Effects of Mountain-Basin System on Chemical Composition, Antioxidant Activity and Volatile Flavor Substances of Cabernet Sauvignon Wines in Xinjiang Region, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Method
2.2. Wine Fermentation
2.3. Determination of Meteorological Indexes and Physiochemical Properties
2.4. Determination of Organic Acids
2.5. Determination of Phenolic Compounds
2.6. Determination of Antioxidant Activities in Vitro
2.7. Determination of Volatile Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Differences in Meteorological Indicators in Different Regions
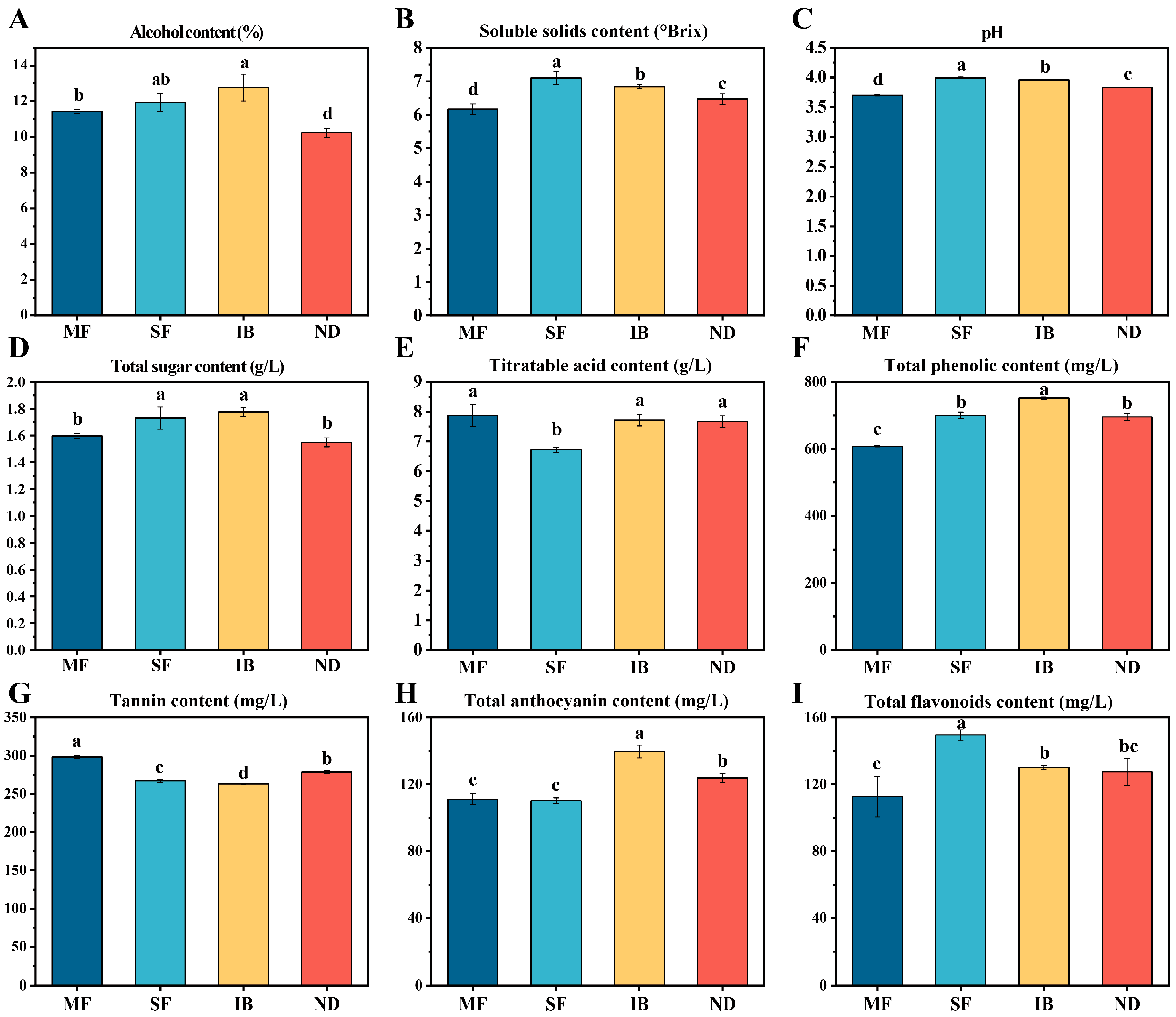
3.2. Differences in Physicochemical Properties of Wine from Different Regions
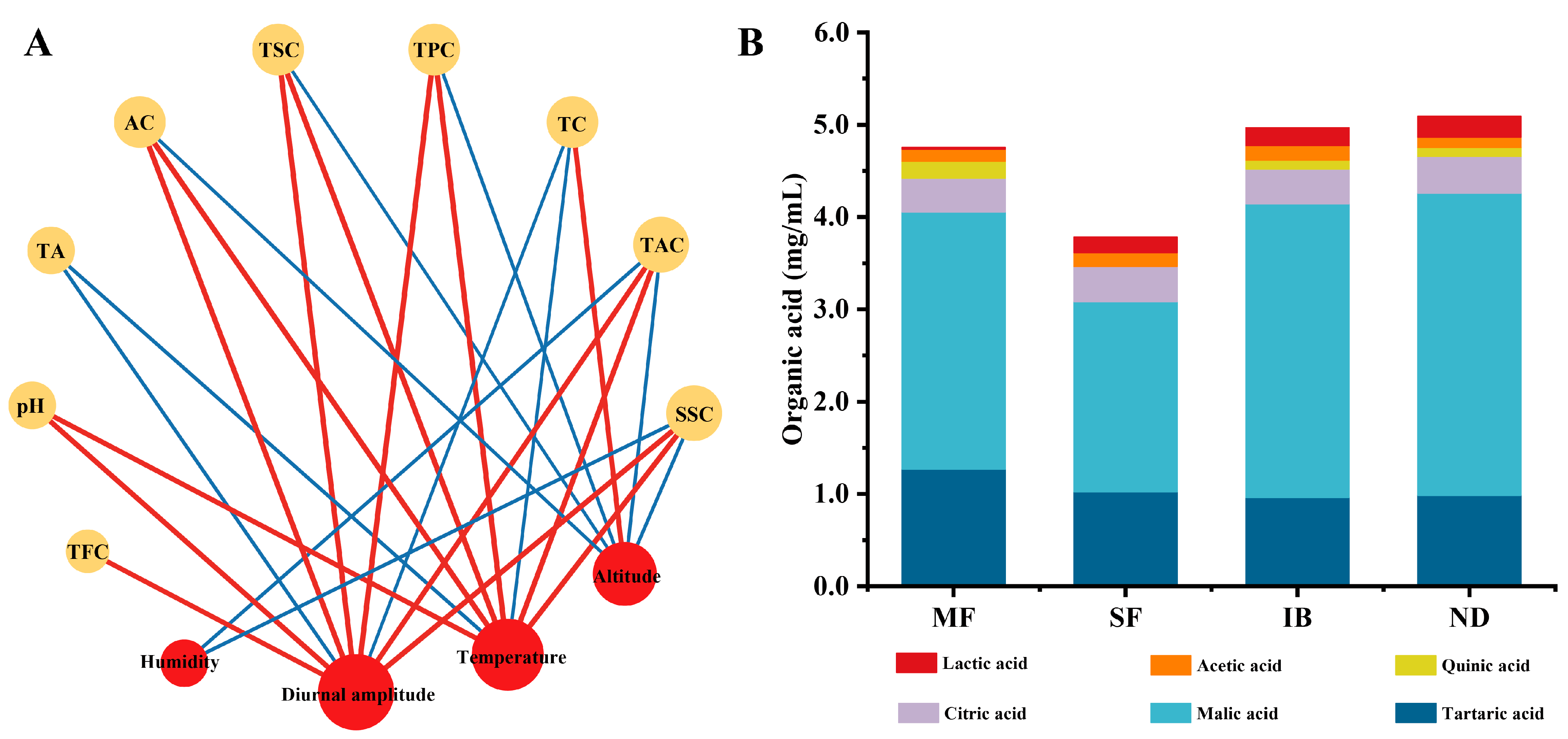
3.3. Correlation Analysis of Environmental Factors and Physicochemical Properties
3.4. Organic Acids
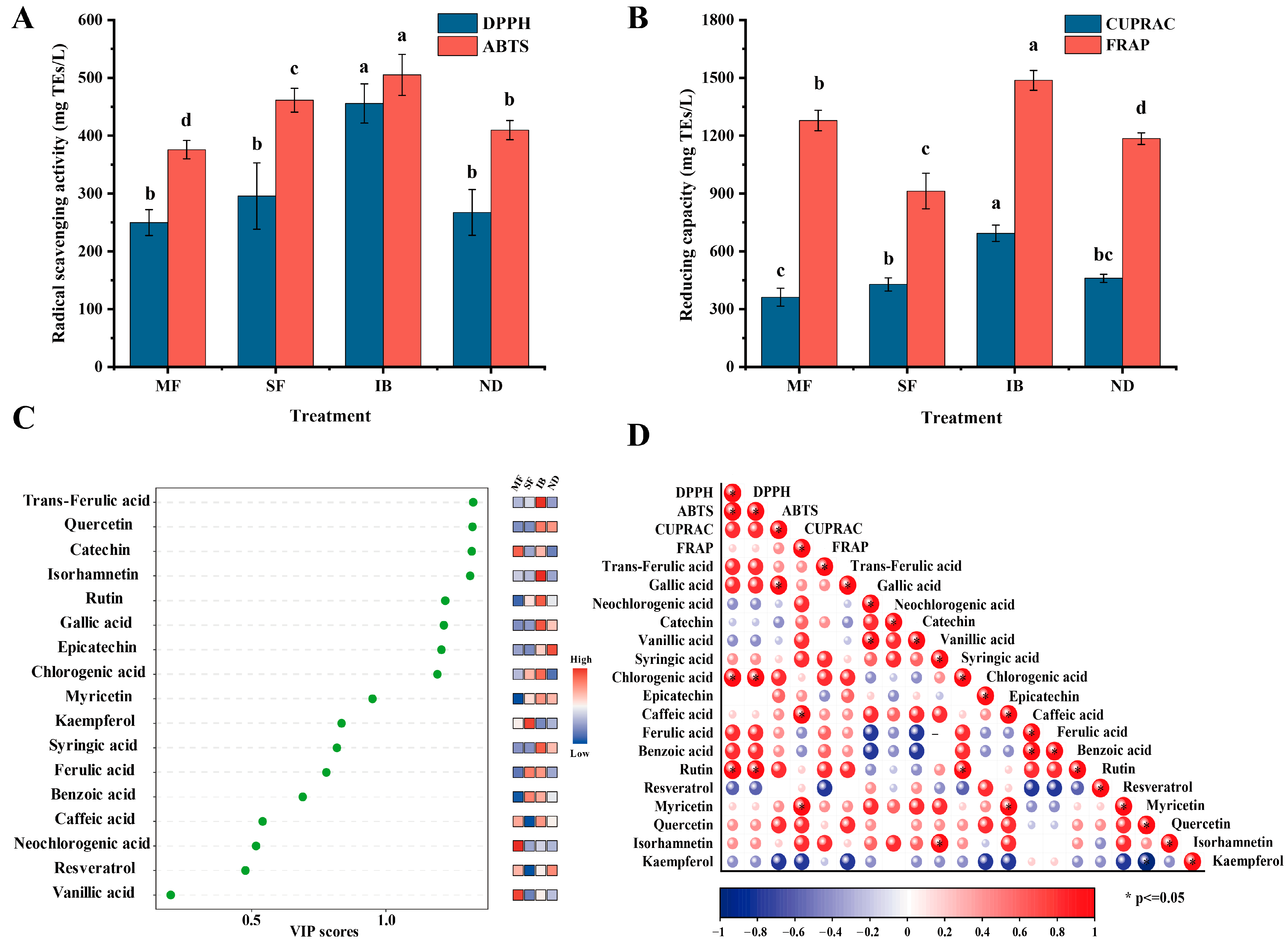
3.5. Analysis of Monomeric Phenol Content and Antioxidant Activity in Different Regions
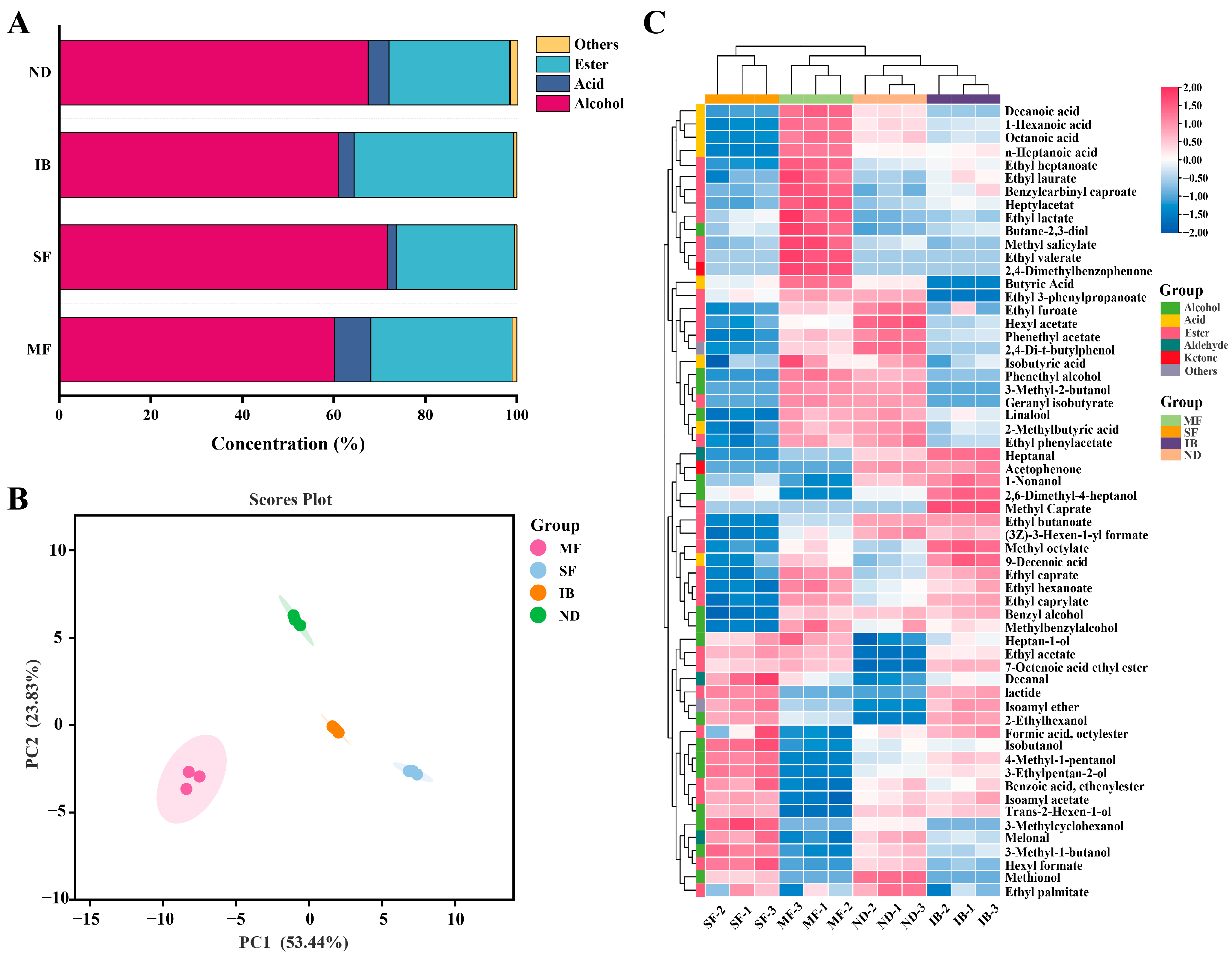
3.6. Differences in Volatile Compounds of Wines from Different Regions
3.7. Analysis of Characteristic Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tai, X.; Epstein, H.E.; Li, B. Elevation and Climate Effects on Vegetation Greenness in an Arid Mountain-Basin System of Central Asia. Remote Sens. 2020, 12, 1665. [Google Scholar] [CrossRef]
- Lidstone, J. The Geography of Wine: Regions, Terroir and Techniques. J. Geogr. 2014, 113, 259–260. [Google Scholar] [CrossRef]
