Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols
Abstract
1. Introduction
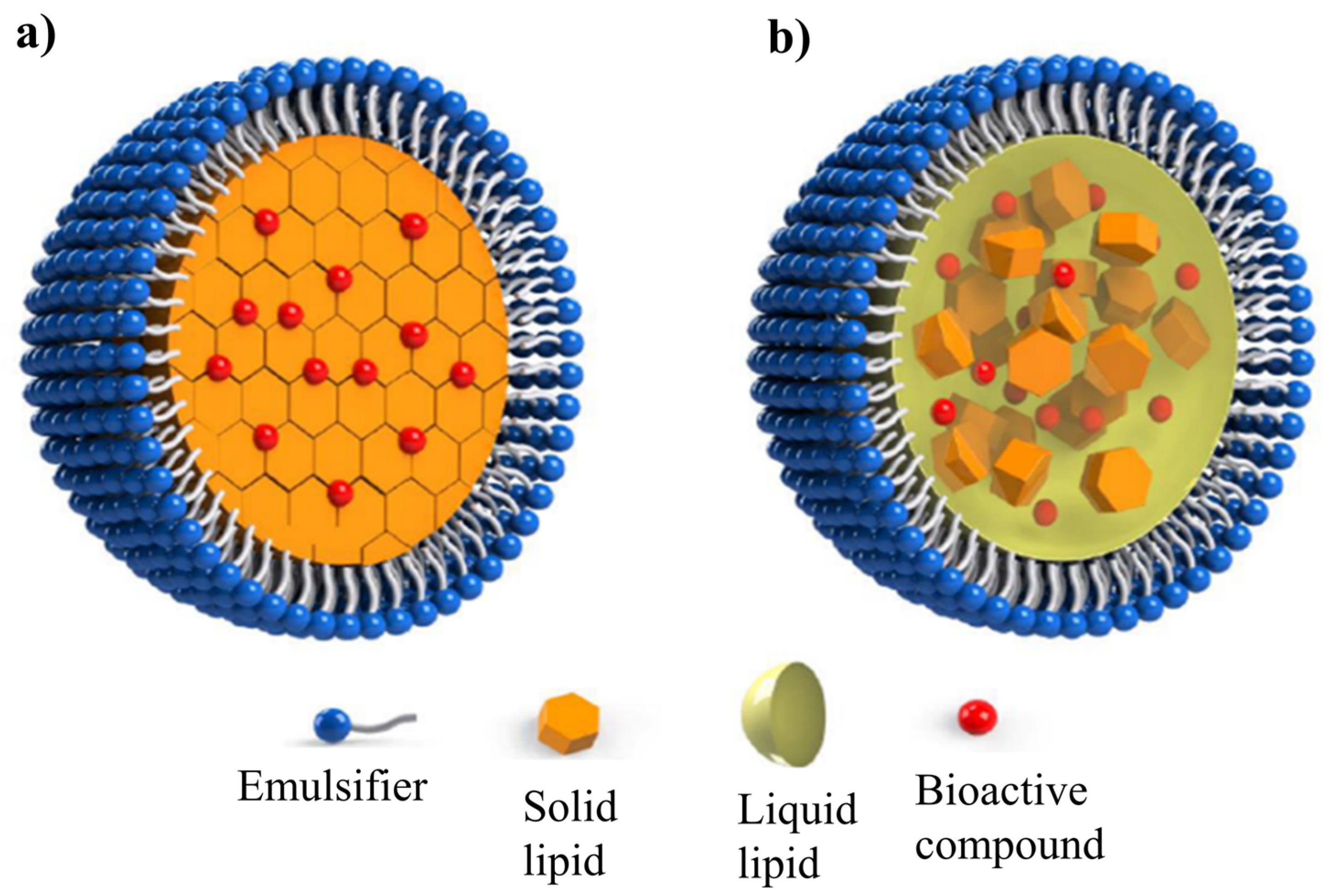
2. Lipid Nanoparticles
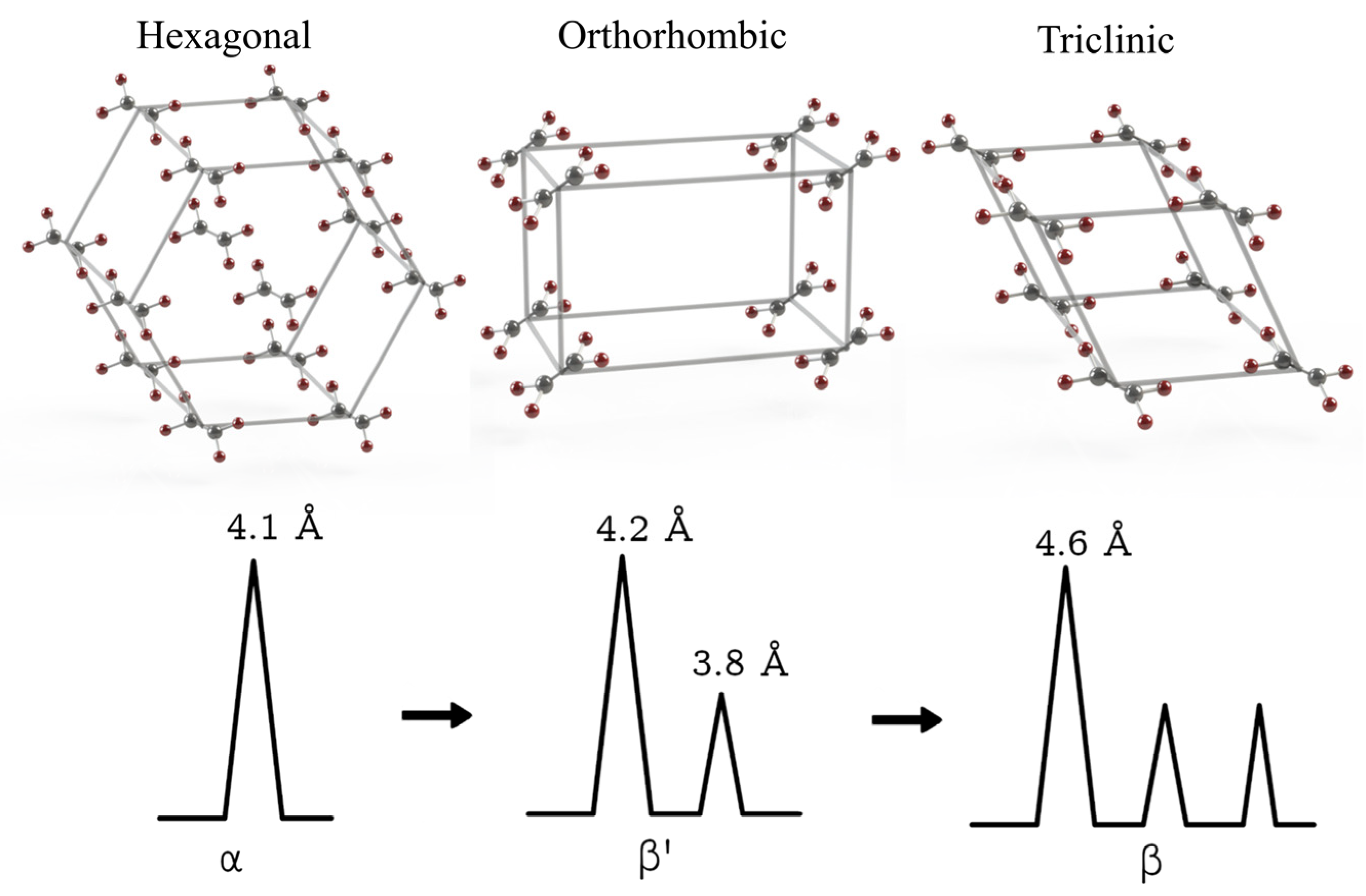
2.1. Lipids and Crystallization Properties
2.2. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
3. Lipid Nanoparticle Formulation
3.1. Lipids
| Lipid Nanostructure | Bioactive Compound | Lipid Matrices | Emulsifiers | Main Outcomes | Reference |
|---|---|---|---|---|---|
| NLCs | β-carotene and α-tocopherol | Murumuru butter | Span 80 Cremophor RH40 | - The β-carotene stability was notably improved by the co-encapsulation of α-tocopherol within mumumuru-based NLCs. - The bioaccessibility of β-carotene after NLCs passage in the in vitro dynamic digestion system was approximately 42%. - The surfactant employed, in combination with the extremely small PS, rendered the produced NLCs toxic to the tested cell cultures. | [54] |
| NLCs | Phytosterols | High oleic sunflower oil Fully hydrogenated canola and crambe oils | Tween 80 | - The entrapment of phytosterols was enhanced by the use of fully hydrogenated canola and crambe oils separately in the NLCs formulation. - The increase in the phytosterol amount within the NLCs formulation led to a higher PS. - The incorporation of phytosterols contributed to an increase in the crystallinity of the developed systems. | [55] |
| NLCs and SLNs | β-carotene | MCT Glyceryl stearate Hydrogenated palm oil | Tween 80 | - The concentration of glyceryl stearate does not affect lipid digestibility. - Hydrogenated palm oil delays the lipolysis kinetics of SLNs. - The composition of the micelle fraction determined the concentration of β-carotene. - Monounsaturated FAs enhanced the solubilization capacity of micelles. | [40] |
| NLCs | Cinnamon essential oil | Corn, sesame, sweet almond, and black seed oil Cocoa butter | Tween 80 | - NLCs formulated with oils with higher concentrations of SFAs (corn and sesame oils) exhibited a larger average PS. - The NLCs formulated with almond oil demonstrated the most favorable physicochemical properties and the highest encapsulation stability. | [56] |
| NLCs | Quercetin and piperine | Glyceryl behenate Squalene | Tween 80 and Span 80 | - The NLCs exhibited a negative surface charge and an average PS of less than 200 nm. - The EE for both quercetin and piperine exceeded 90%. - The safety of the NLCs was demonstrated by the absence of hemolysis in blood samples. | [57] |
| NLCs and SLNs | Lutein | Cocoa butter Corn oil | Zein peptides Tween 80 | - SLN samples exhibited precipitation and during the storage period. - SLN system exhibited the highest FFAs release rate and lutein bioavailability. | [58] |
| NLCs | Lycopene | Cocoa butter Grape seed oil | Span® 80 Plantasens® HE20 | - An increase in the emulsifier amount (from 2.5% to 7.0%) resulted in NLCs with smaller PS and higher ZP. - The NLCs formulation effectively protected lycopene from instability during storage, particularly at 4 °C for a duration of 3 months. | [59] |
| NLCs | Phytosterols | Soybean oil Fully hydrogenated palm and crambe oils (simple and interestefied) | Enzymatic modified soybean lecithin | - NLCs formulated with interesterified lipid blends exhibited greater stability compared to those developed with single lipid blends. - Interesterification led to a reduction in the MP and recrystallization index of the NLCs. - The highest EE and LC were observed in NLCs formulated with interesterified lipid blends. | [60] |
| NLCs | Thymol | Fully hydrogenated palm oil Thymol oil | Tween 80 | - The increase in thymol content in the NLCs formulations did not affect their physical properties. - The NLCs provided a slower release of thymol in active calcium alginate films, with the release rate being slower in the structures containing a higher thymol content. - The NLC-based films demonstrated sustained antimicrobial activity in the model food system (ground beef) over extended periods, attributable to their slow-release profiles. | [61] |
| SLNs and NLCs | Zeaxanthin | Glycerol monostearate and Glycerol distearate MCT | Tween 80 and lecithin | - SLNs exhibited larger PS compared to NLCs. - NLCs exhibited higher EE and LC than SLNs. - The entrapment of zeaxanthin increased the PS of both SLNs and NLCs. - The use of glycerol distearate decreased the viscosity of the dispersed phase of LNs, resulting in a more uniform PS distribution compared to glycerol monostearate. | [37] |
| NLCs | Vitamin D3 | Glycerol monostearate MCT | Rhamnolipids | - An increase in the liquid lipid content resulted in NLCs with larger PS. - The ratio between solid and liquid lipid influenced both the rate and extent of lipid digestion. | [46] |
| NLCs | Vitamin D3 | Anhydrous milk fat Vitamin E acetate | Sodium caseinate | - The PS and PDI increased as the caseinate concentration reached 3%. - The increase in liquid lipid content in the NLCs formulation resulted in a reduction of PS. - The incorporation of vitamin D3 into the NLCs structure enhanced its anticancer effect. | [47] |
| SLNs | Vitamin E tocotrienols | FAs esters | Poloxamer 188 | - SLNs facilitated the increased bioavailability and selective tissue accumulation of tocotrienol. | [62] |
| NLCs and SLNs | Curcumin | Beeswax MCT | Lecithin Tween 80 | - Both NLCs and SLNs demonstrated good PS stability within the beverage throughout the storage period. - The beverage containing NLCs exhibited slightly greater color stability. - The SLNs-based beverage exhibited higher curcumin bioaccessibility compared to the NLCs-based beverage, although it showed reduced curcumin stability. | [63] |
| NLCs | β-carotene | Fully hydrogenated soybean oil High oleic sunflower oil | Tween 80 Enzymatic modified soybean lecithin Whey protein isolate (WPI) | - The β-carotene entrapment into NLCs structure altered the polymorphic habit of NLCs and increased the energy required for melting. - WPI-based NLCs showed larger PS, lower physical stability, and reduced EE and LC. | [64] |
