Exploring the Nutritional Value and Health Benefits of Honey from the Portuguese Protected Area of Montesinho Natural Park
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sampling
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Determination of the Total Phenolic and Flavonoid Content
2.5. Determination of the Phenolic Composition by HPLC-DAD
2.6. Determination of the Amino Acids Profile
2.7. pH Determination
2.8. Hydrogen Peroxide Determination
2.9. Bioactivities
2.9.1. Assessment of the In Vitro Antioxidant Activity
2.9.2. Enzyme Inhibition Activity
2.9.3. Assessment of the Antimicrobial Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Content
3.2. Phenolic Composition by HPLC-DAD
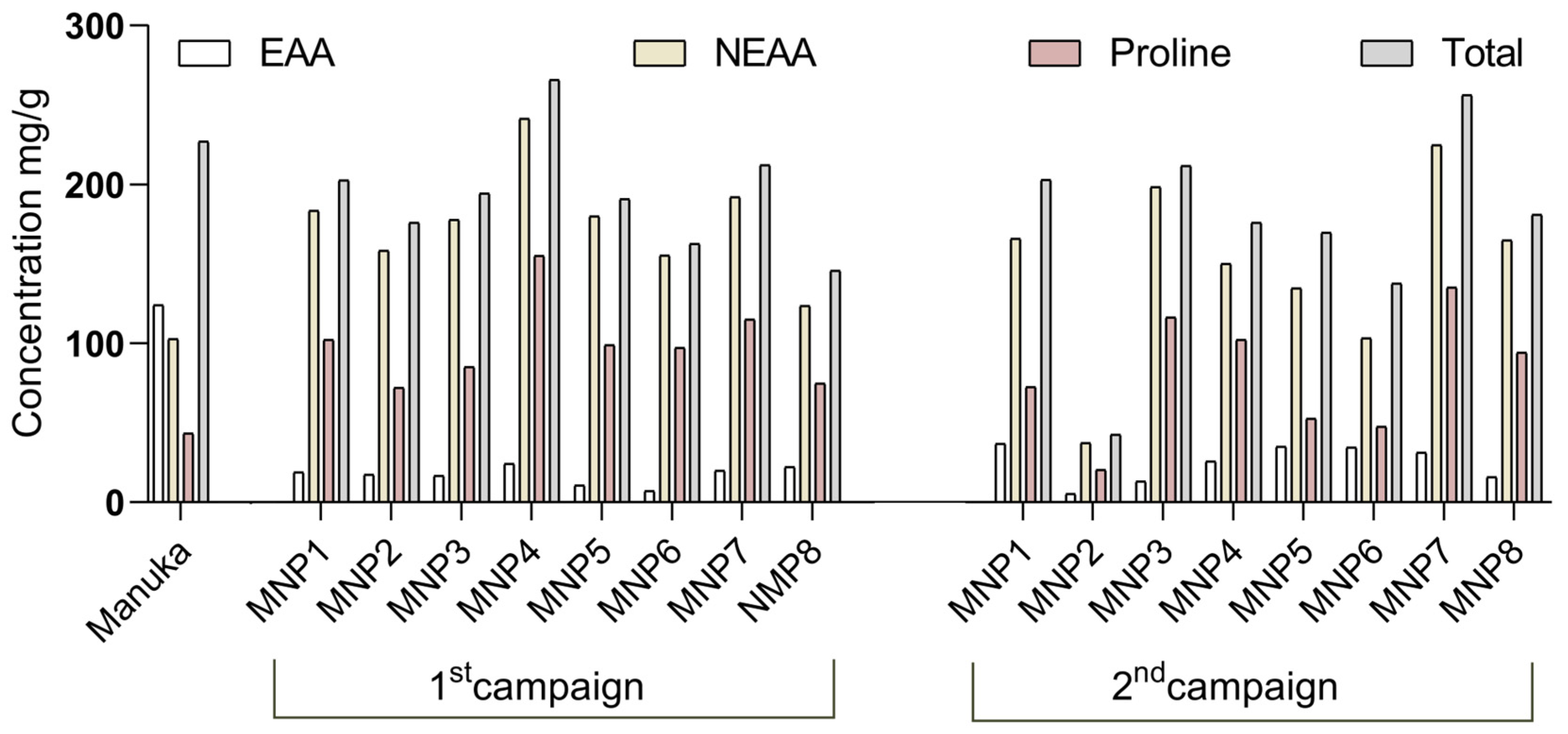
3.3. Amino Acids Profile
3.4. pH and H2O2 Determination
3.5. In Vitro Antioxidant Activity
3.6. Enzyme Inhibition
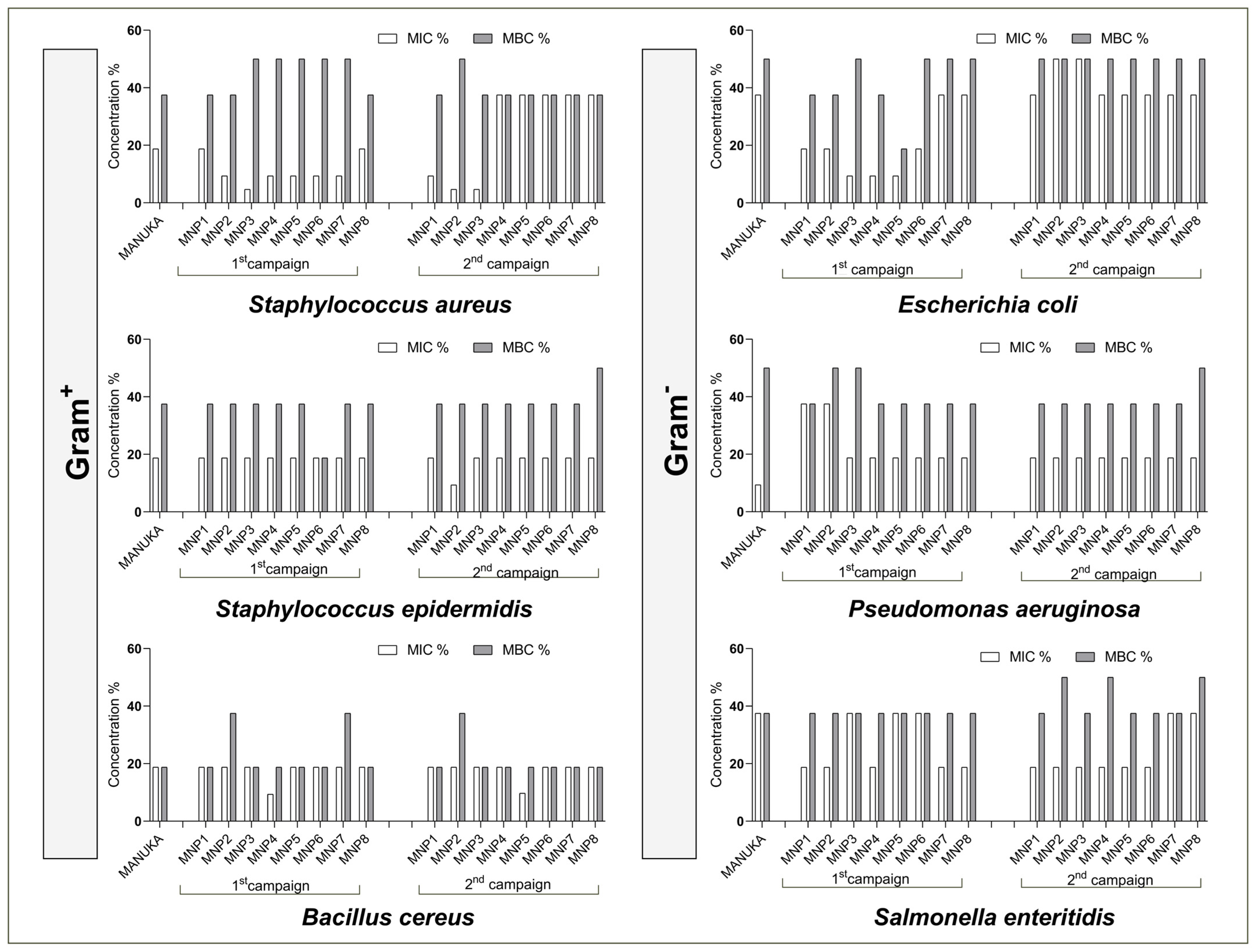
3.7. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, S.; Venugopal, S. Antioxidant and α-Amylase Inhibition Activities of Phenolic Compounds in the Extracts of Indian Honey. Chin. J. Nat. Med. 2012, 10, 255–259. [Google Scholar] [CrossRef]
- Habib, H.M.; Kheadr, E.; Ibrahim, W.H. Inhibitory Effects of Honey from Arid Land on Some Enzymes and Protein Damage. Food Chem. 2021, 364, 130415. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Santos-Buelga, C.; Era, B.; González-Paramás, A.M.; Tuberoso, C.I.G.; Medda, R.; Pintus, F.; Fais, A. Sardinian Honeys as Sources of Xanthine Oxidase and Tyrosinase Inhibitors. Food Sci. Biotechnol. 2018, 27, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Al-Sayaghi, A.M.; Al-Kabsi, A.M.; Abduh, M.S.; Saghir, S.A.M.; Alshawsh, M.A. Antibacterial Mechanism of Action of Two Types of Honey against Escherichia coli through Interfering with Bacterial Membrane Permeability, Inhibiting Proteins, and Inducing Bacterial DNA Damage. Antibiotics 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ghidotti, M.; Fiamegos, Y.; Dumitrascu, C.; de la Calle, M.B. Use of Elemental Profiles to Verify Geographical Origin and Botanical Variety of Spanish Honeys with a Protected Denomination of Origin. Food Chem. 2021, 342, 128350. [Google Scholar] [CrossRef]
- Li, M.; Xiao, H.; Su, Y.; Cheng, D.; Jia, Y.; Li, Y.; Yin, Q.; Gao, J.; Tang, Y.; Bai, Q. Synergistic Inhibitory Effect of Honey and Lactobacillus plantarum on Pathogenic Bacteria and Their Promotion of Healing in Infected Wounds. Pathogens 2023, 12, 501. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Thakur, V.; Brar, S.K. Antibacterial Efficacy of Raw and Processed Honey. Biotechnol. Res. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Magalhães, L.; Moreira, M.M.; Rede, D.; Fernandes, V.C.; Viegas, O.; Pinto, E.; Almeida, A.; Azevedo, R.; Delerue-Matos, C. Exploring Geographical Influences on Physicochemical Characteristics of Honey: The Montesinho Natural Park Scenario. Food Qual. Saf. 2024, 8, fyae015. [Google Scholar] [CrossRef]
- Bayomy, H.M.; Alamri, E.S.; Ozaybi, N.A.; Eid, K.S.A.; Nassar, A.M.K.; Shamsia, S.M. Different Floral Honey Types Are Differentiated Based on Their Antioxidant Activities, Contents of Bioactive Compounds, and Pesticide Residues. Food Biosci. 2024, 60, 104352. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dorosh, O.; Fernandes, V.C.; Delerue-Matos, C.; Moreira, M.M. Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry. Foods 2024, 13, 317. [Google Scholar] [CrossRef]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-Canes as a Source of Value-Added Compounds for Cosmetic Formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Giroud, B.; Albert, M.; Lheritier, J.; Grenier-Loustalot, M.F. Characterization of Honey Amino Acid Profiles Using High-Pressure Liquid Chromatography to Control Authenticity. Anal. Bioanal. Chem. 2004, 378, 1342–1350. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese Coast as a Source of Proteinaceous Material: Total and Free Amino Acid Composition Profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Branco, A.; Soares, C.; Delerue-Matos, C.; Ramalhosa, M.J.; Domingues, V.F. Cosmetic Degradation: A Study on Body Lotions with Botanical Extracts. S. Afr. J. Bot. 2024, 170, 194–204. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Paíga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simões, P.; Costa, L.; Bernardo, A.; Fernández, N.; et al. Multi-Step Subcritical Water Extracts of Fucus vesiculosus L. and Codium tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Silva, A.; Cassani, L.; Carpena, M.; Lourenço-Lopes, C.; Grosso, C.; Chamorro, F.; García-Pérez, P.; Carvalho, A.; Domingues, V.F.; Barroso, M.F.; et al. Exploring the Potential of Invasive Species Sargassum muticum: Microwave-Assisted Extraction Optimization and Bioactivity Profiling. Mar. Drugs 2024, 22, 352. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for Tyrosinase Inhibitors among Extracts of Seashore Plants and Identification of Potent Inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Fernandes, A.; Valentão, P.; Andrade, P.B. Comparing the Phenolic Profile of Pilocarpus Pennatifolius Lem. by HPLC–DAD–ESI/MS n with Respect to Authentication and Enzyme Inhibition Potential. Ind. Crops Prod. 2015, 77, 391–401. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison With Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Fernandes, L.; Ribeiro, H.; Oliveira, A.; Sanches Silva, A.; Freitas, A.; Henriques, M.; Rodrigues, M.E. Portuguese Honeys as Antimicrobial Agents against Candida Species. J. Tradit. Complement. Med. 2021, 11, 130–136. [Google Scholar] [CrossRef]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant Activity, Quality Parameters and Mineral Content of Portuguese Monofloral Honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Estevinho, L.M.; Feás, X.; Seijas, J.A.; Pilar Vázquez-Tato, M. Organic Honey from Trás-Os-Montes Region (Portugal): Chemical, Palynological, Microbiological and Bioactive Compounds Characterization. Food Chem. Toxicol. 2012, 50, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant Activity of Portuguese Honey Samples: Different Contributions of the Entire Honey and Phenolic Extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Živković, J.; Sunarić, S.; Stanković, N.; Mihajilov-Krstev, T.; Spasić, A. Total Phenolic and Flavonoid Contents, Antioxidant and Antibacterial Activities of Selected Honeys against Human Pathogenic Bacteria. Acta Pol. Pharm.-Drug Res. 2019, 76, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical Properties and Phenolic Content of Honey from Different Floral Origins and from Rural versus Urban Landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-Collected Pollen from Five Portuguese Natural Parks: Palynological Origin, Phenolic Content, Antioxidant Properties and Antimicrobial Activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Pedisić, S.; Čulina, P.; Pavlešić, T.; Vahčić, N.; Elez Garofulić, I.; Zorić, Z.; Dragović-Uzelac, V.; Repajić, M. Efficiency of Microwave and Ultrasound-Assisted Extraction as a Green Tool for Polyphenolic Isolation from Monofloral Honeys. Processes 2023, 11, 3141. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic Acids and Flavonoids Profiles of Commercial Honey from Different Floral Sources and Geographic Sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Küçükaydın, S.; Tel-Çayan, G.; Çayan, F.; Taş-Küçükaydın, M.; Eroğlu, B.; Duru, M.E.; Öztürk, M. Characterization of Turkish Astragalus Honeys According to Their Phenolic Profiles and Biological Activities with a Chemometric Approach. Food Biosci. 2023, 53, 102507. [Google Scholar] [CrossRef]
- Hernanz, D.; Jara-Palacios, M.J.; Santos, J.L.; Gómez Pajuelo, A.; Heredia, F.J.; Terrab, A. The Profile of Phenolic Compounds by HPLC-MS in Spanish Oak (Quercus) Honeydew Honey and Their Relationships with Color and Antioxidant Activity. LWT 2023, 180, 114724. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Pacholczyk-Sienicka, B.; Ciepielowski, G.; Modranka, J.; Bartosik, T.; Albrecht, Ł. Classification of Polish Natural Bee Honeys Based on Their Chemical Composition. Molecules 2022, 27, 4844. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R.; et al. Honey Quality and International Regulatory Standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Trepp, R.; Muri, R.; Abgottspon, S.; Bosanska, L.; Hochuli, M.; Slotboom, J.; Rummel, C.; Kreis, R.; Everts, R. Impact of Phenylalanine on Cognitive, Cerebral, and Neurometabolic Parameters in Adult Patients with Phenylketonuria (the PICO Study): A Randomized, Placebo-Controlled, Crossover, Noninferiority Trial. Trials 2020, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Iwata, J. Amino Acid Metabolism and Autophagy in Skeletal Development and Homeostasis. Bone 2021, 146, 115881. [Google Scholar] [CrossRef]
- Donkersley, P.; Rhodes, G.; Pickup, R.W.; Jones, K.C.; Power, E.F.; Wright, G.A.; Wilson, K. Nutritional Composition of Honey Bee Food Stores Vary with Floral Composition. Oecologia 2017, 185, 749–761. [Google Scholar] [CrossRef]
- Hermosín, I.; Chicón, R.M.; Dolores Cabezudo, M. Free Amino Acid Composition and Botanical Origin of Honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Machado, A.M.; Tomás, A.; Russo-Almeida, P.; Duarte, A.; Antunes, M.; Vilas-Boas, M.; Graça Miguel, M.; Cristina Figueiredo, A. Quality Assessment of Portuguese Monofloral Honeys. Physicochemical Parameters as Tools in Botanical Source Differentiation. Food Res. Int. 2022, 157, 111362. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated Production of Hydrogen Peroxide Is Crucial for High Antibacterial Activity of Honeydew Honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Brudzynski, K. A Current Perspective on Hydrogen Peroxide Production in Honey. A Review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Zaheen, Z.; Yatoo, A.M.; Ali, S.; Ali, M.N.; Majid, S.; Rasool, S.; Rashid, S.M.; Ahmad, S.B.; Mir, M.u.R.; Zehra, U. Honey: Types, Composition and Antimicrobial Mechanisms. In Therapeutic Applications of Honey and Its Phytochemicals; Springer: Singapore, 2020; pp. 193–214. [Google Scholar]
