Impact of a Limosilactobacillus fermentum, Quercetin, and Resveratrol Nutraceutical on Fecal Microbiota Composition and Metabolic Activity in Healthy and Hypertensive Subjects
Abstract
1. Introduction
2. Methods
2.1. Microorganisms
2.2. Preparation of Nutraceutical with L. Fermentum, Quercetin, and Resveratrol
2.3. Simulated Gastrointestinal Digestion of the Nutraceutical
2.4. Preparation of Human Fecal Inoculum
2.5. In Vitro Fecal Fermentation of the Nutraceutical
2.6. Identification and Quantification of Bacterial Groups During Fecal Fermentation
2.7. Evaluation of the Metabolic Activity of the Intestinal Microbiota
2.8. Statistical Analysis
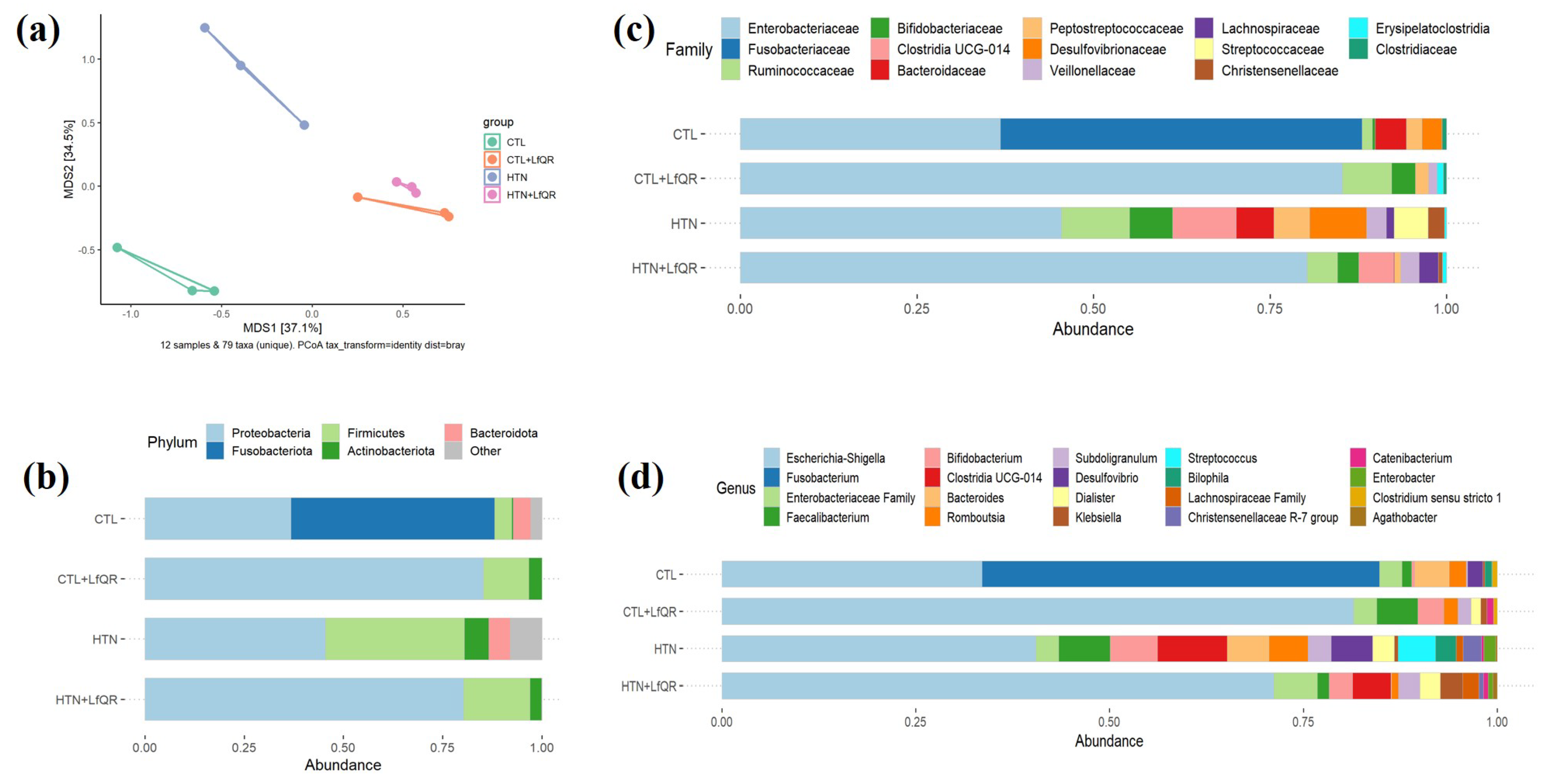
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Brito Alves, J.L.; Costa-Silva, J.H. Maternal protein malnutrition induced-hypertension: New evidence about the autonomic and respiratory dysfunctions and epigenetic mechanisms. Clin. Exp. Pharmacol. Physiol. 2018, 45, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Q.; Liu, Y.; Wang, L.; Ge, Z.; Li, Z.; Feng, S.; Wu, C. Gut microbiota and hypertension: Association, mechanisms and treatment. Clin. Exp. Hypertens. 2023, 45, 2195135. [Google Scholar] [CrossRef]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin. Sci. 2018, 132, 701–718. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Qi, Y.; Zubcevic, J.; Kim, S.; Yang, T.; Shenoy, V.; Cole-Jeffrey, C.T.; Lobaton, G.O.; Stewart, D.C.; Rubiano, A.; et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ. Res. 2017, 120, 312–323. [Google Scholar] [CrossRef]
- de Brito Alves, J.L.; de Sousa, V.P.; Cavalcanti Neto, M.P.; Magnani, M.; Braga, V.A.; da Costa-Silva, J.H.; Leandro, C.G.; Vidal, H.; Pirola, L. New Insights on the Use of Dietary Polyphenols or Probiotics for the Management of Arterial Hypertension. Front. Physiol. 2016, 7, 448. [Google Scholar] [CrossRef]
- Cavalcanti Neto, M.P.; Aquino, J.S.; Romao da Silva, L.F.; de Oliveira Silva, R.; Guimaraes, K.S.L.; de Oliveira, Y.; de Souza, E.L.; Magnani, M.; Vidal, H.; de Brito Alves, J.L. Gut microbiota and probiotics intervention: A potential therapeutic target for management of cardiometabolic disorders and chronic kidney disease? Pharmacol. Res. 2018, 130, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.M.; Li, J.; Stevens, B.R.; Pepine, C.J.; Raizada, M.K. Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 2022, 130, 401–417. [Google Scholar] [CrossRef]