| NLCs | Lutein | Glyceryl distearate MCT | Ethyl lauroyl arginate Rhamnolipid Tea saponin | - Emulsifiers played a regulatory role in the crystallization behavior of NLCs. - Rhamnolipid-stabilized NLCs demonstrated higher EE for lutein, the slowest release of FFAs, and exhibited high bioaccessibility. | [65] |
| NLCs | β-carotene | Fully hydrogenated soybean oil High oleic sunflower oil | Tween 80 Enzymatic modified soybean lecithin | - NLCs stabilized with Tween 80 exhibited superior physical stability during passage through the in vitro dynamic GIT system, leading to an increase in β-carotene bioaccessibility. - Both NLCs systems did not affect cell viability up to a β-carotene concentration of 25 μg. mL−1. | [21] |
| SLNs | Omega-3 Vitamin D3 | Beeswax | Egg yolk lecithin Tween 80 | - The addition of vitamin D3 to the formulations decreased the NLC PS. - The EE of VD3 increased when ω-3 was included in the formulations. - SLNs effectively protected both bioactives compounds against oxidation and high pH levels. | [66] |
| SLNs | α-tocopherol | Fully hydrogenated palm, soybean and crambe oils | Enzymatic modified soybean lecithin | - The entrapment of α-tocopherol modified the thermal behavior of the particles, resulting in increased crystallinity without affecting the polymorphic structure. - α-Tocopherol-loaded SLNs dispersions demonstrated stability, with no significant loss of α-tocopherol. | [30] |
| NLCs | Phytosterols | Walnut oil Stearic acid | Soybean lecithin | - The bioaccessibility of phytosterols significantly increased (four times) when entrapped into NLCs. - The incorporation of phytosterols also effectively reduced lipid oxidation of the NLCs. | [67] |
| NLCs | Cannabidiol | Hemp seed oil Fully hydrogenated soybean oil | Tween 80 Enzymatic modified soybean lecithin | - The use of individual emulsifiers to produce NLCs resulted in smaller PS; - Increasing the proportion of liquid lipid in unloaded NLCs did not significantly affect the PS or PDI. - An increase in the liquid lipid content in CBD-loaded NLCs led to a reduction in PS. - The CBD retention rate remained at 100% over a period of 30 days. | [48] |
| SLNs | Vitamin D3 | Beeswax | Lecithin | - SLNs demonstrated high EE for vitamin D3, achieving a high concentration. - The enrichment of drinks with SLN-Vitamin D3 did not exhibit any negative effects on cell viability. - The supplementation of thickened beverages with SLNs proved to be a viable strategy, without significant impact on the rheological properties of the beverages. | [68] |
| NLCs | Emodin | Beeswax Glycerol di-stearate MCT | Tween 80 | - The incorporation of beeswax in the formulation of NLCs resulted in larger particles and lower EE compared to the use of glyceryl dibehenate. - The EE of emodin was not significantly influenced by the solid and liquid lipid ratio in the NLCs. | [49] |
3.2. Emulsifiers
3.3. Lipophilic Bioactive Compounds
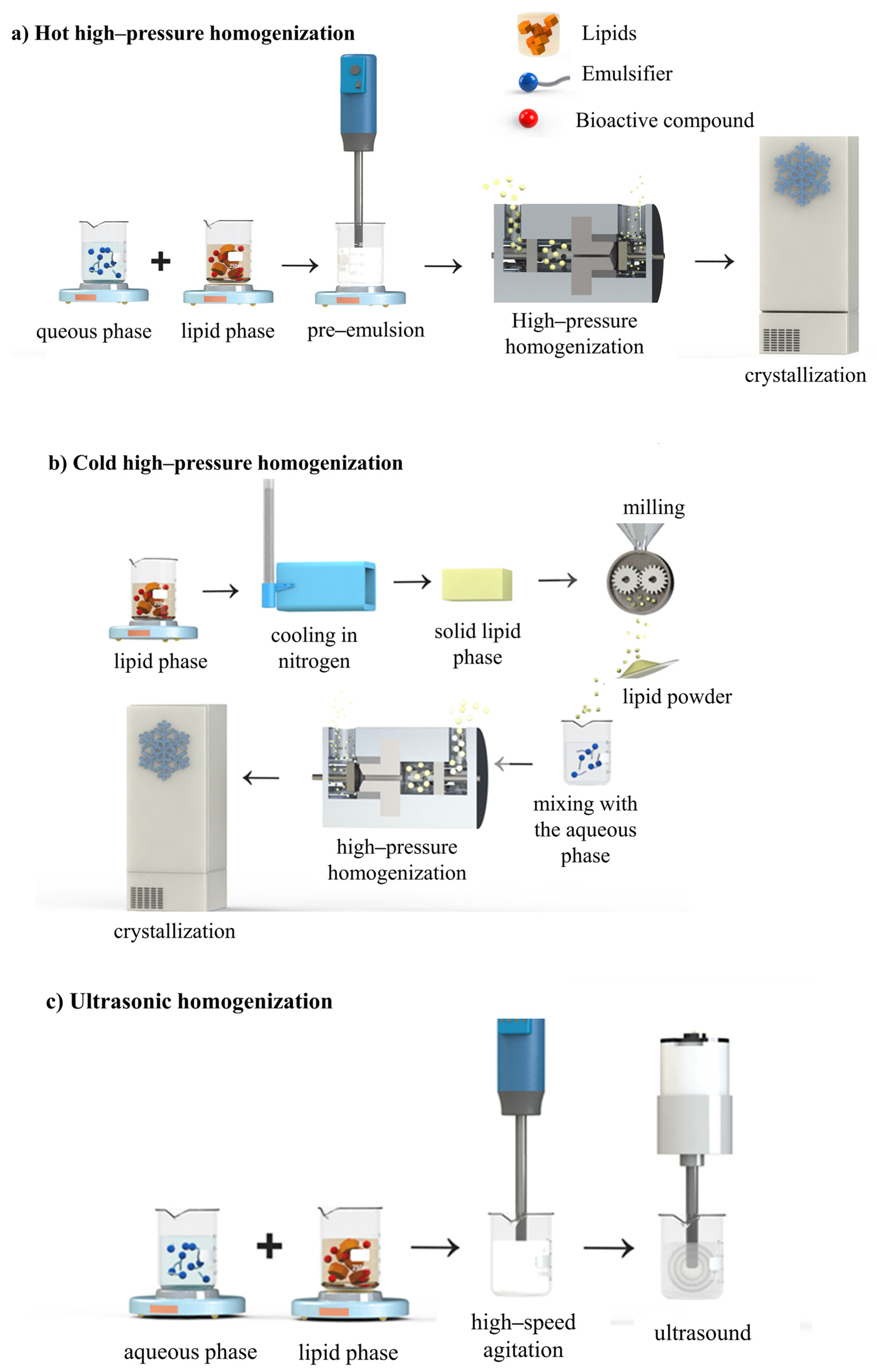
4. Production Methods
| Method | Lipid Nanostructure | Process | Lipid Phase | Emulsifier/Surfactant | Bioactive | Reference | |
|---|---|---|---|---|---|---|---|
| High energy | High-pressure homogenization | SLNs | 700 bar and 2 cycles. | Fully hydrogenated palm, soybean, crambe oils. | Lecithin | α-tocopherol | [30] |
| NLCs | 700 bar and 2 cycles. | Palm, soybean, microalgae, and crambe harfat, soybean oil, interesterified lipid. | Lecithin | - | [60] | ||
| NLCs | 500 bars for 6 cycles | Tristearin, Phosal 53 MCT (lecithin 53% and MCT). | Tween 80 | Ondansetron hydrochloride | [79] | ||
| High-pressure homogenization + Microfluidization | NLCs | Microfluidizer under 50 MPa for three times. | MCT, glyceryl palmitostearate | Rhamnolipid | Emulsifier | [65] | |
| Microfluidization | SLNs | Pre-emulsion and homogenization in a microfluidizer (40–45 °C) for five times at 5000–28,500 psi. | Tripalmitin, cetyl palmitate, pluronic F68 | Lecithin, tween 80 | Trypsin, testosterone | [80] | |
| Complex nanoparticles | 100 MPa for 2 cycles. | MCT | Tea saponin | β-carotene, curcumin | [81] | ||
| Ultrasonic homogenization | SLNs and NLCs | Ultrasonic probe with 25% and 50 cycles (4 s on and 1 s off). | MCT | Lecithin, tween 80 | Zeaxanthin | [37] | |
| NLCs | Ultrasonic for 5 min, amplitude intensity of 60%. | Dynasan 116, capryol 90, lauroglycol 90, miglyol 810, tributyrin | Twen 80, lecithin | Trans-resveratrol | [82] | ||
| SLNs | 100 W of ultrasonic for 3 or 5 min, amplitude 50–100% in a continuous operation or pulse modulation. | Glyceryl monostearate | Lipoid S75 (fat-free soybean phospholipids with 70% phosphatidylcholine), tween 60. | - | [83] | ||
| SLNs | Sonication (90 W) for 2–10 min at 75 °C. | Stearic acid, tripalmitin | Span 80, tween 80 | Curcumin | [84] | ||
| Low energy | Microemulsion | SLNs | Dispersion of hot O/W microemulsion in cold water (2–4 °C) at a 1:50 ratio (microemulsion: water). | Glyceryl monostearate, glyceryl trimyristate, glyceryl behenate, glyceryl palmitostearate, hydrogenated coco-glycerides, cetyl palmitate and stearic acid. | Tween 20 | Tetracycline | [85] |
| SLNs | Cold dilution of microemulsion. | Trimyristin, tristearin, myristic acid, glyceryl dibehenate, and glyceryl monostearate | Epikuron 200, polysorbate (20, 40, 80), cremophor. | - | [86] | ||
| SLNs | Hot microemulsion dispersed in 5 parts (v/v) of cold water (2–4 °C). | Propylene glycol monopalmitate, glyceryl monostearate | Tween 80 | - | [87] | ||
| Spontaneous emulsification | SLNs | Emulsifier at 85 °C for 30 min at 500 rpm. Added to aqueous phase over 5 min at 750 or 1050 rpm. | Beeswax, propolis wax | Tween 80 | - | [88] | |
| LNC | Under stirring. Removal of organic solvents by evaporation and concentration of colloidal dispersions. | MCT | Hydrogenated soybean lecithin, egg lecithin, tween 80 | Jatropha isabellei | [89] | ||