- Qin, L.; Xie, H.; Xiang, N.; Wang, M.; Han, S.; Pan, M.; Guo, X.; Zhang, W. Dynamic Changes in Anthocyanin Accumulation and Cellular Antioxidant Activities in Two Varieties of Grape Berries during Fruit Maturation under Different Climates. Molecules 2022, 27, 384. [Google Scholar] [CrossRef]
- Esteves, M.A.; Orgaz, M. The influence of climatic variability on the quality of wine. Int. J. Biometeorol. 2001, 45, 13–21. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2014, 65, 821–832. [Google Scholar] [CrossRef]
- Balint, G.; Reynolds, A.G. Irrigation Strategies Impact Baco noir Grapevines in Ontario. II. Fruit Composition and Wine Sensory Analysis. Am. J. Enol. Vitic. 2017, 68, 310–324. [Google Scholar] [CrossRef]
- Rastija, V.; Srecnik, G.; Marica-Medic-Saric. Polyphenolic composition of Croatian wines with different geographical origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Nicholas, K.A.; Matthews, M.A.; Lobell, D.B.; Willits, N.H.; Field, C.B. Effect of vineyard-scale climate variability on Pinot noir phenolic composition. Agric. For. Meteorol. 2011, 151, 1556–1567. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Molecules 2012, 17, 8804–8821. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Bowers, J.E.; Meredith, C.P. The parentage of a classic wine grape, Cabernet Sauvignon. Nat. Genet. 1997, 16, 84–87. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, J.; Zhang, F.; Liu, D.; Cheng, W.; Gao, F.; Wang, P. Effect of postharvest grape dehydration on chemical composition, antioxidant activity and sensory characeteristics of Marselan wines. Food Chem. X 2024, 22, 101503. [Google Scholar] [CrossRef] [PubMed]