- Costa, P.C.T.; de Luna Freire, M.O.; de Oliveira Coutinho, D.; Godet, M.; Magnani, M.; Antunes, V.R.; de Souza, E.L.; Vidal, H.; de Brito Alves, J.L. Nutraceuticals in the management of autonomic function and related disorders: A comprehensive review. Pharmacol. Res. 2024, 208, 107368. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Costa, P.; de Souza, E.L.; Lacerda, D.C.; Cruz Neto, J.P.R.; Sales, L.C.S.; Silva Luis, C.C.; Pontes, P.B.; Cavalcanti Neto, M.P.; de Brito Alves, J.L. Evidence for Quercetin as a Dietary Supplement for the Treatment of Cardio-Metabolic Diseases in Pregnancy: A Review in Rodent Models. Foods 2022, 11, 2772. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chang, W.T.; Shiau, D.K.; Chou, P.H.; Wu, M.F.; Hsu, C.L. Antiobesity Efficacy of Quercetin-Rich Supplement on Diet-Induced Obese Rats: Effects on Body Composition, Serum Lipid Profile, and Gene Expression. J. Agric. Food Chem. 2018, 66, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules 2022, 12, 1871. [Google Scholar] [CrossRef]
- de Luna Freire, M.O.; do Nascimento, L.C.P.; de Oliveira, K.A.R.; de Oliveira, A.M.; Napoleao, T.H.; Lima, M.D.S.; Lagranha, C.J.; de Souza, E.L.; de Brito Alves, J.L. Effects of a Mixed Limosilactobacillus fermentum Formulation with Claimed Probiotic Properties on Cardiometabolic Variables, Biomarkers of Inflammation and Oxidative Stress in Male Rats Fed a High-Fat Diet. Foods 2021, 10, 2202. [Google Scholar] [CrossRef]
- Maia, L.A.; de Souza, J.R.; da Silva, L.F.R.; Magnani, M.; de Souza, E.L.; de Brito Alves, J.L. Effects of Probiotics on Inflammatory Biomarkers and Its Associations With Cardiac Autonomic Function in Women With Arterial Hypertension: A Secondary Analysis of a Randomized Clinical Trial. Probiotics Antimicrob. Proteins 2024, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, F.P.B.; de Luna Freire, M.O.; de Oliveira Coutinho, D.; de Santana Cirilo, M.A.; de Brito Alves, J.L. Gut Dysbiosis and Probiotic Therapy in Chronic Kidney Disease: A Comprehensive Review. Probiotics Antimicrob. Proteins 2024, 1–23. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Albuquerque, T.M.R.; Dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- de Luna Freire, M.O.; Cruz Neto, J.P.R.; de Albuquerque Lemos, D.E.; de Albuquerque, T.M.R.; Garcia, E.F.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum Strains as Novel Probiotic Candidates to Promote Host Health Benefits and Development of Biotherapeutics: A Comprehensive Review. Probiotics Antimicrob. Proteins 2024, 16, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Plamada, D.; Vodnar, D.C. Polyphnols-Gut Microbiota Interrelationshipe: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, K.B.; do Nascimento, Y.M.; Tavares, J.F.; Cavalcanti, M.T.; de Brito Alves, J.L.; Garcia, E.F.; de Souza, E.L. Development and in vitro evaluation of novel nutraceutical formulations composed of Limosilactobacillus fermentum, quercetin and/or resveratrol. Food Chem. 2021, 342, 128264. [Google Scholar] [CrossRef]