| Solvent diffusion | rLNP | Lipids dissolved in ethanol at 10 mg/mL, flow rate of 0.1 mL/s, using a syringe. Homogenization of the mixture at 500 rpm. | MCT | - | Curcumin | [90] | |
| NLCs | Dissolved in acetone and added to the aqueous phase (1:2 v/v ratio). | Stearic acid, Capryol PGMC, cholesterol, triolein | Brij 35, Brij 72 | Simvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A | [91] | ||
| Solvent injection | NLCs | All weighted directly, and the final volume adjusted to 1 mL. 1000 rpm + 65 °C + ultrasonic probe (60 W for 90 s) | Precirol® ATO 5, copaiba oil | Tween 80 | - | [92] | |
| NLCs | Hypodermic needle syringe. Mixture stirred at 1500 rpm for 1 h (evaporation of ethanol), and cooled for 30 min. | Stearic acid to oleic acid | Sodium lauryl sulfate | Fexofenadine HCL | [93] | ||
| Phase inversion | NLCs | The hot dispersion (murumuru butter and surfactants) was submitted to two heating/cooling cycles. | Murumuru butter | Span 80, Cremophor RH | α-tocopherol, β-carotene | [54] | |
| SLNs | Oil, surfactant, and water were heated at 50 °C. | Octadecane | Brij 30, C12E4 | - | [94] |
4.1. High-Energy Methods
4.1.1. High-Pressure Homogenization
4.1.2. Microfluidization
4.1.3. Ultrasonic Homogenization
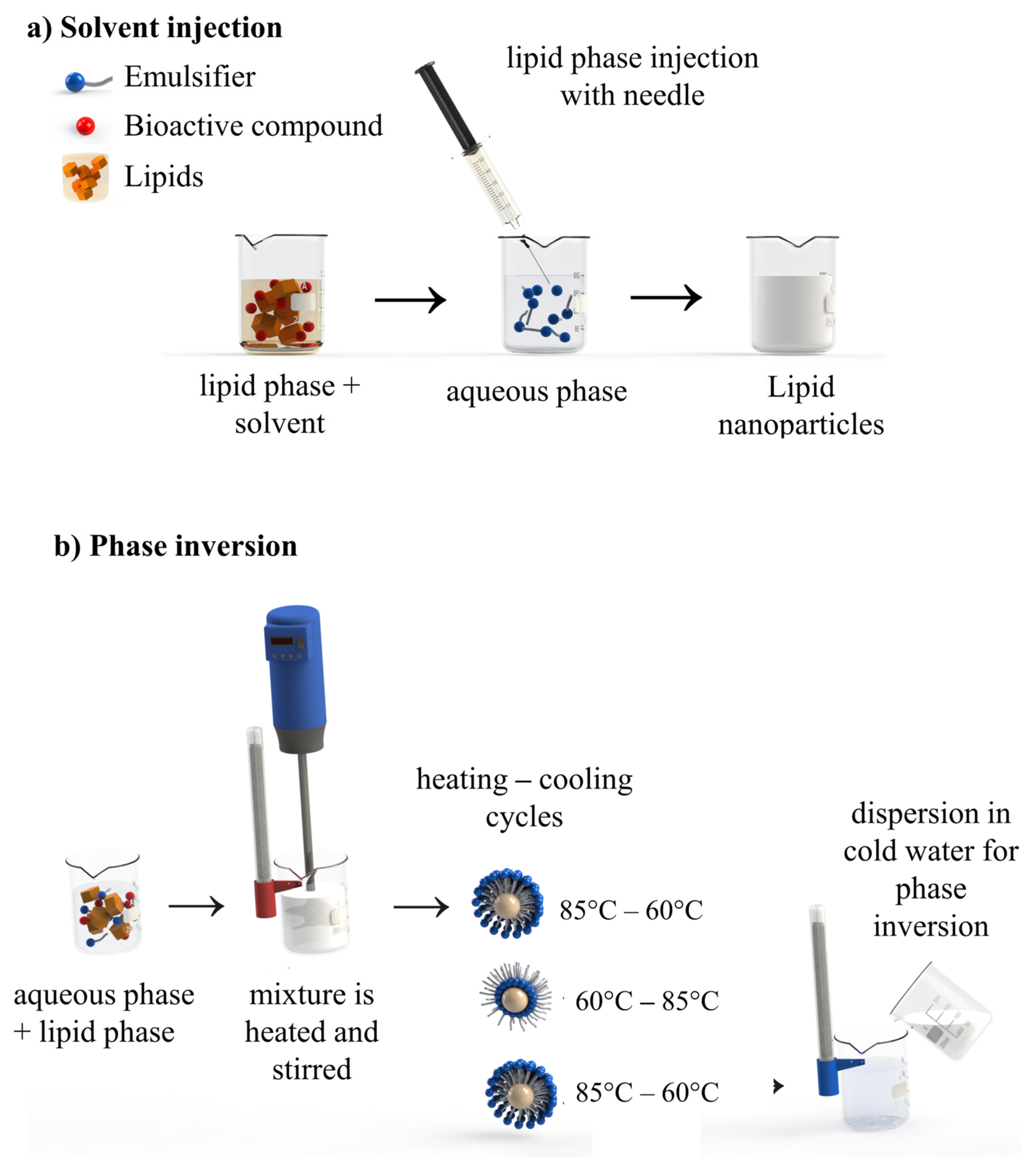
4.2. Low-Energy Methods
4.2.1. Microemulsion
4.2.2. Spontaneous Emulsification
4.2.3. Solvent Diffusion
4.2.4. Solvent Injection
4.2.5. Phase Inversion
5. Characterization Methods
5.1. Particle Size (PS) and Polydispersity Index (PDI)
5.2. Zeta Potential (ZP)
5.3. Crystallinity and Polymorphism
5.4. Thermal Properties and Solid Fat Content (SFC)
5.5. Morphology and Ultrastructure
5.6. Entrapment Efficiency (EE) and Load Capacity (LC)
5.7. Physical Stability
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duncan, T.V. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid. Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.I.; Panchapakesan, C.P.; Huang, Q.; Takhistov, P.; Liu, S.; Kokini, J.L. Nanotechnology: A New Frontier in Food Science. Food Technol. Mag. 2003, 57, 24–29. [Google Scholar]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid Lipid Nanoparticles as Delivery Systems for Bioactive Food Components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Rathee, S.; Nayak, V.; Singh, K.R.; Ojha, A. Nanofortification of Vitamin B-Complex in Food Matrix: Need, Regulations, and Prospects. Food Chem. Mol. Sci. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Granja, A.; Silva, A.M.; Saraiva, J.A.; Reis, S.; Cardoso, M. Nanoencapsulation of Vitamin B12 with Opuntia Ficus-Indica Seed Oil for Food Supplements 2 Uses 3 4. Food Bioprod. Process. 2025, 150, 275–284. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Molaveisi, M.; Zhao, Y.; Shi, Q.; Fang, Z. Function of Vitamin D3-Loaded Lipid-Based Nanocarriers in Food Industry: Principles, Applications, and Challenges. Trends Food Sci. Technol. 2025, 155, 104798. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Stahl, M.A.; Grimaldi, R.; Cardoso, L.P.; Gigante, M.L.; Ribeiro, A.P.B. High Oleic Sunflower Oil and Fully Hydrogenated Soybean Oil Nanostructured Lipid Carriers: Development and Characterization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130039. [Google Scholar] [CrossRef]
- Scrimgeour, C. Chemistry of Fatty Acids. In Bailey’s Industrial Oil and Fat Products. Edible Oil and Fat Products: Chemistry, Chemical Properties, and Health Effects; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 2. [Google Scholar]
- Sato, K. Crystallization Behaviour of Fats and Lipids—A Review. Chem. Eng. Sci. 2001, 56, 2255–2265. [Google Scholar] [CrossRef]
- Lawler, P.; Dimick, P. Crystallization and Polymorphism of Fats. In Food Lipids; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Martini, S.; Awad, T.; Marangoni, A.G. Structure and Properties of Fat Crystal Networks. In Modifying Lipids for Use in Food; Gunstone, F., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 142–169. [Google Scholar]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and Kinetic Aspects of Fat Crystallization. Adv. Colloid. Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- da Silva Santos, V.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid Lipid Nanoparticles as Carriers for Lipophilic Compounds for Applications in Foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.B.; Basso, R.C.; Grimaldi, R.; Gioielli, L.A.; dos Santos, A.O.; Cardoso, L.P.; Guaraldo Gonçalves, L.A. Influence of Chemical Interesterification on Thermal Behavior, Microstructure, Polymorphism and Crystallization Properties of Canola Oil and Fully Hydrogenated Cottonseed Oil Blends. Food Res. Int. 2009, 42, 1153–1162. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Stahl, M.A.; Grimaldi, R.; Cardoso, L.P.; Ribeiro, A.P.B. Characterization of Lipid Systems Based on Fully Hydrogenated Soybean and High Oleic Sunflower Oils to Obtain Nanostructured Lipid Carriers. Food Biosci. 2023, 53, 102634. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Martins, J.T.; Abrunhosa, L.; Baixinho, J.; Matias, A.A.; Vicente, A.A.; Pinheiro, A.C. Lipid-Based Nanostructures as a Strategy to Enhance Curcumin Bioaccessibility: Behavior under Digestion and Cytotoxicity Assessment. Food Res. Int. 2021, 143, 110278. [Google Scholar] [CrossRef]
- Luisa Lüdtke, F.; Aparecida Stahl, M.; Grimaldi, R.; Bruno Soares Forte, M.; Lúcia Gigante, M.; Paula Badan Ribeiro, A. Opti-mization of High Pressure Homogenization Conditions to Produce Nanostructured Lipid Carriers Using Natural and Synthetic Emulsifiers. Food Res. Int. 2022, 160, 111746. [Google Scholar] [CrossRef]
