baking. Food Control 2013, 33, 287–292. [Google Scholar] [CrossRef]
- Amra, H.A.; Mahmoud, S.A.; Tana, A.H.; El-Azab, M.A. Destruction of aflatoxins B1 and G 1 in bread making. Mycotoxin Res. 1996, 12, 73–78. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Köppen, R.; Koch, M.; Siegel, D.; Merkel, S.; Maul, R.; Nehls, I. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 2010, 86, 1595–1612. [Google Scholar] [CrossRef]
- Annunziata, L.; Stramenga, A.; Visciano, P.; Schirone, M.; De Colli, L.; Colagrande, M.N.; Campana, G.; Scortichini, G. Simultaneous determination of aflatoxins, T-2 and HT-2 toxins, and fumonisins in cereal-derived products by QuEChERS extraction coupled with LC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 5143–5155. [Google Scholar] [CrossRef]
- Pasqualone, A.; Vurro, F.; Summo, C.; Abd-El-Khalek, M.H.; Al-Dmoor, H.H.; Grgic, T.; Ruiz, M.; Magro, C.; Deligeorgakis, C.; Helou, C.; et al. The Large and Diverse Family of Mediterranean Flat Breads: A Database. Foods 2022, 11, 2326. [Google Scholar] [CrossRef]
- Garzon, R.; Gasparre, N.; Pasqualone, A.; Papageorgiou, M.; Grgic, T.; Le-Bail, P.; Pablos, I.M.; Tomb, C.E.; Magro, C.; Rosell, C.M. Flatbreads on the Rise, What about their Nutritional Quality? The Current State of the Mediterranean Market. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Rausch, A.