- Brito Sampaio, K.; Luiz de Brito Alves, J.; Mangueira do Nascimento, Y.; Fechine Tavares, J.; Sobral da Silva, M.; Dos Santos Nascimento, D.; Dos Santos Lima, M.; Priscila de Araujo Rodrigues, N.; Fernandes Garcia, E.; Leite de Souza, E. Nutraceutical formulations combining Limosilactobacillus fermentum, quercetin, and or resveratrol with beneficial impacts on the abundance of intestinal bacterial populations, metabolite production, and antioxidant capacity during colonic fermentation. Food Res. Int. 2022, 161, 111800. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- de Araujo Henriques Ferreira, G.; Magnani, M.; Cabral, L.; Brandao, L.R.; Noronha, M.F.; de Campos Cruz, J.; de Souza, E.L.; de Brito Alves, J.L. Potentially Probiotic Limosilactobacillus fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins 2022, 14, 349–359. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Huus, K.E.; Youngblut, N.D.; Escobar, J.S.; Ley, R.E. Obesity is the main driver of altered gut microbiome functions in the metabolically unhealthy. Gut Microbes 2023, 15, 2246634. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.S.; Ramphall, S.; Rijal, S.; Prakash, V.; Ekladios, H.; Mulayamkuzhiyil Saju, J.; Mandal, N.; Kham, N.I.; Shahid, R.; Venugopal, S. Association of Gut Microbial Dysbiosis and Hypertension: A Systematic Review. Cureus 2022, 14, e29927. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamashita, T.; Watanabe, H.; Kami, K.; Yoshida, N.; Tabata, T.; Emoto, T.; Sasaki, N.; Mizoguchi, T.; Irino, Y.; et al. Gut Microbiome and Plasma Microbiome-Related Metabolites in Patients With Decompensated and Compensated Heart Failure. Circ. J. 2018, 83, 182–192. [Google Scholar] [CrossRef]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Lan, Y.; Ning, K.; Ma, Y.; Zhao, J.; Ci, C.; Yang, X.; An, F.; Zhang, Z.; An, Y.; Cheng, M. High-Density Lipoprotein Cholesterol as a Potential Medium between Depletion of Lachnospiraceae Genera and Hypertension under a High-Calorie Diet. Microbiol. Spectr. 2022, 10, e0234922. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A Six-Day, Lifestyle-Based Immersion Program Mitigates Cardiovascular Risk Factors and Induces Shifts in Gut Microbiota, Specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium prausnitzii: A Pilot Study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Xu, X.D.; Tang, Y.Y.; Jiang, H.L.; Li, L.; Xia, W.J.; Cui, N.; Bai, J.; Dai, Z.M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, e120–e134. [Google Scholar] [CrossRef]