- Hussain, M.B. Role of Honey in Topical and Systemic Bacterial Infections. J. Altern. Complement. Med. 2018, 24, 15–24. [Google Scholar] [CrossRef]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The Role of Hydrogen Peroxide in the Antibacterial Activity of Different Floral Honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Lehmann, D.M.; Krishnakumar, K.; Batres, M.A.; Hakola-Parry, A.; Cokcetin, N.; Harry, E.; Carter, D.A. A Cost-Effective Colourimetric Assay for Quantifying Hydrogen Peroxide in Honey. Access Microbiol. 2019, 1, e000065. [Google Scholar] [CrossRef]
- Guttentag, A.; Krishnakumar, K.; Cokcetin, N.; Harry, E.; Carter, D. Factors Affecting the Production and Measurement of Hydrogen Peroxide in Honey Samples. Access Microbiol. 2021, 3, 000198. [Google Scholar] [CrossRef]
- Feng, Y. Antibacterial Properties of Manuka Honey and the Role of Methylglyoxal. J. Stud. Res. 2023, 12, 1–12. [Google Scholar] [CrossRef]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Singh Nigam, P. Antibacterial Activity of Manuka Honey and Its Components: An Overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Monofloral Honeys by Sicilian Black Honeybee (Apis Mellifera Ssp. Sicula) Have High Reducing Power and Antioxidant Capacity. Heliyon 2016, 2, e00193. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and Antimicrobial Effects of Phenolic Compounds Extracts of Northeast Portugal Honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Boutoub, O.; El-Guendouz, S.; Manhita, A.; Dias, C.B.; Estevinho, L.M.; Paula, V.B.; Carlier, J.; Costa, M.C.; Rodrigues, B.; Raposo, S.; et al. Comparative Study of the Antioxidant and Enzyme Inhibitory Activities of Two Types of Moroccan Euphorbia Entire Honey and Their Phenolic Extracts. Foods 2021, 10, 1909. [Google Scholar] [CrossRef]
- Boutoub, O.; El-Guendouz, S.; Estevinho, L.M.; Paula, V.B.; Aazza, S.; El Ghadraoui, L.; Rodrigues, B.; Raposo, S.; Carlier, J.; Costa, M.C.; et al. Antioxidant Activity and Enzyme Inhibitory Potential of Euphorbia Resinifera and E. Officinarum Honeys from Morocco and Plant Aqueous Extracts. Environ. Sci. Pollut. Res. 2021, 28, 503–517. [Google Scholar] [CrossRef]
- Elamine, Y.; Aazza, S.; Lyoussi, B.; Dulce Antunes, M.; Estevinho, L.M.; Anjos, O.; Resende, M.; Faleiro, M.L.; Miguel, M.G. Preliminary Characterization of a Moroccan Honey with a Predominance of Bupleurum spinosum Pollen. J. Apic. Res. 2018, 57, 153–165. [Google Scholar] [CrossRef]
- Rodrigues da Silva, L.; Campos Chisté, R.; Fernandes, E. Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna Vulgaris. Separations 2021, 8, 177. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Kanamori, N.; Suzuki, N.; Nagashima, T. Characterization of Honey from Different Floral Sources. Its Functional Properties and Effects of Honey Species on Storage of Meat. Food Chem. 2006, 97, 256–262. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Suppasat, T.; Silprasit, K. Glucosidase Inhibition and Compound Identification of Stingless Bee Honey and Preserved Fruits of Citrus Japonica. Heliyon 2024, 10, e29740. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Abu Bakar, M.F.; Majid, M.; Muhammad, N.; Lim, S.Y. In Vitro Anti-Diabetic Activity of Stingless Bee Honey from Different Botanical Origins. Food Res. 2020, 4, 1421–1426. [Google Scholar] [CrossRef]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Serralheiro, M.L.; Araujo, M.E. Biological Properties of Phenolic Compound Extracts in Selected Algerian Honeys—The Inhibition of Acetylcholinesterase and α-Glucosidase Activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Romário-Silva, D.; Alencar, S.M.; Bueno-Silva, B.; Sardi, J.D.C.O.; Franchin, M.; de Carvalho, R.D.P.; Ferreira, T.E.d.S.A.; Rosalen, P.L. Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges and Solutions. Microorganisms 2022, 10, 2325. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 2018, 1–10. [Google Scholar] [CrossRef]


| Parameter | MNP1 | MNP2 | MNP3 | MNP4 | MNP5 | MNP6 | MNP7 | MNP8 | Manuka | |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/100 g) | 1st campaign | 68.72 ± 2.38 *** | 72.77 ± 1.54 **** | 71.01 ± 3.81 **** | 71.66 ± 1.01 **** | 64.17 ± 0.58 * | 65.20 ± 1.09 * | 70.21 ± 1.77 **** | 55.58 ± 0.89 ns | 57.16 ± 1.84 |
| 2nd campaign | 70.88 ± 0.64 **** | 225.43 ± 1.89 **** | 68.82 ± 4.23 *** | 64.85 ± 0.52 * | 62.16 ± 2.93 ns | 67.23 ± 3.41 ** | 73.40 ± 2.14 **** | 77.31 ± 2.80 **** | ||
| TFC (mg EE/100 g) | 1st campaign | 7.24 ± 0.32 **** | 6.86 ± 0.71 **** | 7.74 ± 0.42 **** | 4.33 ± 0.45 ns | 4.71 ± 0.32 ns | 4.25 ± 0.28 ns | 4.47 ± 0.17 ns | 4.37 ± 0.32 ns | 3.52 ± 0.08 |
| 2nd campaign | 6.60 ± 0.27 **** | 35.2 ± 0.6 **** | 5.66 ± 0.27 **** | 4.09 ± 0.08 ns | 4.17 ± 0.58 ns | 6.11 ± 0.17 **** | 3.70 ± 0.18 ns | 5.86 ± 0.52 **** | ||
| pH | 1st campaign | 4.20 | 4.50 | 4.41 | 4.14 | 4.49 | 4.33 | 4.14 | 3.96 | 3.23 |
| 2nd campaign | 4.44 | 4.15 | 4.98 | 4.44 | 5.03 | 4.84 | 4.63 | 4.75 | ||