-K.; Brockmeyer, R.; Schwerdtle, T. Development and Validation of a QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Multi-Method for the Determination of 38 Native and Modified Mycotoxins in Cereals. J. Agric. Food Chem. 2020, 68, 4657–4669. [Google Scholar] [CrossRef]
- Rubert, J.; Dzuman, Z.; Vaclavikova, M.; Zachariasova, M.; Soler, C.; Hajslova, J. Analysis of mycotoxins in barley using ultra high liquid chromatography high resolution mass spectrometry: Comparison of efficiency and efficacy of different extraction procedures. Talanta 2012, 99, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B. The fate of mycotoxins during thermal food processing. J. Sci. Food Agric. 2009, 89, 549–554. [Google Scholar] [CrossRef]
- Torres, P.; Guzmán-Ortiz, M.; Ramírez-Wong, B. Revising the Role of pH and Thermal Treatments in Aflatoxin Content Reduction During the Tortilla and Deep Frying Processes. J. Agric. Food Chem. 2001, 49, 2825–2829. [Google Scholar] [CrossRef]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, B. Evaluation on levels and conversion profiles of DON, 3-ADON, and 15-ADON during bread making process. Food Chem. 2015, 185, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Stadler, D.; Lambertini, F.; Woelflingseder, L.; Schwartz-Zimmermann, H.; Marko, D.; Suman, M.; Berthiller, F.; Krska, R. The Influence of Processing Parameters on the Mitigation