- Kritsunankul, O.; Pramote, B.; Jakmunee, J. Flow injection on-line dialysis coupled to high performance liquid chromatography for the determination of some organic acids in wine. Talanta 2009, 79, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. Aoac Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Mitic, M.N.; Obradovic, M.V.; Grahovac, Z.B.; Pavlovic, A.N. Antioxidant Capacities and Phenolic Levels of Different Varieties of Serbian White Wines. Molecules 2010, 15, 2016–2027. [Google Scholar] [CrossRef]
- Akalin, A.C.; Bayram, M.; Anli, R.E. Antioxidant phenolic compounds of pomegranate wines produced by different maceration methods. J. Inst. Brew. 2018, 124, 38–44. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Kerrihard, A.L.; Pegg, R.B. Characterization of the Volatile Compounds in Raw and Roasted Georgia Pecans by HS-SPME-GC-MS. J. Food Sci. 2018, 83, 2753–2760. [Google Scholar] [CrossRef]
- Xu, X.; Xu, R.; Jia, Q.; Feng, T.; Huang, Q.; Ho, C.; Song, S. Identification of dihydro-β-ionone as a key aroma compound in addition to C8 ketones and alcohols in Volvariella volvacea mushroom. Food Chem. 2019, 293, 333–339. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, J.; Yin, H.; Wang, J.; Wang, X.; Yang, Y.; Xi, Z. Microclimate diversity drives grape quality difference at high-altitude: Observation using PCA analysis and structural equation modeling (SEM). Food Res. Int. 2024, 191, 114644. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.B.; Egipto, R.; Laureano, O.; De Castro, R.; Pereira, G.E.; Ricardo-Da-Silva, J.M. Climate effects on physicochemical composition of Syrah grapes at low and high altitude sites from tropical grown regions of Brazil. Food Res. Int. 2019, 121, 870–879. [Google Scholar] [CrossRef]
- Koerner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Plotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. Trac-Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Geros, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Gao, F.; Guan, L.; Zeng, G.; Hao, X.; Li, H.; Wang, H. Preliminary characterization of chemical and sensory attributes for grapes and wines of different cultivars from the Weibei Plateau region in China. Food Chem. X 2024, 21, 101091. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Lapczynski, A.; Scognamiglio, J.; Letizia, C.S.; Api, A.M. Fragrance materials review on isoamyl alcohol. Food Chem. Toxicol. 2010, 48, S102–S109. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ling, M.; Li, D.; Zhu, B.; Shi, Y.; Duan, C.; Lan, Y. Volatile Profiles and Sensory Characteristics of Cabernet Sauvignon Dry Red Wines in the Sub-Regions of the Eastern Foothills of Ningxia Helan Mountain in China. Molecules 2022, 27, 8817. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Lan, Y.; Cui, Y.; Shi, T.; Hua, Y.; Duan, C.; Pan, Q. Differences in Aroma Profile of Cabernet Sauvignon Grapes and Wines from Four Plots in Jieshi Mountain Region of Eastern China. Foods 2023, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M.; Chen, K.; Zhang, J.; Xue, T.; Cheng, Z.; Zhang, B.; Zhang, K.; Fang, Y. The roles of different photoselective nets in the targeted regulation of metabolite accumulation, wine aroma and sensory profiles in warm viticulture regions. Food Chem. 2022, 396, 133629. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, G.; Duan, L.; Jiang, R.; Pan, Q.; Duan, C.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef]
- Giannattasio, S.; Guaragnella, N.; Zdralevic, M.; Marra, E. Molecular mechanisms of Saccharomyces cereyisiae stress adaptation and programmed cell death in response to acetic acid. Front. Microbiol. 2013, 4, 33. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Saito, T.; Tomiyama, H.; Ishioka, M.; Hashimoto, N.; Thunyamada, S.; Ohkawa, K.; Ohara, H.; Ikeura, H.; Kondo, S. Retar-dation of Endogenous ABA Synthesis by NDGA in Leaves Affects Anthocyanin, Sugar, and Aroma Volatile Concentrations in ‘Kyoho’ Grape Berries. Hortic. J. 2022, 91, 186–194. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascues, E.; Calderon, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media (Second Enlarged and Revised Edition); Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011; ISBN 978-7-03-058320-8. [Google Scholar]