| H2O2 (μg/100 g) | 1st campaign | 32.23 ± 0.35 **** | 34.62 ± 0.38 **** | 34.49 ± 0.28 **** | 32.04 ± 0.41 **** | 33.37 ± 0.80 **** | 33.81 ± 0.29 **** | 33.56 ± 0.90 **** | 31.32 ± 0.29 **** | 0.00 |
| 2nd campaign | 33.03 ± 0.32 **** | 33.18 ± 1.27 **** | 34.85 ± 0.13 **** | 31.32 ± 0.13 **** | 34.02 ± 0.42 **** | 33.56 ± 0.26 **** | 34.59 ± 0.37 **** | 33.29 ± 0.63 **** |
| Compounds | MNP1 | MNP2 | MNP3 | MNP4 | MNP5 | MNP6 | MNP7 | MNP8 | Manuka | |
|---|---|---|---|---|---|---|---|---|---|---|
| Naringin | 1st campaign | 0.56 ± 0.03 **** | 0.51 ± 0.03 **** | 0.45 ± 0.02 **** | 0.15 ± 0.01 **** | 0.44 ± 0.02 **** | 0.31 ± 0.02 **** | 0.25 ± 0.01 **** | 0.08 ± 0.001 * | <LOD |
| 2nd campaign | 1.67 ± 0.08 **** | <LOD ns | 0.90 ± 0.05 **** | 0.48 ± 0.02 **** | <LOD ns | 0.32 ± 0.02 **** | <LOQ | 0.70 ± 0.03 **** | ||
| Kaempferol | 1st campaign | 0.17 ± 0.01 **** | 0.20 ± 0.01 **** | <LOD ns | 0.05 ± 0.00 **** | 0.18 ± 0.01 **** | 0.07 ± 0.00 **** | 0.17 ± 0.01 **** | 0.16 ± 0.01 **** | <LOD |
| 2nd campaign | 0.19 ± 0.01 **** | <LOD ns | 0.16 ± 0.01 **** | 0.44 ± 0.02 **** | 0.15 ± 0.01 **** | <LOD ns | 0.19 ± 0.01 **** | <LOQ ns | ||
| Naringenin | 1st campaign | <LOQ ns | 4.05 ± 0.20 **** | <LOD ns | 2.46 ± 0.12 **** | <LOQ ns | <LOQ ns | 2.72 ± 0.14 **** | 0.11 ± 0.01 ns | <LOD |
| 2nd campaign | <LOD ns | 5.60 ± 0.28 **** | 0.05 ± 0.00 ns | 0.10 ± 0.01 ns | <LOQ ns | 0.13 ± 0.01 ns | 0.02 ± 0.001 ns | 0.16 ± 0.01 **** | ||
| Quercetin-3-O-galactoside | 1st campaign | 3.21 ± 0.16 **** | 1.20 ± 0.06 ns | 6.53 ± 0.33 **** | 1.66 ± 0.08 ns | <LOQ ns | 2.62 ± 0.13 **** | 1.62 ± 0.08 ns | 5.84 ± 0.29 **** | 1.29 ± 0.06 |
| 2nd campaign | 3.91 ± 0.20 **** | <LOD ns | 1.39 ± 0.07 ns | 2.57 ± 0.13 **** | 4.02 ± 0.20 **** | <LOD ns | 1.91 ± 0.10 *** | 5.00 ± 0.25 **** | ||
| Isorhamnetin-3-O-glucoside | 1st campaign | ND ns | ND ns | ND ns | 2.07 ± 0.10 **** | ND ns | ND ns | ND ns | ND ns | ND |
| 2nd campaign | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ||
| Isorhamnetin-3-O-rutinoside | 1nd campaign | 0.27 ± 0.01 **** | 0.10 ± 0.00 **** | <LOD ns | 0.13 ± 0.01 **** | 0.15 ± 0.01 **** | 0.21 ± 0.01 **** | <LOQ ns | 0.18 ± 0.01 **** | <LOQ |
| 2nd campaign | 0.20 ± 0.01 **** | <LOD ns | 0.05 ± 0.00 **** | 0.13 ± 0.01 **** | 0.08 ± 0.00 **** | ND ns | <LOD ns | 0.14 ± 0.01 **** | ||
| Tiliroside | 1st campaign | 0.91 ± 0.05 **** | 0.20 ± 0.01 *** | 0.30 ± 0.01 **** | 0.50 ± 0.02 **** | 0.67 ± 0.03 **** | 0.49 ± 0.02 **** | 0.46 ± 0.02 **** | 0.22 ± 0.01 *** | <LOQ |
| 2nd campaign | 2.50 ± 0.12 **** | 1.13 ± 0.06 **** | 0.73 ± 0.04 **** | 1.98 ± 0.10 **** | 2.08 ± 0.10 **** | 0.51 ± 0.03 **** | 0.85 ± 0.04 **** | 0.50 ± 0.03 **** | ||
| Apigenin | 1st campaign | 0.49 ± 0.02 **** | 0.28 ± 0.01 **** | <LOQ ns | 0.06 ± 0.00 * | 0.06 ± 0.00 * | 0.48 ± 0.02 **** | 0.26 ± 0.01 **** | 0.22 ± 0.01 **** | <LOD |
| 2nd campaign | 0.30 ± 0.01 **** | 0.22 ± 0.01 **** | 1.43 ± 0.07 **** | 0.12 ± 0.01 **** | 0.14 ± 0.01 **** | <LOD ns | 0.93 ± 0.05 **** | <LOD | ||
| Chrysin | 1st campaign | 0.82 ± 0.04 **** | 0.56 ± 0.03 **** | 0.41 ± 0.02 **** | 0.05 ± 0.00 ns | 0.29 ± 0.01 **** | 0.60 ± 0.03 **** | 1.31 ± 0.07 **** | 0.22 ± 0.01 **** | <LOQ |
| 2nd campaign | 0.74 ± 0.04 **** | <LOD ns | 0.16 ± 0.01 **** | 0.40 ± 0.02 **** | 0.06 ± 0.00 ns | <LOQ ns | 0.41 ± 0.02 **** | <LOQ ns | ||
| Kaempferol-3-O-rutinoside | 1st campaign | <LOQ ns | 0.10 ± 0.00 ns | <LOQ ns | 0.40 ± 0.02 **** | <LOQ ns | <LOD ns | <LOQ ns | <LOD ns | <LOD |
| 2nd campaign | 1.04 ± 0.05 **** | <LOD ns | 2.93 ± 0.15 **** | 0.82 ± 0.04 **** | 0.89 ± 0.04 **** | <LOD ns | <LOD ns | 0.23 ± 0.01 **** | ||
| Quercetin | 1st campaign | 0.50 ± 0.03 **** | 1.12 ± 0.06 **** | <LOD **** | 0.49 ± 0.02 **** | 0.08 ± 0.001 *** | 0.46 ± 0.02 *** | 0.31 ± 0.02 ns | 0.22 ± 0.01 ns | 0.26 ± 0.01 |
| 2nd campaign | 2.79 ± 0.14 **** | 0.97 ± 0.05 **** | 0.64 ± 0.03 **** | 0.25 ± 0.01 ns | <LOQ **** | 0.60 ± 0.03 **** | 1.67 ± 0.08 **** | 0.95 ± 0.05 **** | ||
| Catechin | 1st campaign | 6.99 ± 0.35 **** | 4.29 ± 0.21 **** | 5.37 ± 0.27 **** | 6.30 ± 0.31 **** | 6.48 ± 0.32 **** | 5.35 ± 0.27 **** | 4.81 ± 0.24 **** | 7.41 ± 0.37 **** | 1.21 ± 0.06 |
| 2nd campaign | 5.36 ± 0.27 **** | 42.56 ± 2.13 **** | 4.99 ± 0.25 **** | 3.05 ± 0.15 *** | 3.21 ± 0.16 *** | 7.30 ± 0.36 **** | 4.94 ± 0.25 **** | 4.60 ± 0.23 **** | ||
| Epicatechin | 1st campaign | <LOD **** | 0.06 ± 0.001 **** | <LOD **** | ND **** | 1.22 ± 0.06 ns | 0.18 ± 0.01 **** | 4.15 ± 0.21 **** | 0.33 ± 0.02 **** | 1.33 ± 0.07 |
| 2nd campaign | 0.83 ± 0.04 **** | 3.84 ± 0.19 **** | 0.28 ± 0.01 **** | 1.98 ± 0.10 **** | 0.50 ± 0.03 **** | 2.30 ± 0.12 **** | 0.99 ± 0.05 *** | 1.24 ± 0.06 ns | ||
| Phloridzin | 1st campaign | 3.42 ± 0.17 **** | 4.69 ± 0.23 **** | 4.14 ± 0.21 **** | 1.64 ± 0.08 **** | 3.99 ± 0.20 **** | <LOD **** | 2.81 ± 0.14 **** | 1.34 ± 0.07 ** | 0.79 ± 0.04 |
| 2nd campaign | 3.10 ± 0.16 **** | 4.82 ± 0.24 **** | 5.25 ± 0.26 **** | 3.77 ± 0.19 **** | 3.89 ± 0.19 **** | 4.13 ± 0.21 **** | 2.64 ± 0.13 **** | 3.37 ± 0.17 **** | ||
| Phloretin | 1st campaign | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND |
| 2nd campaign | <LOD ns | 0.59 ± 0.03 **** | <LOD ns | <LOD ns | <LOD ns | 0.12 ± 0.01 *** | <LOQ ns | <LOD ns | ||
| Myricetin | 1st campaign | 0.86 ± 0.04 ns | 0.73 ± 0.04 ns | 0.52 ± 0.03 **** | 0.80 ± 0.04 ns | 0.67 ± 0.03 ns | 0.48 ± 0.02 **** | 0.34 ± 0.02 **** | 0.31 ± 0.02 **** | 0.77 ± 0.04 |
| 2nd campaign | 1.85 ± 0.09 **** | 1.62 ± 0.08 **** | 1.37 ± 0.07 **** | 0.57 ± 0.03 *** | 1.45 ± 0.07 **** | 1.67 ± 0.08 **** | 0.80 ± 0.04 ns | 1.06 ± 0.05 **** | ||
| Kaempferol-3-O-glucoside | 1st campaign | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND |
| 2nd campaign | <LOD ns | ND ns | 0.11 ± 0.01 **** | ND ns | 0.07 ± 0.00 *** | 0.36 ± 0.02 **** | 0.51 ± 0.03 **** | 0.05 ± 0.00 ** | ||
| Rutin | 1st campaign | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND |
| 2nd campaign | 0.88 ± 0.04 **** | 2.72 ± 0.14 **** | 1.87 ± 0.09 **** | 2.51 ± 0.13 **** | 1.49 ± 0.07 **** | 0.95 ± 0.05 **** | 1.23 ± 0.06 **** | 0.99 ± 0.05 **** | ||
| Gallic acid | 1st campaign | 5.49 ± 0.27 **** | 5.69 ± 0.28 **** | 5.89 ± 0.29 **** | 9.68 ± 0.48 **** | 5.32 ± 0.27 **** | 5.25 ± 0.26 **** | 7.28 ± 0.36 **** | 8.61 ± 0.43 **** | 37.14 ± 1.86 |
| 2nd campaign | 6.09 ± 0.30 **** | 18.20 ± 0.91 **** | 4.75 ± 0.24 **** | 3.32 ± 0.17 **** | 5.73 ± 0.29 **** | 3.91 ± 0.20 **** | 5.25 ± 0.26 **** | 6.77 ± 0.34 **** | ||
| Protocatechuic acid | 1st campaign | 1.97 ± 0.10 **** | 1.69 ± 0.08 **** | 1.82 ± 0.09 **** | 3.40 ± 0.17 **** | 1.76 ± 0.09 **** | 1.58 ± 0.08 **** | 2.89 ± 0.14 **** | 1.43 ± 0.07 **** | 0.14 ± 0.01 |
| 2nd campaign | 4.14 ± 0.21 **** | 14.94 ± 0.75 **** | 1.25 ± 0.06 **** | 4.43 ± 0.22 **** | 3.33 ± 0.17 **** | 4.76 ± 0.24 **** | 7.94 ± 0.40 **** | 5.24 ± 0.26 **** | ||
| Neochlorogenic acid | 1st campaign | 1.92 ± 0.10 **** | 4.48 ± 0.22 **** | 5.45 ± 0.27 **** | 10.86 ± 0.54 **** | 1.77 ± 0.09 **** | 1.49 ± 0.07 **** | 8.27 ± 0.41 **** | 2.60 ± 0.13 **** | 0.14 ± 0.01 |
| 2nd campaign | 2.07 ± 0.10 **** | 14.77 ± 0.74 **** | 2.06 ± 0.10 **** | 5.04 ± 0.25 **** | 2.85 ± 0.14 **** | 5.52 ± 0.28 **** | 4.16 ± 0.21 **** | 4.70 ± 0.23 **** | ||
| Caftaric acid | 1st campaign | 0.21 ± 0.01 ns | 0.38 ± 0.02 ns | 0.49 ± 0.02 ns | 0.82 ± 0.04 **** | 3.53 ± 0.18 **** | 0.32 ± 0.02 ns | 0.32 ± 0.02 ns | 0.75 ± 0.04 *** | 0.21 ± 0.01 |
| 2nd campaign | 0.46 ± 0.02 ns | 9.49 ± 0.47 **** | 0.31 ± 0.02 ns | 0.20 ± 0.01 ns | 0.39 ± 0.02 ns | 0.18 ± 0.01 ns | 0.20 ± 0.01 ns | 0.78 ± 0.04 **** | ||
| Chlorogenic acid | 1st campaign | 1.04 ± 0.05 ns | 2.54 ± 0.13 **** | 5.79 ± 0.29 **** | 1.78 ± 0.09 ** | 1.03 ± 0.05 ns | 0.98 ± 0.05 ns | 5.71 ± 0.29 **** | 1.07 ± 0.05 ns | 0.90 ± 0.05 |
| 2nd campaign | 1.21 ± 0.06 ns | 20.09 ± 1.00 **** | 0.81 ± 0.04 ns | 1.00 ± 0.05 ns | 1.00 ± 0.05 ns | 1.21 ± 0.06 ns | 0.77 ± 0.04 ns | 1.33 ± 0.07 ns | ||
| 4-O-caffeyolquinic acid | 1st campaign | 0.32 ± 0.02 ns | 1.18 ± 0.06 **** | 0.18 ± 0.01 ns | 1.19 ± 0.06 **** | 0.71 ± 0.04 **** | 0.59 ± 0.03 ** | 4.71 ± 0.24 **** | 1.11 ± 0.06 **** | 0.33 ± 0.02 |
| 2nd campaign | 0.58 ± 0.03 ** | 2.39 ± 0.12 **** | 0.91 ± 0.05 **** | 0.47 ± 0.02 ns | 0.58 ± 0.03 ** | 0.71 ± 0.04 **** | 0.41 ± 0.02 ns | 0.44 ± 0.02 ns | ||
| Vanillic acid | 1st campaign | 4.10 ± 0.20 **** | 7.51 ± 0.38 **** | 2.03 ± 0.10 **** | 3.30 ± 0.16 **** | 2.83 ± 0.14 **** | 2.84 ± 0.14 **** | 1.26 ± 0.06 ** | 2.02 ± 0.10 **** | 0.68 ± 0.03 |
| 2nd campaign | 8.20 ± 0.41 **** | 7.54 ± 0.38 **** | 0.95 ± 0.05 ns | 1.49 ± 0.07 *** | 0.48 ± 0.02 ns | 0.83 ± 0.04 ns | 0.52 ± 0.03 ns | 0.56 ± 0.03 ns | ||
| Caffeic acid | 1st campaign | 0.21 ± 0.01 **** | 0.02 ± 0.001 ns | <LOD ns | 0.09 ± 0.001 *** | 0.19 ± 0.01 **** | 0.40 ± 0.02 **** | 0.11 ± 0.01 **** | 0.10 ± 0.00 **** | <LOD |
| 2nd campaign | 0.42 ± 0.02 **** | 1.48 ± 0.07 **** | 0.86 ± 0.04 **** | 0.41 ± 0.02 **** | 0.35 ± 0.02 **** | <LOD ns | <LOD ns | 0.33 ± 0.02 **** | ||
| Syringic acid | 1st campaign | 0.11 ± 0.01 ns | 0.12 ± 0.01 ns | 0.11 ± 0.01 ns | 8.53 ± 0.43 **** | 0.08 ± 0.001 ns | 0.26 ± 0.01 ns | 0.19 ± 0.01 ns | 0.13 ± 0.01 ns | 0.12 ± 0.01 |
| 2nd campaign | 0.20 ± 0.01 ns | 3.17 ± 0.16 **** | 0.50 ± 0.02 ** | 1.16 ± 0.06 **** | 0.20 ± 0.01 ns | 1.89 ± 0.09 **** | 0.86 ± 0.04 **** | ND ns | ||
| p-Coumaric acid | 1st campaign | 0.75 ± 0.04 **** | 0.72 ± 0.04 **** | 0.67 ± 0.03 **** | 0.86 ± 0.04 **** | 0.72 ± 0.04 **** | 1.29 ± 0.06 **** | 0.17 ± 0.01 ** | 0.08 ± 0.00 ns | <LOD |
| 2nd campaign | 1.09 ± 0.05 **** | 0.92 ± 0.05 **** | 1.43 ± 0.07 **** | 0.99 ± 0.05 **** | 1.04 ± 0.05 **** | 1.61 ± 0.08 **** | 1.30 ± 0.07 **** | 1.09 ± 0.05 **** | ||
| trans-Ferulic acid | 1st campaign | 0.37 ± 0.02 ns | ND **** | ND **** | ND **** | ND **** | 0.85 ± 0.04 **** | ND **** | 0.18 ± 0.01 **** | 0.39 ± 0.02 |
| 2nd campaign | 0.57 ± 0.03 **** | 0.75 ± 0.04 **** | 0.81 ± 0.04 **** | 0.44 ± 0.02 ns | 0.73 ± 0.04 **** | 0.68 ± 0.03 **** | 0.56 ± 0.03 **** | 0.70 ± 0.04 **** | ||
| Sinapic acid | 1st campaign | 2.32 ± 0.12 **** | 1.72 ± 0.09 **** | 1.27 ± 0.06 **** | ND ns | 1.48 ± 0.07 **** | 2.31 ± 0.12 **** | 1.07 ± 0.05 **** | 5.31 ± 0.27 **** | 0.19 ± 0.01 |