- Oliveira, D.R.B.; de Furtado, G.F.; Cunha, R.L. Solid Lipid Nanoparticles Stabilized by Sodium Caseinate and Lactoferrin. Food Hydrocoll. 2019, 90, 321–329. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Fernandes, J.; Gonçalves, R.F.S.; Martins, J.T.; Berni, P.; Ribeiro, A.P.B.; Vicente, A.A.; Pinheiro, A.C. Performance of Β-carotene-loaded Nanostructured Lipid Carriers under Dynamic in Vitro Digestion System: Influence of the Emulsifier Type. J. Food Sci. 2024, 89, 3290–3305. [Google Scholar] [CrossRef]
- Vardanega, R.; Lüdtke, F.L.; Loureiro, L.; Toledo Hijo, A.A.C.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A. Enhancing Cannabidiol Bioaccessibility Using Ionic Liquid as Emulsifier to Produce Nanosystems: Characterization of Structures, Cytotoxicity Assessment, and in Vitro Digestion. Food Res. Int. 2024, 188, 114498. [Google Scholar] [CrossRef]
- Stahl, M.A.; Lüdtke, F.L.; Grimaldi, R.; Gigante, M.L.; Ribeiro, A.P.B. Characterization and Stability of Solid Lipid Nanoparticles Produced from Different Fully Hydrogenated Oils. Food Res. Int. 2024, 176, 113821. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Madalena, D.A.; Fernandes, J.M.; Marques, M.; Vicente, A.A.; Pinheiro, A.C. Application of Nanostructured Delivery Systems in Food: From Incorporation to Detection and Characterization. Trends Food Sci. Technol. 2022, 129, 111–125. [Google Scholar] [CrossRef]
- Barroso, L.; Viegas, C.; Vieira, J.; Ferreira-Pêgo, C.; Costa, J.; Fonte, P. Lipid-Based Carriers for Food Ingredients Delivery. J. Food Eng. 2021, 295, 110451. [Google Scholar] [CrossRef]
- de Serra, M.L.G.; Hernández, L.A.; Vázquez, R.M.L.; Villafuerte, R.L.; García, F.B. Revista Mexicana de Ciencias Farmacéuticas. Rev. Mex. De. Cienc. Farm. 2008, 39, 50–66. [Google Scholar]
- Souto, E.B.; Santana, M.H.A.; Pinho, S.C. Nanopartículas de lipídios sólidos: Métodos clássicos de produção laboratorial. Química Nova 2011, 34, 1762–1769. [Google Scholar] [CrossRef]
- Sharma, V.K.; Diwan, A.; Sardana, S.; Dhall, V. Solid lipid nano-particles system: An overview. Int. J. Res. Pharm. Sci. 2011, 2, 450–461. [Google Scholar]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured Lipid Carriers (NLC): A Potential Delivery System for Bioactive Food Molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Aparecida Stahl, M.; Luisa Lüdtke, F.; Grimaldi, R.; Lúcia Gigante, M.; Paula Badan Ribeiro, A. Characterization and Stability of α-Tocopherol Loaded Solid Lipid Nanoparticles Formulated with Different Fully Hydrogenated Vegetable Oils. Food Chem. 2024, 439, 138149. [Google Scholar] [CrossRef]
- Boonme, P.; Souto, E.B.; Wuttisantikul, N.; Jongjit, T.; Pichayakorn, W. Influence of Lipids on the Properties of Solid Lipid Nanoparticles from Microemulsion Technique. Eur. J. Lipid Sci. Technol. 2013, 115, 820–824. [Google Scholar] [CrossRef]
- Mohammadi, M.; Assadpour, E.; Jafari, S.M. Encapsulation of Food Ingredients by Nanostructured Lipid Carriers (NLCs). In Lipid-Based Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–270. [Google Scholar]
- Yoon, G.; Park, J.W.; Yoon, I.S. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs): Recent Advances in Drug Delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Ashkar, A.; Sosnik, A.; Davidovich-Pinhas, M. Structured Edible Lipid-Based Particle Systems for Oral Drug-Delivery. Biotechnol. Adv. 2021, 54, 107789. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured Lipid Matrices for Improved Microencapsulation of Drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Osanlou, R.; Emtyazjoo, M.; Banaei, A.; Hesarinejad, M.A.; Ashrafi, F. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers Containing Zeaxanthin and Evaluation of Physicochemical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128588. [Google Scholar] [CrossRef]
- Tan, Y.; McClements, D.J. Improving the Bioavailability of Oil-Soluble Vitamins by Optimizing Food Matrix Effects: A Review. Food Chem. 2021, 348, 129148. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Modulating β-Carotene Bioaccessibility by Controlling Oil Composition and Concentration in Edible Nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef]
- Abreu-Martins, H.H.d.; Artiga-Artigas, M.; Hilsdorf Piccoli, R.; Martín-Belloso, O.; Salvia-Trujillo, L. The Lipid Type Affects the in Vitro Digestibility and β-Carotene Bioaccessibility of Liquid or Solid Lipid Nanoparticles. Food Chem. 2020, 311, 126024. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Charleer, L.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion Stability during Gastrointestinal Conditions Effects Lipid Digestion Kinetics. Food Chem. 2018, 246, 179–191. [Google Scholar] [CrossRef]
- Bonnaire, L.; Sandra, S.; Helgason, T.; Decker, E.A.; Weiss, J.; McClements, D.J. Influence of Lipid Physical State on the in Vitro Digestibility of Emulsified Lipids. J. Agric. Food Chem. 2008, 56, 3791–3797. [Google Scholar] [CrossRef]
- Nik, A.M.; Langmaid, S.; Wright, A.J. Nonionic Surfactant and Interfacial Structure Impact Crystallinity and Stability of β-Carotene Loaded Lipid Nanodispersions. J. Agric. Food Chem. 2012, 60, 4126–4135. [Google Scholar] [CrossRef]
- Katouzian, I.; Faridi Esfanjani, A.; Jafari, S.M.; Akhavan, S. Formulation and Application of a New Generation of Lipid Nano-Carriers for the Food Bioactive Ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Doktorovová, S.; Araújo, J.; Garcia, M.L.; Rakovský, E.; Souto, E.B. Formulating Fluticasone Propionate in Novel PEG-Containing Nanostructured Lipid Carriers (PEG-NLC). Colloids Surf. B Biointerfaces 2010, 75, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.A.; Cerqueira, M.A.; Gonçalves, C.; Amado, I.R.; Teixeira, J.A.; Pastrana, L. Encapsulation of Vitamin D3 Using Rhamnolipids-Based Nanostructured Lipid Carriers. Food Chem. 2023, 427, 136654. [Google Scholar] [CrossRef] [PubMed]
- Barri, A.; Ghanbarzadeh, B.; Mohammadi, M.; Pezeshki, A. Application of Sodium Caseinate as Protein Based Coating/Stabilizing Agent in Development and Optimization of Vitamin D3-Loaded Nanostructured Lipid Carriers (NLCs). Food Hydrocoll. 2023, 144, 108747. [Google Scholar] [CrossRef]