- Robles-Vera, I.; de la Visitacion, N.; Toral, M.; Sanchez, M.; Romero, M.; Gomez-Guzman, M.; Yang, T.; Izquierdo-Garcia, J.L.; Guerra-Hernandez, E.; Ruiz-Cabello, J.; et al. Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. FASEB J. 2020, 34, 13626–13640. [Google Scholar] [CrossRef]
- Machado, A.S.; Oliveira, J.R.; Lelis, D.F.; de Paula, A.M.B.; Guimaraes, A.L.S.; Andrade, J.M.O.; Brandi, I.V.; Santos, S.H.S. Oral Probiotic Bifidobacterium Longum Supplementation Improves Metabolic Parameters and Alters the Expression of the Renin-Angiotensin System in Obese Mice Liver. Biol. Res. Nurs. 2021, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhao, J.; Wang, Y.; Bai, M.; Sun, S. Specific Alterations of Gut Microbiota in Chinese Patients with Hypertension: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2022, 47, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.J. Functional genomics of the lactic acid bacterium Limosilactobacillus fermentum LAB-1: Metabolic, probiotic and biotechnological perspectives. Heliyon 2022, 8, e11412. [Google Scholar] [CrossRef]
- Okada, T.; Fukuda, S.; Hase, K.; Nishiumi, S.; Izumi, Y.; Yoshida, M.; Hagiwara, T.; Kawashima, R.; Yamazaki, M.; Oshio, T.; et al. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat. Commun. 2013, 4, 1654. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Andres, S.; Snelling, T.J.; Lopez-Ferreras, L.; Yanez-Ruiz, D.R.; Garcia-Estrada, C.; Lopez, S. Effect of Sunflower and Marine Oils on Ruminal Microbiota, In vitro Fermentation and Digesta Fatty Acid Profile. Front. Microbiol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
| Donor | Sex | Age (Years) | Antibiotic Use (Last Six Months) | Probiotic/Prebiotic/Symbiotic Use (Last Six Months) | Number of Antihypertensive (Daily Use) | |
|---|---|---|---|---|---|---|
| 1 | Male | 37 | No | No | - | |
| Healthy donors | 2 | Male | 29 | No | No | - |
| 3 | Male | 28 | No | No | - | |
| 4 | Female | 28 | No | No | - | |
| 5 | Female | 42 | No | No | - | |
| 6 | Female | 25 | No | No | - | |
| 7 | Male | 37 | No | No | 1 | |
| Hypertensive donors | 8 | Male | 36 | No | No | 2 |
| 9 | Male | 42 | No | No | 2 | |
| 10 | Female | 54 | No | No | 1 | |
| 11 | Female | 53 | No | No | 1 | |
| 12 | Female | 47 | No | No | 1 |