| 2nd campaign | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ||
| 3,5-di-caffeoylquinic acid | 1st campaign | 0.16 ± 0.01 **** | 0.08 ± 0.00 *** | <LOQ ns | 0.42 ± 0.02 **** | <LOQ ns | <LOD ns | <LOQ ns | 0.27 ± 0.01 **** | <LOQ |
| 2nd campaign | 0.77 ± 0.04 **** | <LOD ns | 0.48 ± 0.02 **** | 0.28 ± 0.01 **** | 0.90 ± 0.04 **** | 0.31 ± 0.02 **** | 0.53 ± 0.03 **** | 0.30 ± 0.02 **** | ||
| Ellagic acid | 1st campaign | 1.79 ± 0.09 **** | 2.52 ± 0.13 **** | 2.21 ± 0.11 **** | <LOD ns | 1.99 ± 0.10 **** | <LOD ns | 1.60 ± 0.08 **** | 0.66 ± 0.03 **** | <LOQ |
| 2nd campaign | 0.70 ± 0.03 **** | <LOD ns | 0.61 ± 0.03 **** | 0.23 ± 0.01 *** | <LOD ns | <LOD ns | ND ns | <LOD ns | ||
| 3,4-Di-caffeyolquinic acid | 1st campaign | 0.48 ± 0.02 **** | 0.36 ± 0.02 **** | 0.63 ± 0.03 **** | 0.20 ± 0.01 **** | 0.52 ± 0.03 **** | 0.47 ± 0.02 **** | 0.31 ± 0.02 **** | 0.09 ± 0.00 * | <LOD |
| 2nd campaign | 0.84 ± 0.04 **** | 0.69 ± 0.03 **** | 0.89 ± 0.04 **** | 0.87 ± 0.04 **** | 0.84 ± 0.04 **** | 0.44 ± 0.02 **** | 0.78 ± 0.04 **** | 1.07 ± 0.05 **** | ||
| Cinnamic acid | 1st campaign | 0.35 ± 0.02 **** | 0.24 ± 0.01 **** | 0.16 ± 0.01 **** | <LOD ns | 0.20 ± 0.01 **** | 0.15 ± 0.01 **** | 0.06 ± 0.001 **** | 0.13 ± 0.01 **** | ND |
| 2nd campaign | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ND ns | ||
| trans-Epsilon viniferin | 1st campaign | <LOD ns | 0.05 ± 0.001 **** | <LOD ns | <LOD ns | <LOD ns | <LOD ns | <LOD ns | <LOD ns | <LOD |
| 2nd campaign | 0.26 ± 0.01 **** | 0.27 ± 0.01 **** | <LOD ns | 0.26 ± 0.01 **** | <LOD ns | <LOD ns | <LOQ ns | <LOD ns | ||
| Total | 1st campaign | 39.80 | 47.37 | 44.44 | 57.86 | 36.36 | 30.02 | 53.17 | 41.19 | 45.87 |
| 2nd campaign | 52.96 | 158.76 | 38.92 | 39.78 | 36.45 | 40.46 | 42.01 | 42.32 |
| MNP1 | MNP2 | MNP3 | MNP4 | MNP5 | MNP6 | MNP7 | MNP8 | Manuka | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Asp | 1st campaign | 16.01 ± 0.05 **** | 21.56 ± 0.44 **** | 23.76 ± 0.23 **** | 11.09 ± 0.29 **** | 19.12 ± 0.43 **** | 11.44 ± 0.72 **** | 13.32 ± 0.18 **** | 8.33 ± 0.01 ns | 8.00 ± 0.14 |
| 2nd campaign | 13.49 ± 0.01 **** | 6.42 ± 0.26 **** | 13.60 ± 0.70 **** | 5.64 ± 0.06 **** | 9.95 ± 0.65 **** | 7.03 ± 0.33 ** | 10.08 ± 0.62 **** | 11.96 ± 0.48 **** | ||
| Glu | 1st campaign | 14.12 ± 0.01 **** | 20.46 ± 0.43 **** | 22.71 ± 0.17 **** | 12.54 ± 0.42 **** | 19.79 ± 0.36 **** | 10.76 ± 0.58 ns | 12.72 ± 0.21 **** | 6.78 ± 0.01 **** | 9.73 ± 0.01 |
| 2nd campaign | 19.51 ± 0.17 **** | 0.49 ± 0.01 **** | 22.73 ± 1.30 **** | 10.43 ± 0.04 ns | 17.30 ± 0.89 **** | 13.51 ± 0.74 **** | 18.50 ± 1.25 **** | 17.08 ± 0.97 **** | ||
| Asn | 1st campaign | 7.04 ± 0.04 **** | 6.57 ± 0.15 **** | 7.35 ± 0.04 **** | 7.11 ± 0.35 **** | 5.29 ± 0.12 **** | 2.28 ± 0.14 **** | 4.08 ± 0.04 **** | 3.84 ± 0.02 * | 3.51 ± 0.02 |
| 2nd campaign | 8.05 ± 0.09 **** | 1.32 ± 0.06 **** | 6.70 ± 0.33 **** | 2.22 ± 0.01 **** | 5.54 ± 0.02 **** | 3.31 ± 0.08 ns | 4.98 ± 0.41 **** | 8.14 ± 0.32 **** | ||
| Ser | 1st campaign | 2.55 ± 0.03 ns | 2.30 ± 0.13 **** | 2.24 ± 0.01 **** | 2.49 ± 0.03 ns | 2.14 ± 0.01 **** | 1.13 ± 0.09 **** | 2.24 ± 0.03 **** | 1.51 ± 0.06 **** | 2.64 ± 0.06 |
| 2nd campaign | 2.41 ± 0.09 ** | 0.14 ± 0.01 **** | 2.24 ± 0.21 **** | 1.68 ± 0.12 **** | 2.93 ± 0.16 *** | 2.76 ± 0.13 ns | 2.45 ± 0.17 * | 1.67 ± 0.14 **** | ||
| Gln | 1st campaign | 3.07 ± 0.02 **** | 2.37 ± 0.01 **** | 2.52 ± 0.17 **** | 2.68 ± 0.01 **** | 2.23 ± 0.09 **** | 1.16 ± 0.10 **** | 2.37 ± 0.01 **** | 1.97 ± 0.06 **** | 5.57 ± 0.01 |
| 2nd campaign | 9.20 ± 0.11 **** | 0.06 ± 0.01 **** | 6.47 ± 0.40 **** | 4.09 ± 0.18 **** | 6.01 ± 0.35 ** | 4.34 ± 0.26 **** | 5.34 ± 0.40 ns | 5.94 ± 0.26 * | ||
| His | 1st campaign | 1.30 ± 0.06 ** | 1.24 ± 0.10 **** | 1.64 ± 0.14 ** | 0.44 ± 0.04 **** | 1.48 ± 0.13 ns | 0.78 ± 0.04 **** | 0.35 ± 0.02 **** | 0.13 ± 0.01 **** | 1.47 ± 0.02 |
| 2nd campaign | 0.84 ± 0.06 **** | 0.32 ± 0.02 **** | 1.88 ± 0.15 **** | 0.28 ± 0.02 **** | 0.84 ± 0.04 **** | 0.38 ± 0.01 **** | 0.32 ± 0.02 **** | 0.44 ± 0.03 **** | ||
| Thr | 1st campaign | 0.31 ± 0.01 **** | 0.29 ± 0.01 **** | 0.29 ± 0.03 **** | 0.22 ± 0.02 **** | 0.09 ± 0.01 **** | <LOD **** | 0.06 ± 0.01 **** | <LOD **** | 1.04 ± 0.08 |
| 2nd campaign | 0.39 ± 0.01 **** | <LOD **** | 0.33 ± 0.01 **** | 0.04 ± 0.01 **** | 1.20 ± 0.10 **** | 0.99 ± 0.03 ns | 0.11 ± 0.01 **** | 0.06 ± 0.01 **** | ||
| Tau | 1st campaign | 19.84 ± 0.01 **** | 16.93 ± 0.20 **** | 16.74 ± 0.69 **** | 25.84 ± 1.36 **** | 16.92 ± 0.38 **** | 18.23 ± 1.08 **** | 19.30 ± 0.16 **** | 7.88 ± 0.28 *** | 5.92 ± 0.38 |
| 2nd campaign | 15.56 ± 0.16 **** | 2.48 ± 0.08 **** | 10.21 ± 1.11 **** | 6.34 ± 0.14 ns | 15.76 ± 0.65 **** | 7.71 ± 0.45 *** | 10.08 ± 0.94 **** | 7.54 ± 0.53 ** | ||
| Arg | 1st campaign | 6.13 ± 0.05 **** | 6.11 ± 0.14 **** | 6.19 ± 0.15 **** | 5.71 ± 0.20 **** | 6.03 ± 0.06 **** | 4.78 ± 0.27 **** | 5.58 ± 0.05 **** | 4.01 ± 0.10 **** | 2.70 ± 0.08 |