- Vardanega, R.; Lüdtke, F.L.; Loureiro, L.; Gonçalves, R.F.S.; Pinheiro, A.C.; Vicente, A.A. Development and Characterization of Nanostructured Lipid Carriers for Cannabidiol Delivery. Food Chem. 2024, 441, 138295. [Google Scholar] [CrossRef]
- Song, Z.; Tian, W.; Wang, C.; You, Y.; Li, Y.; Xiao, J. Enhancing Supersaturation Maintenance of Hydrophobic Ingredients Using Nanostructured Lipid Carriers: The Role of Solid Lipid Type and Level. Food Chem. 2025, 465, 142057. [Google Scholar] [CrossRef]
- Eltayeb, M.; Bakhshi, P.K.; Stride, E.; Edirisinghe, M. Preparation of Solid Lipid Nanoparticles Containing Active Compound by Electrohydrodynamic Spraying. Food Res. Int. 2013, 53, 88–95. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of Stearic Acid on Plasma Lipid and Lipoproteins in Humans. Lipids 2005, 40, 1201–1205. [Google Scholar] [CrossRef]
- Wang, J.L.; Dong, X.Y.; Wei, F.; Zhong, J.; Liu, B.; Yao, M.H.; Yang, M.; Zheng, C.; Quek, S.Y.; Chen, H. Preparation and Characterization of Novel Lipid Carriers Containing Microalgae Oil for Food Applications. J. Food Sci. 2014, 79, E169–E177. [Google Scholar] [CrossRef]
- Aditya, N.P.; Ko, S. Solid Lipid Nanoparticles (SLNs): Delivery Vehicles for Food Bioactives. RSC Adv. 2015, 5, 30902–30911. [Google Scholar] [CrossRef]
- Gomes, G.V.L.; Sola, M.R.; Rochetti, A.L.; Fukumasu, H.; Vicente, A.A.; Pinho, S.C. β-Carotene and α-Tocopherol Coencapsulated in Nanostructured Lipid Carriers of Murumuru (Astrocaryum murumuru) Butter Produced by Phase Inversion Temperature Method: Characterisation, Dynamic in Vitro Digestion and Cell Viability Study. J. Microencapsul. 2019, 36, 43–52. [Google Scholar] [CrossRef]
- Santos, V.d.S.; Braz, B.B.; Silva, A.Á.; Cardoso, L.P.; Ribeiro, A.P.B.; Santana, M.H.A. Nanostructured Lipid Carriers Loaded with Free Phytosterols for Food Applications. Food Chem. 2019, 298, 125053. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, S.; Ghanbarzadeh, B.; Ayaseh, A.; Dehghannya, J.; Ehsani, A.; Ozyurt, H. Essential Oil-Loaded Nanostructured Lipid Carriers: The Effects of Liquid Lipid Type on the Physicochemical Properties in Beverage Models. Food Biosci. 2020, 35, 100526. [Google Scholar] [CrossRef]
- Chaudhari, V.S.; Murty, U.S.; Banerjee, S. Nanostructured Lipid Carriers as a Strategy for Encapsulation of Active Plant Constituents: Formulation and in Vitro Physicochemical Characterizations. Chem. Phys. Lipids 2021, 235, 105037. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, F.; Pu, C.; Tang, W.; Sun, Q. Nanoencapsulation of Lutein within Lipid-Based Delivery Systems: Characterization and Comparison of Zein Peptide Stabilized Nano-Emulsion, Solid Lipid Nanoparticle, and Nano-Structured Lipid Carrier. Food Chem. 2021, 358, 129840. [Google Scholar] [CrossRef]
- Sirikhet, J.; Chanmahasathien, W.; Raiwa, A.; Kiattisin, K. Stability Enhancement of Lycopene in Citrullus lanatus Extract via Nanostructured Lipid Carriers. Food Sci. Nutr. 2021, 9, 1750–1760. [Google Scholar] [CrossRef]
- da Silva, M.G.; de Godoi, K.R.R.; Gigante, M.L.; Cardoso, L.P.; Ribeiro, A.P.B. Nanostructured Lipid Carriers for Delivery of Free Phytosterols: Effect of Lipid Composition and Chemical Interesterification on Physical Stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128425. [Google Scholar] [CrossRef]
- Karimi-Khorrami, N.; Radi, M.; Amiri, S.; Abedi, E.; McClements, D.J. Fabrication, Characterization, and Performance of Antimicrobial Alginate-Based Films Containing Thymol-Loaded Lipid Nanoparticles: Comparison of Nanoemulsions and Nanostructured Lipid Carriers. Int. J. Biol. Macromol. 2022, 207, 801–812. [Google Scholar] [CrossRef]
- Fu, J.Y.; Meganathan, P.; Gunasegaran, N.; Tan, D.M.Y. Effect of Nano-Delivery Systems on the Bioavailability and Tissue Biodistribution of Vitamin E Tocotrienols. Food Res. Int. 2023, 171, 113048. [Google Scholar] [CrossRef]
- Gonçalves, R.F.S.; Vicente, A.A.; Pinheiro, A.C. Incorporation of Curcumin-Loaded Lipid-Based Nano Delivery Systems into Food: Release Behavior in Food Simulants and a Case Study of Application in a Beverage. Food Chem. 2023, 405, 134740. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Grimaldi, R.; Cardoso, L.P.; Gigante, M.L.; Vicente, A.A.; Ribeiro, A.P.B. Development and Characterization of Fully Hydrogenated Soybean Oil and High Oleic Sunflower Oil β-Carotene Loaded Nanostructured Lipid Carriers. Food Biophys. 2023, 18, 338–352. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured Lipid Carriers (NLCs) Stabilized by Natural or Synthetic Emulsifiers for Lutein Delivery: Improved Physicochemical Stability, Antioxidant Activity, and Bioaccessibility. Food Chem. 2023, 403, 134465. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Ghobadi, R.; Sohrabvandi, S.; Khanniri, E.; Mollakhalili-Meybodi, N. Co-Encapsulation of Omega-3 and Vitamin D3 in Beeswax Solid Lipid Nanoparticles to Evaluate Physicochemical and in Vitro Release Properties. Front. Nutr. 2024, 11, 1323067. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huai, Q.; Li, D.; Wu, X.; Li, Y.; Zheng, H. Investigation on the Structure and Properties of Nanostructured Lipid Carriers Loaded with Different Contents of Phytosterols and Their Impact on Lipid Oxidation. Food Biosci. 2024, 62, 105033. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Vieira, J.M.; Gonçalves, R.F.S.; Martins, J.T.; Vicente, A.A.; Pinheiro, A.C. Development of Novel Functional Thickened Drinks Enriched with Vitamin D3 for the Older Adult Population—Behaviour under Dynamic in Vitro Digestion. Food Hydrocoll. 2025, 158, 110572. [Google Scholar] [CrossRef]