| Paremeters | CTL | CTL + LfQR | HTN | HTN + LfQR | F Value | p-Value |
|---|---|---|---|---|---|---|
| Sugars | ||||||
| Maltose | 0.00 ± 0.00 | 0.93 ± 0.00 #α | 0.00 ± 0.00 | 0.66 ± 0.03 * | 2228.25 | <0.001 |
| Glucose | 0.00 ± 0.00 | 0.75 ± 0.00 #α | 0.00 ± 0.00 | 0.54 ± 0.05 * | 722.52 | <0.001 |
| Fructose | 0.04 ± 0.03 | 3.41 ± 0.02 #α | 0.00 ± 0.00 | 3.65 ± 0.03 * | 17,264.83 | <0.001 |
| Rhaminose | 0.11 ± 0.05 | 0.00 ± 0.00 # | 0.09 ± 0.02 | 0.00 ± 0.00 * | 11.32 | <0.001 |
| Organic acids | ||||||
| Lactic acid | 0.00 ± 0.00 | 1.70 ± 0.01 #α | 0.00 ± 0.00 | 1.75 ± 0.02 * | 35,593.64 | <0.001 |
| Acetic acid | 0.32 ± 0.01 * | 0.65 ± 0.00 #α | 0.37 ± 0.01 | 0.67 ± 0.00 * | 2371.93 | <0.001 |
| Propionic acid | 0.05 ± 0.09 * | 0.04 ± 0.08 | 0.45 ± 0.05 | 0.21 ± 0.00 * | 26.49 | <0.001 |
| Butyric acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.06 ± 0.10 | 0.00 ± 0.00 | 1.00 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brasil, J.M.A.; Melo, N.C.d.O.; Sampaio, K.B.; Costa, P.C.T.d.; Duman, H.; Karav, S.; Lima, M.d.S.; de Souza, E.L.; Alves, J.L.d.B. Impact of a Limosilactobacillus fermentum, Quercetin, and Resveratrol Nutraceutical on Fecal Microbiota Composition and Metabolic Activity in Healthy and Hypertensive Subjects. Foods 2025, 14, 986. https://doi.org/10.3390/foods14060986
Brasil JMA, Melo NCdO, Sampaio KB, Costa PCTd, Duman H, Karav S, Lima MdS, de Souza EL, Alves JLdB. Impact of a Limosilactobacillus fermentum, Quercetin, and Resveratrol Nutraceutical on Fecal Microbiota Composition and Metabolic Activity in Healthy and Hypertensive Subjects. Foods. 2025; 14(6):986. https://doi.org/10.3390/foods14060986
Chicago/Turabian StyleBrasil, Jéssica Maria Alves, Nathalia Caroline de Oliveira Melo, Karoliny Brito Sampaio, Paulo César Trindade da Costa, Hatice Duman, Sercan Karav, Marcos dos Santos Lima, Evandro Leite de Souza, and José Luiz de Brito Alves. 2025. "Impact of a Limosilactobacillus fermentum, Quercetin, and Resveratrol Nutraceutical on Fecal Microbiota Composition and Metabolic Activity in Healthy and Hypertensive Subjects" Foods 14, no. 6: 986. https://doi.org/10.3390/foods14060986
APA StyleBrasil, J. M. A., Melo, N. C. d. O., Sampaio, K. B., Costa, P. C. T. d., Duman, H., Karav, S., Lima, M. d. S., de Souza, E. L., & Alves, J. L. d. B. (2025). Impact of a Limosilactobacillus fermentum, Quercetin, and Resveratrol Nutraceutical on Fecal Microbiota Composition and Metabolic Activity in Healthy and Hypertensive Subjects. Foods, 14(6), 986. https://doi.org/10.3390/foods14060986