| 2nd campaign | 7.84 ± 0.007 **** | 1.18 ± 0.03 **** | 7.37 ± 0.48 **** | 2.92 ± 0.08 ns | 4.89 ± 0.12 **** | 3.10 ± 0.14 * | 4.93 ± 0.49 **** | 4.91 ± 0.33 **** | ||
| Ala | 1st campaign | 6.19 ± 0.09 **** | 7.12 ± 0.10 **** | 7.39 ± 0.14 **** | 6.91 ± 0.21 **** | 5.91 ± 0.01 **** | 4.01 ± 0.20 ns | 5.98 ± 0.17 **** | 4.27 ± 0.07 ** | 3.83 ± 0.03 |
| 2nd campaign | 4.53 ± 0.01 **** | 1.28 ± 0.03 **** | 4.54 ± 0.20 **** | 2.98 ± 0.02 **** | 4.52 ± 0.34 **** | 4.54 ± 0.34 **** | 4.13 ± 0.36 ns | 3.69 ± 0.21 ns | ||
| Tyr | 1st campaign | 3.81 ± 0.02 ns | 0.98 ± 0.02 **** | 1.04 ± 0.04 **** | 8.98 ± 0.33 **** | 0.98 ± 0.02 **** | 1.54 ± 0.06 **** | 8.46 ± 0.12 **** | 7.22 ± 0.02 **** | 4.19 ± 0.18 |
| 2nd campaign | 9.28 ± 0.03 **** | 1.18 ± 0.01 **** | 2.23 ± 0.11 **** | 4.17 ± 0.05 ns | 1.99 ± 0.12 **** | 2.85 ± 0.17 **** | 20.91 ± 1.43 **** | 3.00 ± 0.22 **** | ||
| Cys | 1st campaign | 2.76 ± 0.01 **** | 2.01 ± 0.08 ns | 2.63 ± 0.25 **** | 3.05 ± 0.16 **** | 2.83 ± 0.03 **** | 2.76 ± 0.11 **** | 2.99 ± 0.21 **** | 3.00 ± 0.34 **** | 1.83 ± 0.02 |
| 2nd campaign | 3.73 ± 0.27 **** | 2.30 ± 0.22 * | 6.03 ± 0.30 **** | 7.33 ± 0.31 **** | 1.67 ± 0.04 ns | 2.69 ± 0.19 **** | 8.22 ± 0.52 **** | 6.84 ± 0.26 **** | ||
| Gly | 1st campaign | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | 0.70 ± 0.02 |
| 2nd campaign | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | ||
| Val | 1st campaign | 1.96 ± 0.10 ns | 1.15 ± 0.07 **** | 1.70 ± 0.18 * | 2.17 ± 0.01 **** | 1.18 ± 0.10 **** | 0.83 ± 0.03 **** | 1.58 ± 0.01 **** | 1.39 ± 0.01 **** | 1.87 ± 0.01 |
| 2nd campaign | 2.42 ± 0.10 **** | 0.93 ± 0.02 **** | 1.96 ± 0.11 ns | 2.05 ± 0.06 * | 1.38 ± 0.08 **** | 1.51 ± 0.05 **** | 2.33 ± 0.11 **** | 2.13 ± 0.13 *** | ||
| Met | 1st campaign | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | 0.11 ± 0.01 |
| 2nd campaign | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | ||
| B-Ala | 1st campaign | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | <LOD **** | 10.76 ± 0.48 |
| 2nd campaign | <LOD **** | <LOD **** | <LOD **** | <LOD **** | 11.39 ± 0.72 *** | 3.81 ± 0.13 **** | <LOD **** | <LOD **** | ||
| Lys | 1st campaign | 4.91 ± 0.18 **** | 12.89 ± 0.94 **** | 10.90 ± 1.17 **** | 2.59 ± 0.16 **** | 5.54 ± 0.35 **** | 2.08 ± 0.19 **** | 1.61 ± 0.04 **** | <LOD **** | 98.62 ± 4.95 |
| 2nd campaign | 0.98 ± 0.08 **** | 0.59 ± 0.06 **** | <LOD **** | <LOD **** | 23.98 ± 1.71 **** | 18.21 ± 1.01 **** | <LOD **** | <LOD **** | ||
| Trp | 1st campaign | 0.22 ± 0.01 **** | 0.32 ± 0.03 **** | 0.32 ± 0.01 **** | 0.11 ± 0.01 **** | 0.18 ± 0.01 **** | 0.10 ± 0.01 **** | 0.16 ± 0.01 **** | 0.17 ± 0.01 **** | 0.44 ± 0.01 |
| 2nd campaign | 1.13 ± 0.04 **** | 0.008 ± 0.0001 **** | 1.51 ± 0.10 **** | 0.23 ± 0.02 **** | 0.64 ± 0.02 | 0.21 ± 0.02 **** | 0.63 ± 0.01 **** | 0.68 ± 0.05 **** | ||
| Phe | 1st campaign | 8.84 ± 0.03 **** | 0.74 ± 0.04 **** | 0.94 ± 0.02 **** | 17.00 ± 0.58 ns | 1.45 ± 0.02 **** | 2.77 ± 0.19 **** | 14.73 ± 0.14 **** | 18.78 ± 0.10 ns | 17.83 ± 0.16 |
| 2nd campaign | 28.30 ± 0.38 **** | 2.93 ± 0.09 **** | 5.82 ± 0.28 **** | 21.28 ± 0.19 **** | 5.62 ± 0.30 **** | 11.64 ± 0.88 **** | 25.73 ± 1.83 **** | 11.06 ± 0.59 **** | ||
| Ile | 1st campaign | 0.87 ± 0.01 **** | 0.54 ± 0.01 **** | 0.58 ± 0.02 **** | 1.13 ± 0.06 * | 0.57 ± 0.02 **** | 0.43 ± 0.04 **** | 0.96 ± 0.03 **** | 0.78 ± 0.01 **** | 1.33 ± 0.08 |
| 2nd campaign | 1.67 ± 0.04 **** | 0.48 ± 0.02 **** | 1.10 ± 0.06 **** | 1.21 ± 0.02 **** | 0.84 ± 0.07 **** | 1.07 ± 0.09 **** | 1.32 ± 0.13 ns | 1.03 ± 0.08 **** | ||
| Leu | 1st campaign | 0.49 ± 0.02 **** | 0.37 ± 0.01 **** | 0.27 ± 0.01 **** | 0.75 ± 0.06 **** | 0.27 ± 0.02 **** | 0.26 ± 0.02 **** | 0.58 ± 0.01 **** | 1.02 ± 0.01 **** | 1.51 ± 0.09 |
| 2nd campaign | 1.25 ± 0.02 **** | 0.16 ± 0.01 **** | 0.65 ± 0.04 **** | 0.81 ± 0.07 **** | 0.53 ± 0.04 **** | 0.56 ± 0.05 **** | 0.80 ± 0.03 **** | 0.61 ± 0.04 **** | ||
| Pro | 1st campaign | 102.20 ± 6.72 **** | 72.01 ± 4.24 **** | 85.12 ± 8.49 **** | 155.10 ± 6.60 **** | 98.92 ± 2.21 **** | 97.30 ± 6.46 **** | 115.17 ± 5.78 **** | 74.79 ± 7.46 **** | 43.42 ± 3.65 |
| 2nd campaign | 72.49 ± 2.28 **** | 20.46 ± 1.42 **** | 116.44 ± 3.82 **** | 102.34 ± 1.99 **** | 52.77 ± 3.56 * | 47.66 ± 0.03 ns | 135.36 ± 4.54 **** | 94.31 ± 8.06 **** | ||
| Total | 1st campaign | 202.63 | 175.95 | 194.36 | 265.93 | 190.93 | 162.66 | 212.26 | 145.87 | 227.04 |
| 2nd campaign | 203.08 | 42.73 | 211.81 | 176.04 | 169.72 | 137.89 | 256.25 | 181.12 | ||
| EEA | 1st campaign | 18.90 | 17.53 | 16.65 | 24.41 | 10.75 | 7.26 | 20.03 | 22.27 | 124.22 |
| 2nd campaign | 36.99 | 5.42 | 13.25 | 25.90 | 35.02 | 34.56 | 31.26 | 16.02 | ||
| NEEA | 1st campaign | 183.72 | 158.42 | 177.71 | 241.51 | 180.18 | 155.40 | 192.22 | 123.60 | 102.82 |
| 2nd campaign | 166.10 | 37.31 | 198.56 | 150.15 | 134.70 | 103.33 | 224.99 | 165.10 | ||
| EEA/Total% | 1st campaign | 9.33 | 9.96 | 8.57 | 9.18 | 5.63 | 4.46 | 9.44 | 15.26 | 54.71 |
| 2nd campaign | 18.21 | 12.68 | 6.26 | 14.71 | 20.63 | 25.06 | 12.20 | 8.85 | ||
| NEEA/Total % | 1st campaign | 90.67 | 90.04 | 91.43 | 90.82 | 94.37 | 95.54 | 90.56 | 84.74 | 45.29 |