- Xie, Y.; Jiang, J.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Yin, R.; Ma, X.; Niu, J.; Cheng, W. Influence of different geographical origins and bottle storage times on the chemical composition, antioxidant activity and sensory properties of Cabernet Sauvignon wine. Food Chem. X 2024, 24, 101978. [Google Scholar] [CrossRef]

| Characteristics | MF | SF | IB | ND |
|---|---|---|---|---|
| Temperature (°C) | 18.45 ± 0.20 b | 17.27 ± 0.11 d | 17.57 ± 0.26 c | 18.81 ± 0.01 a |
| Humidity (%) | 68.99 ± 2.31 a | 67.79 ± 1.43 b | 67.21 ± 0.82 c | 66.69 ± 0.89 d |
| Diurnal amplitude (°C) | 19.32 ± 0.16 c | 18.86 ± 0.12 d | 19.88 ± 0.23 a | 19.67 ± 0.1 b |
| Altitude (m) | 617 | 492 | 384 | 296 |
| Volatile Compounds | RI | Threshold (µg/L) | MF | SF | IB | ND | Flavor Description |
|---|---|---|---|---|---|---|---|
| Ethyl butanoate | 1021 | 20 | 3.48 | 0 | 27.06 | 24.22 | Apple, pineapple |
| Isoamyl acetate | 1115 | 30 | 188.09 | 278.63 | 268.63 | 256.29 | Banana, pear |
| Ethyl hexanoate | 1238 | 5 | 482.69 | 281.11 | 431.33 | 374.73 | Pineapple, banana |
| Ethyl caprylate | 1440 | 580 | 891.07 | 565.2 | 872.37 | 731.68 | Brandy |
| Decanal | 1496 | 0.1 | 6.2 | 8.14 | 6.15 | 4.79 | Citrus peel |
| Ethyl caprate | 1625 | 122 | 161.01 | 78.27 | 149.9 | 104.16 | Pear, brandy |
| Methionol | 1715 | 8 | 0 | 3.62 | 0 | 11.33 | Onion, meat stink |
| Methyl salicylate | 1730 | 40 | 43.54 | 19.24 | 19.21 | 21.92 | Medicinal herb |