- da Silva, M.G.; de Godoi, K.R.R.; Gigante, M.L.; Pavie Cardoso, L.; Paula Badan Ribeiro, A. Developed and Characterization of Nanostructured Lipid Carriers Containing Food-Grade Interesterified Lipid Phase for Food Application. Food Res. Int. 2022, 155, 111119. [Google Scholar] [CrossRef]
- Kharat, M.; McClements, D.J. Fabrication and Characterization of Nanostructured Lipid Carriers (NLC) Using a Plant-Based Emulsifier: Quillaja Saponin. Food Res. Int. 2019, 126, 108601. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle-and Microparticle-Based Delivery Systems; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482233162. [Google Scholar]
- Alam, S.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation Utilizing the HLB Concept for the Development of Moisturizing Cream and Lotion: In-Vitro Characterization and Stability Evaluation. Cosmetics 2020, 7, 43. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Salvia-Trujillo, L.; Verkempinck, S.; Rijal, S.K.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Lipid Nanoparticles with Fats or Oils Containing β-Carotene: Storage Stability and in Vitro Digestibility Kinetics. Food Chem. 2019, 278, 396–405. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zhou, H.; Xiao, H.; McClements, D.J. Factors Impacting Lipid Digestion and SS-Carotene Bioaccessibility Assessed by Standardized Gastrointestinal Model (INFOGEST): Oil Droplet Concentration. Food Funct. 2020, 11, 7126–7137. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Influence of Mandarin Fiber Addition on Physico-Chemical Properties of Nanoemulsions Containing β-Carotene under Simulated Gastrointestinal Digestion Conditions. LWT Food Sci. Technol. 2017, 84, 331–337. [Google Scholar] [CrossRef]
- Dan, N. Compound Release from Nanostructured Lipid Carriers (NLCs). J. Food Eng. 2016, 171, 37–43. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Nanostructured Lipid Carriers Containing Ondansetron Hydrochloride by Cold High-Pressure Homogenization Method: Preparation, Characterization, and Pharmacokinetic Evaluation. J. Drug Deliv. Sci. Technol. 2019, 53, 101185. [Google Scholar] [CrossRef]
- Helgason, T.; Salminen, H.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Formation of Transparent Solid Lipid Nanoparticles by Microfluidization: Influence of Lipid Physical State on Appearance. J. Colloid. Interface Sci. 2015, 448, 114–122. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, M.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Co-Encapsulation of Curcumin and β-Carotene in Pickering Emulsions Stabilized by Complex Nanoparticles: Effects of Microfluidization and Thermal Treatment. Food Hydrocoll. 2022, 122, 107064. [Google Scholar] [CrossRef]
- Khan, I.; Sunita, S.; Hussein, N.R.; Omer, H.K.; Elhissi, A.; Houacine, C.; Khan, W.; Yousaf, S.; Rathore, H.A. Development and Characterization of Novel Combinations and Compositions of Nanostructured Lipid Carrier Formulations Loaded with Trans-Resveratrol for Pulmonary Drug Delivery. Pharmaceutics 2024, 16, 1589. [Google Scholar] [CrossRef]
- Pucek-Kaczmarek, A. Influence of Process Design on the Preparation of Solid Lipid Nanoparticles by an Ultrasonic-Nanoemulsification Method. Processes 2021, 9, 1265. [Google Scholar] [CrossRef]
- Behbahani, E.S.; Ghaedi, M.; Abbaspour, M.; Rostamizadeh, K. Optimization and Characterization of Ultrasound Assisted Preparation of Curcumin-Loaded Solid Lipid Nanoparticles: Application of Central Composite Design, Thermal Analysis and X-Ray Diffraction Techniques. Ultrason. Sonochem. 2017, 38, 271–280. [Google Scholar] [CrossRef]
- Shah, R.M.; Malherbe, F.; Eldridge, D.; Palombo, E.A.; Harding, I.H. Physicochemical Characterization of Solid Lipid Nanoparticles (SLNs) Prepared by a Novel Microemulsion Technique. J. Colloid. Interface Sci. 2014, 428, 286–294. [Google Scholar] [CrossRef]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials 2019, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, A.; Goli, S.A.H.; Varshosaz, J.; Salvia-Trujillo, L.; Martín-Belloso, O. Fabrication of Edible Solid Lipid Nanoparticle from Beeswax/Propolis Wax by Spontaneous Emulsification: Optimization, Characterization and Stability. Food Chem. 2022, 387, 132934. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.K.; Meyer, P.A.; Stein, T.; Tonussi, C.R.; Lemos-Senna, E. Development and in Vivo Evaluation of Lipid-Based Nanocarriers Containing Jatropha Isabellei Dry Extract from the Dichloromethane Fraction Intended for Oral Treatment of Arthritic Diseases. Braz. J. Pharm. Sci. 2022, 58, e19178. [Google Scholar] [CrossRef]
- Han, D.; Ji, L.; Lu, M.; Li, D.; Sheng, X.; Zhang, J.; Wang, C. Pickering Emulsion Stabilized by Egg Derived Reconstituted Lipid Nanoparticles for Encapsulation and Oral Delivery of Curcumin. Food Chem. 2025, 472, 142912. [Google Scholar] [CrossRef]
- Mousavi-Simakani, S.M.; Azadi, A.; Tanideh, N.; Omidifar, N.; Ghasemiyeh, P.; Mohammadi-Samani, S. Simvastatin-Loaded Nanostructured Lipid Carriers as Topical Drug Delivery System for Wound Healing Purposes: Preparation, Characterization, and In Vivo Histopathological Studies. Adv. Pharm. Bull. 2023, 13, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Uchôa, A.F.C.; Formiga, A.L.D.; Alves, Á.E.F.; Cardoso, A.L.M.R.; de Araujo Pereira, G.M.; Carvalho, L.M.M.; da Silva, L.F.A.; da Silva Pereira, P.S.; de Souza, P.H.O.; Jales, S.T.L.; et al. Optimization and Functionalization of Copaiba Oil-Loaded Nanostructured Lipid Carriers to Improve Cytotoxicity against Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2025, 105, 106575. [Google Scholar] [CrossRef]
- Abdelhameed, A.H.; Abdelhafez, W.A.; Saleh, K.H.I.; Mohamed, M.S. Formulation, Optimization, and in-Vivo Evaluation of Nanostructured Lipid Carriers Loaded with Fexofenadine HCL for Oral Delivery. J. Drug Deliv. Sci. Technol. 2022, 74, 103607. [Google Scholar] [CrossRef]