| 2nd campaign | 81.79 | 87.32 | 93.74 | 85.29 | 79.37 | 74.94 | 87.80 | 91.15 | ||
| Pro/Total % | 1st campaign | 50.44 | 40.93 | 43.80 | 58.32 | 51.81 | 59.82 | 54.26 | 51.27 | 19.12 |
| 2nd campaign | 35.69 | 47.89 | 54.98 | 58.14 | 31.09 | 34.56 | 52.82 | 52.07 |
| Parameter | MNP1 | MNP2 | MNP3 | MNP4 | MNP5 | MNP6 | MNP7 | MNP8 | Manuka | |
|---|---|---|---|---|---|---|---|---|---|---|
| Antioxidant activity | ||||||||||
| FRAP (mg AAE/100 g honey) | 1st campaign | 73.24 ± 3.85 **** | 81.73 ± 2.36 **** | 84.73 ± 1.79 **** | 53.13 ± 1.66 **** | 70.95 ± 2.69 **** | 64.28 ± 1.10 **** | 68.47 ± 2.36 **** | 53.96 ± 2.34 **** | 24.59 ± 1.27 |
| 2nd campaign | 38.49 ± 0.30 **** | 128.11 ± 2.85 **** | 38.56 ± 0.48 **** | 33.72 ± 0.33 *** | 34.13 ± 1.06 *** | 24.82 ± 0.51 ns | 26.46 ± 0.55 ns | 35.49 ± 0.21 **** | ||
| DPPH (mg TE/100 g honey) | 1st campaign | 51.78 ± 2.78 **** | 60.35 ± 2.15 **** | 57.89 ± 2.36 **** | 36.02 ± 0.11 **** | 49.53 ± 3.77 **** | 44.29 ± 0.72 **** | 42.01 ± 0.67 **** | 37.46 ± 0.55 **** | 98.65 ± 2.23 |
| 2nd campaign | 56.81 ± 2.93 **** | 84.65 ± 4.62 **** | 47.25 ± 1.37 **** | 32.54 ± 1.26 **** | 33.75 ± 2.17 **** | 31.22 ± 0.50 **** | 40.85 ± 0.54 **** | 39.01 ± 1.96 **** | ||
| ABTS (mg TE/100 g honey) | 1st campaign | 48.03 ± 1.21 **** | 53.57 ± 0.63 **** | 51.08 ± 1.06 **** | 36.57 ± 1.21 *** | 48.42 ± 1.80 **** | 42.90 ± 1.73 **** | 41.55 ± 1.17 **** | 32.48 ± 0.39 ns | 29.99 ± 0.62 |
| 2nd campaign | 35.40 ± 1.89 ** | 137.69 ± 0.98 **** | 52.98 ± 0.81 **** | 43.38 ± 0.66 **** | 44.11 ± 1.21 **** | 48.61 ± 1.34 **** | 32.63 ± 0.57 ns | 52.32 ± 2.18 **** | ||
| Antiradical activity | ||||||||||
| •NO scavenging activity (% at 2 mg/mL) | 1st campaign | 32.38 ± 5.02 **** | 33.54 ± 0.64 **** | 33.62 ± 6.12 **** | 24.60 ± 3.39 **** | 31.58 ± 0.56 **** | 30.85 ± 2.58 **** | 26.42 ± 2.05 **** | 15.93 ± 0.69 ** | 7.72 ± 1.42 |
| 2nd campaign | 15.68 ± 0.69 ** | 18.56 ± 3.13 *** | 30.13 ± 0.87 **** | 7.33 ± 1.41 ns | n.a. | n.a. | n.a. | 4.31 ± 0.90 ns | ||
| •OH scavenging activity (% at 2 mg/mL) | 1st campaign | 8.06 ± 0.95 ns | 15.02 ± 1.16 **** | 12.35 ± 1.20 **** | 9.91 ± 0.37 ** | 8.74 ± 0.82 ns | 8.58 ± 0.84 ns | 13.96 ± 0.66 **** | 9.37 ± 0.41 * | 6.90 ± 0.88 |
| 2nd campaign | 9.37 ± 0.41 * | 13.87 ± 0.28 **** | 9.96 ± 0.72 ** | 6.67 ± 0.73 ns | 9.13 ± 0.65 * | 6.84 ± 0.92 ns | 8.56 ± 1.56 ns | 9.37 ± 0.41 ns | ||
| O2•− scavenging activity (% at 2 mg/mL)/ [IC50, mg/mL] | 1st campaign | 64.98 ± 0.68 ****/[1.74] | 82.61 ± 0.38 ****/[1.33] | 79.55 ± 1.51 ****/[1.36] | 66.93 ± 1.06 ****/[1.76] | 77.32 ± 0.81 ****/[1.58] | 66.09 ± 1.73 ****/[1.74] | 70.63 ± 3.33 ****/[1.56] | 69.38 ± 2.28 ****/[1.51] | 17.30 ± 7.20/[>2.00] |
| 2nd campaign | 62.80 ± 4.69 ****/[1.69] | 84.59 ± 1.35 ****/[0.79] | 65.48 ± 4.56 ****/[1.18] | 64.64 ± 2.77 ****/[1.61] | 66.39 ± 1.24 ****/[1.46] | 60.27 ± 3.40 ****/[1.67] | 66.32 ± 6.11 ****/[1.61] | 68.48 ± 0.33 ****/[1.56] | ||
| Enzymatic inhibitory activity | ||||||||||
| Tyrosinase inhibition (% at 2 mg/mL) | 1st campaign | 34.94 ± 4.91 **** | 19.05 ± 3.04 **** | 19.80 ± 1.89 **** | 17.53 ± 1.18 **** | 16.19 ± 1.57 **** | 21.82 ± 2.25 **** | 11.72 ± 3.58 *** | 17.38 ± 2.89 **** | n.a. |
| 2nd campaign | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| α-Glucosidase inhibition (% at 2mg/mL) | 1st campaign | 39.38 ± 10.10 ns | 43.18 ± 9.14 ns | 38.18 ± 3.55 ns | 48.28 ± 10.32 ns | 40.67 ± 8.08 ns | 31.68 ± 4.90 ns | 49.30 ± 9.28 ns | 46.24 ± 1.00 ns | 34.91 ± 8.47 |
| 2nd campaign | 58.42 ± 7.79 ** | 38.05 ± 4.44 ns | 41.47 ± 6.93 ns | 39.48 ± 3.40 ns | 43.91 ± 5.02 ns | 55.51 ± 6.25 ** | 53.16 ± 4.82 * | 40.48 ± 2.81 ns | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosso, C.; Soares, S.; Silva, A.; Soares, C.; Moreira, M.M.; Dorosh, O.; Barroso, M.F.; Prieto, M.A.; Delerue-Matos, C. Exploring the Nutritional Value and Health Benefits of Honey from the Portuguese Protected Area of Montesinho Natural Park. Foods 2025, 14, 963. https://doi.org/10.3390/foods14060963
Grosso C, Soares S, Silva A, Soares C, Moreira MM, Dorosh O, Barroso MF, Prieto MA, Delerue-Matos C. Exploring the Nutritional Value and Health Benefits of Honey from the Portuguese Protected Area of Montesinho Natural Park. Foods. 2025; 14(6):963. https://doi.org/10.3390/foods14060963
Chicago/Turabian StyleGrosso, Clara, Sónia Soares, Aurora Silva, Cristina Soares, Manuela M. Moreira, Olena Dorosh, M. Fátima Barroso, Miguel A. Prieto, and Cristina Delerue-Matos. 2025. "Exploring the Nutritional Value and Health Benefits of Honey from the Portuguese Protected Area of Montesinho Natural Park" Foods 14, no. 6: 963. https://doi.org/10.3390/foods14060963
APA StyleGrosso, C., Soares, S., Silva, A., Soares, C., Moreira, M. M., Dorosh, O., Barroso, M. F., Prieto, M. A., & Delerue-Matos, C. (2025). Exploring the Nutritional Value and Health Benefits of Honey from the Portuguese Protected Area of Montesinho Natural Park. Foods, 14(6), 963. https://doi.org/10.3390/foods14060963