| Phenethyl acetate | 1804 | 98 | 105.64 | 64.87 | 84.93 | 125.73 | Rose, honey |
| Phenethyl alcohol | 1920 | 1000 | 1881.52 | 667.07 | 820.54 | 1508.46 | Rose |
| 9-Decenoic acid | 2305 | 4.3 | 87.77 | 70.06 | 101.7 | 76.84 | Fatty, wax fragrance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Qi, Y.; Han, R.; Cui, M.; Gao, F.; Wang, P.; Sun, Q. Effects of Mountain-Basin System on Chemical Composition, Antioxidant Activity and Volatile Flavor Substances of Cabernet Sauvignon Wines in Xinjiang Region, China. Foods 2025, 14, 1086. https://doi.org/10.3390/foods14071086
Zhang J, Qi Y, Han R, Cui M, Gao F, Wang P, Sun Q. Effects of Mountain-Basin System on Chemical Composition, Antioxidant Activity and Volatile Flavor Substances of Cabernet Sauvignon Wines in Xinjiang Region, China. Foods. 2025; 14(7):1086. https://doi.org/10.3390/foods14071086
Chicago/Turabian StyleZhang, Junbo, Yapeng Qi, Ruiyang Han, Miao Cui, Feifei Gao, Ping Wang, and Qinming Sun. 2025. "Effects of Mountain-Basin System on Chemical Composition, Antioxidant Activity and Volatile Flavor Substances of Cabernet Sauvignon Wines in Xinjiang Region, China" Foods 14, no. 7: 1086. https://doi.org/10.3390/foods14071086
APA StyleZhang, J., Qi, Y., Han, R., Cui, M., Gao, F., Wang, P., & Sun, Q. (2025). Effects of Mountain-Basin System on Chemical Composition, Antioxidant Activity and Volatile Flavor Substances of Cabernet Sauvignon Wines in Xinjiang Region, China. Foods, 14(7), 1086. https://doi.org/10.3390/foods14071086