- Gao, S.; McClements, D.J. Formation and Stability of Solid Lipid Nanoparticles Fabricated Using Phase Inversion Temperature Method. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 79–87. [Google Scholar] [CrossRef]
- Inguva, P.; Grasselli, S.; Heng, P.W.S. High Pressure Homogenization—An Update on Its Usage and Understanding. Chem. Eng. Res. Des. 2024, 202, 284–302. [Google Scholar] [CrossRef]
- Fathi, F.; Machado, T.O.X.; Kodel, H.d.A.C.; Portugal, I.; Ferreira, I.O.; Zielinska, A.; Oliveira, M.B.P.P.; Souto, E.B. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for the Delivery of Bioactives Sourced from Plants: Part I—Composition and Production Methods. Expert. Opin. Drug Deliv. 2024, 21, 1479–1490. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A Review on Novel Methodologies for Drug Nanoparticle Preparation: Microfluidic Approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Özaslan, Z.T.; Sahni, P.; Khanashyam, A.C.; Kutlu, N.; Yilmaz, M.S.; Isleroglu, H.; Ramniwas, S.; Rustagi, S. High Pressure Homogenization for Preservation of Liquid Foods- Mechanisms, Molecular Modifications and Recent Developments. Future Foods 2024, 10, 100488. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Ahmadi, F.; Hashemi, S.M.H.; Babaei, A.; Yaddollahi, S.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: A Review of the Methods of Manufacture and Routes of Administration. Pharm. Dev. Technol. 2022, 27, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Effectiveness of Encapsulating Biopolymers to Produce Sub-Micron Emulsions by High Energy Emulsification Techniques. Food Res. Int. 2007, 40, 862–873. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, L. Nanoparticles Fabricated from Bulk Solid Lipids: Preparation, Properties, and Potential Food Applications. Adv. Colloid. Interface Sci. 2019, 273, 102033. [Google Scholar] [CrossRef]
- Khairnar, B.A.; Dabhane, H.A.; Dashpute, R.S.; Girase, M.S.; Nalawade, P.M.; Gaikwad, V.B. Study of Biogenic Fabrication of Zinc Oxide Nanoparticles and Their Applications: A Review. Inorg. Chem. Commun. 2022, 146, 110155. [Google Scholar] [CrossRef]
- Abedi, E.; Akhavan, H.-R.; Mohammadi, H.; Banasaz, S. Structure-Based Modifications of Nano Lipid Carriers: Comparative Review on Release Properties and Anti-Microbial Activities of Bioactive Compounds. Food Control 2024, 159, 110237. [Google Scholar] [CrossRef]
- Kavinila, S.; Nimbkar, S.; Moses, J.A.; Anandharamakrishnan, C. Emerging Applications of Microfluidization in the Food Industry. J. Agric. Food Res. 2023, 12, 100537. [Google Scholar] [CrossRef]
- Yuan, H.; Wei, X.; Chen, C.; Yu, H.; Huang, J.; Tian, H. Recent Developments in Cellulose-Based Emulsion Systems for Food Applications: A Review. Food Hydrocoll. 2025, 163, 111089. [Google Scholar] [CrossRef]
- Salminen, H.; Helgason, T.; Aulbach, S.; Kristinsson, B.; Kristbergsson, K.; Weiss, J. Influence of Co-Surfactants on Crystallization and Stability of Solid Lipid Nanoparticles. J. Colloid. Interface Sci. 2014, 426, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Karthivashan, G.; Park, S.Y.; Kim, J.; Choi, D.-K. Microfluidization Trends in the Development of Nanodelivery Systems and Applications in Chronic Disease Treatments. Int. J. Nanomed. 2018, 13, 6109–6121. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Li, L.-W.; Chen, X.-Y.; Liu, L.-C.; Yang, Y.; Wu, Y.-J.; Chen, G.; Zhang, Z.-F.; Luo, P. Oil-in-Water Camellia Seeds Oil Nanoemulsions via High Pressure Microfluidization: Formation and Evaluation. LWT 2021, 140, 110815. [Google Scholar] [CrossRef]
- Severino, P.; Santana, M.H.A.; Souto, E.B. Optimizing SLN and NLC by 2 2 Full Factorial Design: Effect of Homogenization Technique. Mater. Sci. Eng. C 2012, 32, 1375–1379. [Google Scholar] [CrossRef]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Prepared by Microwave and Ultrasound-Assisted Synthesis: Promising Green Strategies for the Nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef]
- Stefanov, S.; Gugleva, V.; Andonova, V. Technological Strategies for the Preparation of Lipid Nanoparticles: An Updated Review. Pharmacia 2023, 70, 449–463. [Google Scholar] [CrossRef]
- Woo, J.O.; Misran, M.; Lee, P.F.; Tan, L.P. Development of a Controlled Release of Salicylic Acid Loaded Stearic Acid-Oleic Acid Nanoparticles in Cream for Topical Delivery. Sci. World J. 2014, 2014, 205703. [Google Scholar] [CrossRef]
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N. Nanostructured Lipid Carriers for the Topical Delivery of Tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261. [Google Scholar] [CrossRef]
- Masiiwa, W.L.; Gadaga, L.L. Intestinal Permeability of Artesunate-Loaded Solid Lipid Nanoparticles Using the Everted Gut Method. J. Drug Deliv. 2018, 2018, 3021738. [Google Scholar] [CrossRef]
- Beloqui, A.; des Rieux, A.; Préat, V. Mechanisms of Transport of Polymeric and Lipidic Nanoparticles across the Intestinal Barrier. Adv. Drug Deliv. Rev. 2016, 106, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.K.; Upadhyay, S.C.; Kumar, M.; Pathak, K.; Kaushik, D.; Verma, R.; Bhatt, S.; Massoud, E.E.S.; Rahman, M.H.; Cavalu, S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers 2022, 14, 3545. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.C.; Ghebremeskel, K.; White, K.; Minelli, C.; Tewfik, I.; Thapa, P.; Tewfik, S. Formulation and Characterization of Phytostanol Ester Solid Lipid Nanoparticles for the Management of Hypercholesterolemia: An Ex Vivo Study. Int. J. Nanomed. 2021, 16, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Krishnasailaja, A.; Gazi, A.S. Formulation of Methotrexate Loaded Solid Lipid Nanoparticles by Micro Emulsion Technique. Curr. Nanomater. 2023, 8, 153–161. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Onaizi, S.A. Effects of Emulsification Factors on the Characteristics of Crude Oil Emulsions Stabilized by Chemical and Biosurfactants: A Review. Fuel 2024, 361, 130604. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous Emulsification. Curr. Opin. Colloid. Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent Emulsification Evaporation and Solvent Emulsification Diffusion Techniques for Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer International Publishing: Cham, Switzerland, 2021; pp. 287–300. [Google Scholar]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef]