of Deoxynivalenol during Industrial Baking. Toxins 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Samarajeewa, U.; Sen, A.C.; Cohen, M.D.; Wei, C.I. Detoxification of Aflatoxins in Foods and Feeds by Physical and Chemical Methods. J. Food Prot. 1990, 53, 489–501. [Google Scholar] [CrossRef]


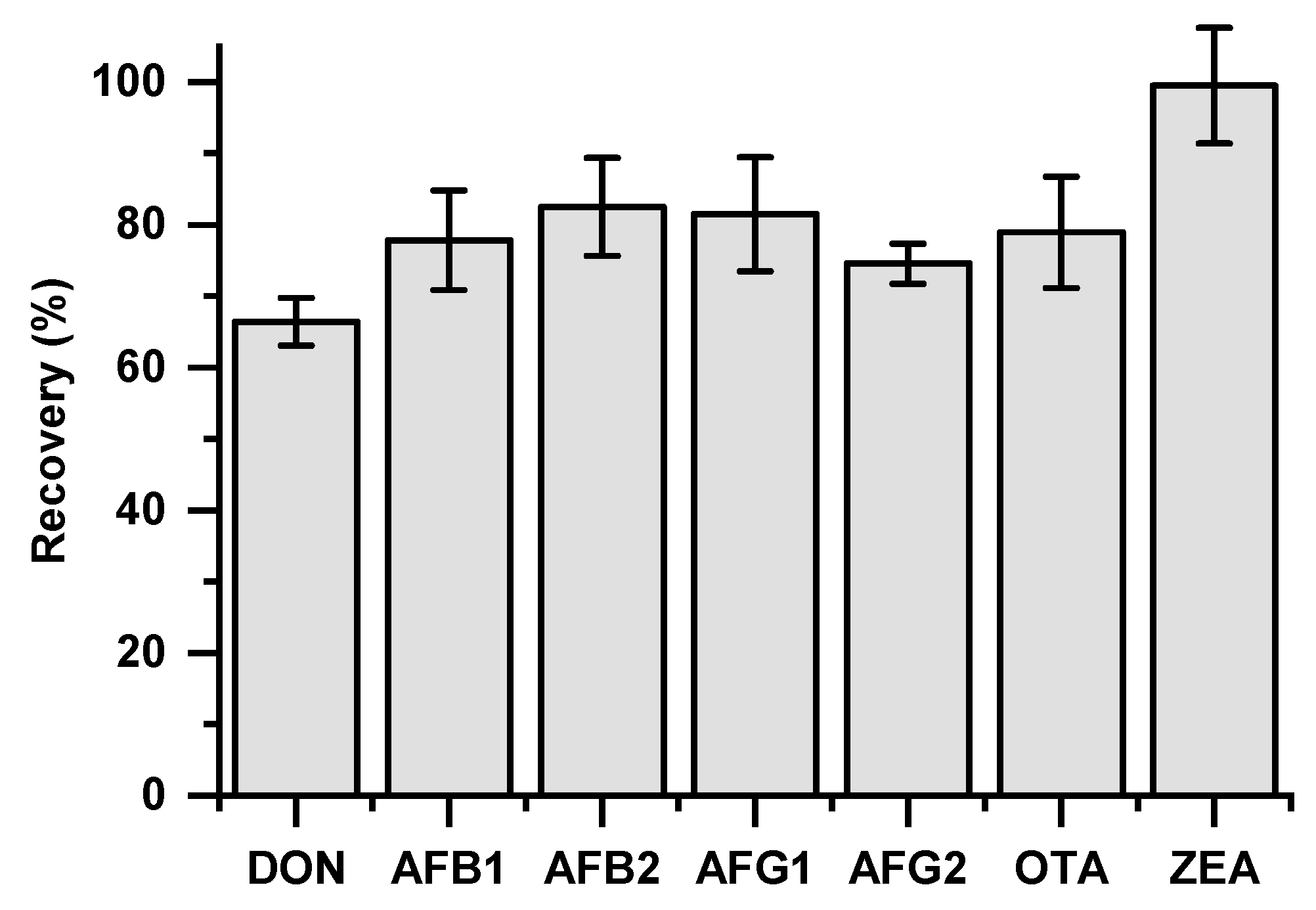
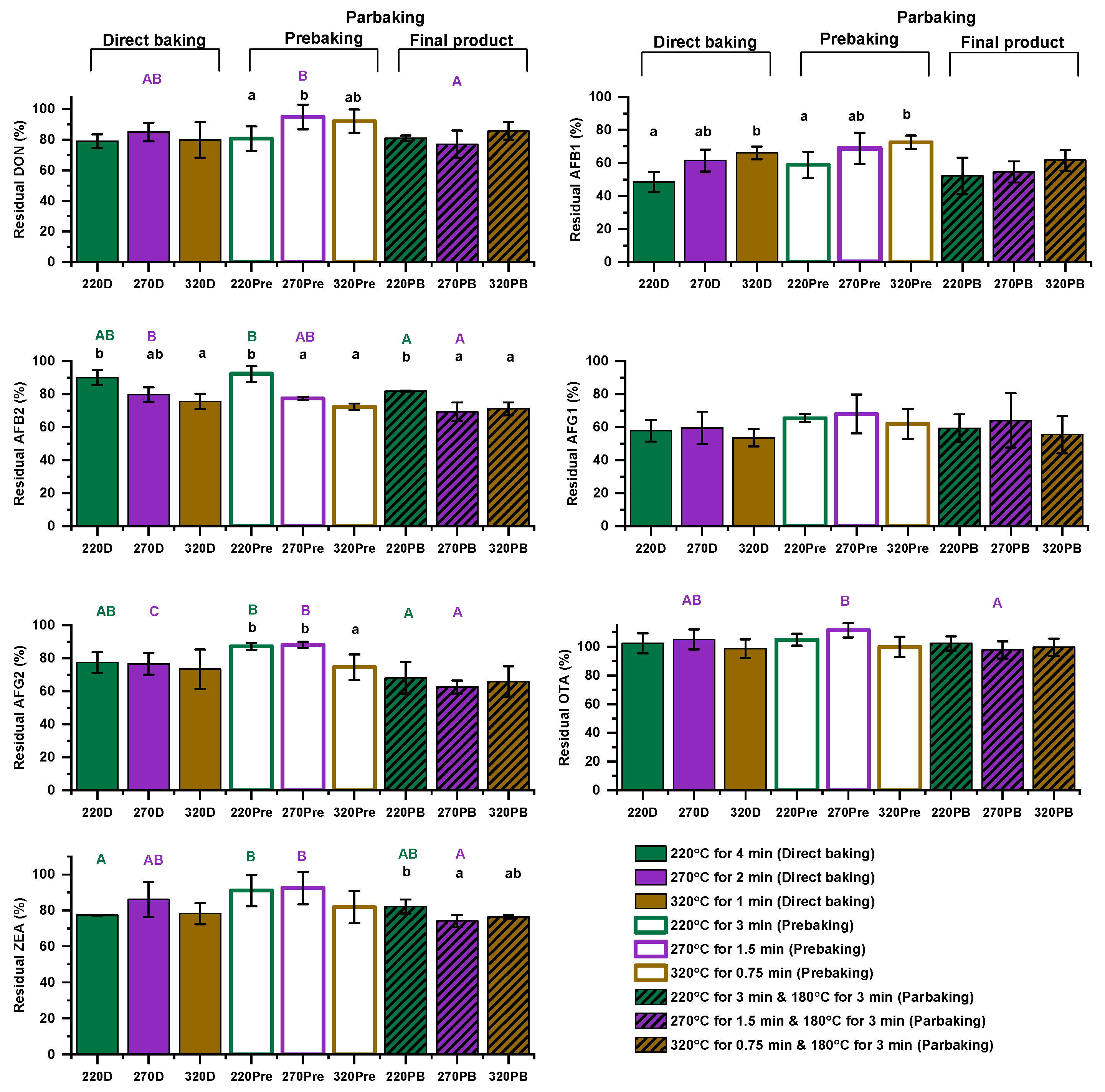
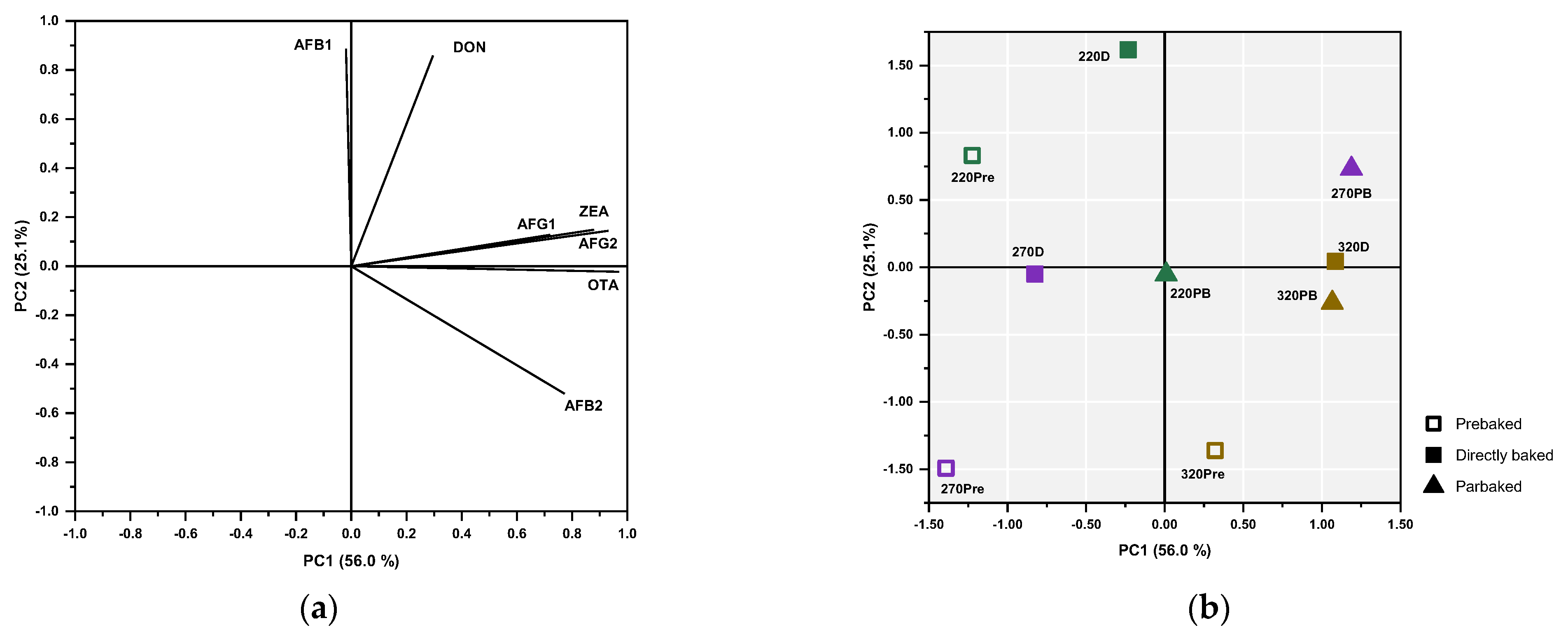

| Sample Code | 1st Baking | 2nd Baking | Final Moisture (%) |
|---|---|---|---|
| 220D | 4 min/220 °C | - | 34.6 (±1.8) |
| 200Pre | 3 min/220 °C | - | 36.4 (±1.7) |
| 220PB | 3 min/220 °C | 3 min/180 °C | 33.3 (±1.2) |
| 270D | 2 min/270 °C | - | 37.0 (±1.6) |
| 270Pre | 1.5 min/270 °C | - | 37.9 (±1.7) |
| 270PB | 1.5 min/270 °C | 3 min/180 °C | 33.5 (±1.3) |
| 320D | 1 min/320 °C | - | 37.3 (±1.7) |
| 320Pre | 45 s/320 °C | - | 38.1 (±1.6) |
| 320PB | 45 s/320 °C | 3 min/180 °C | 35.2 (±1.5) |
| Analyte | Retention Time (min) | Precursor Ion | Product Ion | Collision Energy (V) | Polarity |
|---|---|---|---|---|---|
| Deoxynivalenol (DON) | 8.43 | 297 | 249 * | 10 | + |
| 231 ** | 14 | + | |||
| 175 ** | 19 | + | |||
| Aflatoxin B1 (AFB1) | 11.61 | 313.1 | 285.1 * | 23 | + |
| 270 ** | 27 | + | |||
| 241 ** | 37 | + | |||
| Aflatoxin B2 (AFB2) | 11.42 | 315.1 | 287.1 * | 26 | + |
| 271 ** | 32 | + | |||
| 243 ** | 39 | + | |||