- Schubert, H.; Engel, R. Product and Formulation Engineering of Emulsions. Chem. Eng. Res. Des. 2004, 82, 1137–1143. [Google Scholar] [CrossRef]
- de Queiroz, M.C.V.; Muehlmann, L.A. Characteristics and Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. J. Nanotheranostics 2024, 5, 188–211. [Google Scholar] [CrossRef]
- Satya Lakshmi, S.; Persis Joni, K. Nano structured lipid carrier system-a novel targeting carrier. Int. J. Pharm. Sci. Res. 2021, 12, 6128. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef] [PubMed]
- Jintapattanakit, A.; Hasan, H.M.; Junyaprasert, V.B. Vegetable Oil-Based Nanoemulsions Containing Curcuminoids: Formation Optimization by Phase Inversion Temperature Method. J. Drug Deliv. Sci. Technol. 2018, 44, 289–297. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Jannin, V.; Musakhanian, J.; Marchaud, D. Approaches for the Development of Solid and Semi-Solid Lipid-Based Formulations. Adv. Drug Deliv. Rev. 2008, 60, 734–746. [Google Scholar] [CrossRef]
- Bunjes, H. Characterization of Solid Lipid Nano-and Microparticles. In Lipospheres in Drug Targets and Delivery; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of Particles in Solution—A Perspective on Light Scattering and Comparative Technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Lakshmi, P.; Kumar, G.A. Nanosuspension Technology: A Review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Montes, C.; Villaseñor, M.J.; Ríos, Á. Analytical Control of Nanodelivery Lipid-Based Systems for Encapsulation of Nutraceuticals: Achievements and Challenges. Trends Food Sci. Technol. 2019, 90, 47–62. [Google Scholar] [CrossRef]
- Leopoldo, V.R.; Beatriz, G.F.; de Lourdes, G.S.M.; Alejandra, H.L.; Luisa, V.R.M. Nanopartículas lipídicas sólidas. Rev. Mex. De Cienc. Farm. 2008, 39, 38–52. [Google Scholar]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of Astaxanthin-Loaded Nanostructured Lipid Carriers in Beverage Systems. J. Sci. Food Agric. 2018, 98, 511–518. [Google Scholar] [CrossRef]
- McClements, J.; McClements, D.J. Standardization of Nanoparticle Characterization: Methods for Testing Properties, Stability, and Functionality of Edible Nanoparticles. Crit. Rev. Food Sci. Nutr. 2016, 56, 1334–1362. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-Concentration Zeta Potential Measurements Using Light-Scattering Techniques. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Mehnert, W.; Müller, R.H. Polymorphic Behaviour of Compritol®888 ATO as Bulk Lipid and as SLN and NLC. J. Microencapsul. 2006, 23, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; Decker, E.A.; Mcclements, D.J.; Weiss, J. Impact of Surfactant Properties on Oxidative Stability of β-Carotene Encapsulated within Solid Lipid Nanoparticles. J. Agric. Food Chem. 2009, 57, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- AOCS. American Oil Chemists’ Society Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion Delivery Systems: Influence of Carrier Oil on β-Carotene Bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Shukat, R.; Bourgaux, C.; Relkin, P. Crystallisation Behaviour of Palm Oil Nanoemulsions Carrying Vitamin E: DSC and Synchrotron X-Ray Scattering Studies. J. Therm. Anal. Calorim. 2012, 108, 153–161. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Andreani, T.; Nunes, F.M.; Cassimiro, D.L.; de Almeida, A.E.; Ribeiro, C.A.; Sarmento, V.H.V.; Gremião, M.P.D.; Silva, A.M.; Souto, E.B. Loading of Praziquantel in the Crystal Lattice of Solid Lipid Nanoparticles. J. Therm. Anal. Calorim. 2012, 108, 353–360. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, C.T. Optimization of Nanostructured Lipid Carriers for Lutein Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Hwang, I.C.; Park, J.W.; Park, H.J. Enhanced Payload and Photo-Protection for Pesticides Using Nanostructured Lipid Carriers with Corn Oil as Liquid Lipid. J. Microencapsul. 2012, 29, 596–604. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of Food Grade Nanostructured Lipid Carrier (NLC) for Potential Applications in Medicinal-Functional Foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Lips, A. Comparison of Solid Particles, Globular Proteins and Surfactants as Emulsifiers. Phys. Chem. Chem. Phys. 2008, 10, 1608. [Google Scholar] [CrossRef]
- Sanfeld, A.; Steinchen, A. Emulsions Stability, from Dilute to Dense Emulsions—Role of Drops Deformation. Adv. Colloid. Interface Sci. 2008, 140, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Excipient Foods: Designing Food Matrices That Improve the Oral Bioavailability of Pharmaceuticals and Nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüdtke, F.L.; Silva, T.J.; da Silva, M.G.; Hashimoto, J.C.; Ribeiro, A.P.B. Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols. Foods 2025, 14, 973. https://doi.org/10.3390/foods14060973
Lüdtke FL, Silva TJ, da Silva MG, Hashimoto JC, Ribeiro APB. Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols. Foods. 2025; 14(6):973. https://doi.org/10.3390/foods14060973
Chicago/Turabian StyleLüdtke, Fernanda L., Thaís Jordânia Silva, Mayanny Gomes da Silva, Juliana Campos Hashimoto, and Ana Paula B. Ribeiro. 2025. "Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols" Foods 14, no. 6: 973. https://doi.org/10.3390/foods14060973
APA StyleLüdtke, F. L., Silva, T. J., da Silva, M. G., Hashimoto, J. C., & Ribeiro, A. P. B. (2025). Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols. Foods, 14(6), 973. https://doi.org/10.3390/foods14060973