| AflatoxinG1 (AFG1) | 11.11 | 329 | 243.1 * | 27 | + |
| 215 ** | 32 | + | |||
| 199 ** | 41 | + | |||
| AflatoxinG2 (AFG2) | 10.85 | 331.1 | 313 * | 24 | + |
| 285 ** | 28 | + | |||
| 189 ** | 42 | + | |||
| Ochratoxin A (OTA) | 13.12 | 404 | 239 * | 15 | + |
| 358 ** | 24 | + | |||
| 220.9 ** | 15 | + | |||
| Zearalenone (ZEA) | 13.16 | 317.1 | 131 * | 19 | - |
| 175 ** | 24 | - | |||
| 273 ** | 29 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsiou, K.; Terzidis, M.A.; Papageorgiou, M. Effect of Baking Conditions on Mycotoxin Levels in Flatbreads Prepared from Artificially Contaminated Doughs. Foods 2025, 14, 910. https://doi.org/10.3390/foods14060910
Kotsiou K, Terzidis MA, Papageorgiou M. Effect of Baking Conditions on Mycotoxin Levels in Flatbreads Prepared from Artificially Contaminated Doughs. Foods. 2025; 14(6):910. https://doi.org/10.3390/foods14060910
Chicago/Turabian StyleKotsiou, Kali, Michael A. Terzidis, and Maria Papageorgiou. 2025. "Effect of Baking Conditions on Mycotoxin Levels in Flatbreads Prepared from Artificially Contaminated Doughs" Foods 14, no. 6: 910. https://doi.org/10.3390/foods14060910
APA StyleKotsiou, K., Terzidis, M. A., & Papageorgiou, M. (2025). Effect of Baking Conditions on Mycotoxin Levels in Flatbreads Prepared from Artificially Contaminated Doughs. Foods, 14(6), 910. https://doi.org/10.3390/foods14060910







