Integrated Metabolomic and Microbial Analysis of Quality Dynamics in Channel Catfish (Ictalurus punctatus) Under Refrigerated and Frozen Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sensory Evaluation
2.3. Measurement of pH
2.4. Measurement of TVB-N
2.5. Measurement of TBARS
2.6. Microbiota Analysis
2.6.1. DNA Extraction and Sequencing
2.6.2. Sequencing Data Analysis
2.7. Untargeted Metabolomics Analysis
2.7.1. Metabolite Extraction
2.7.2. UHPLC-MS/MS Analysis
2.7.3. Metabolite Analysis and Identification
2.8. Statistical Analysis
3. Results and Discussions
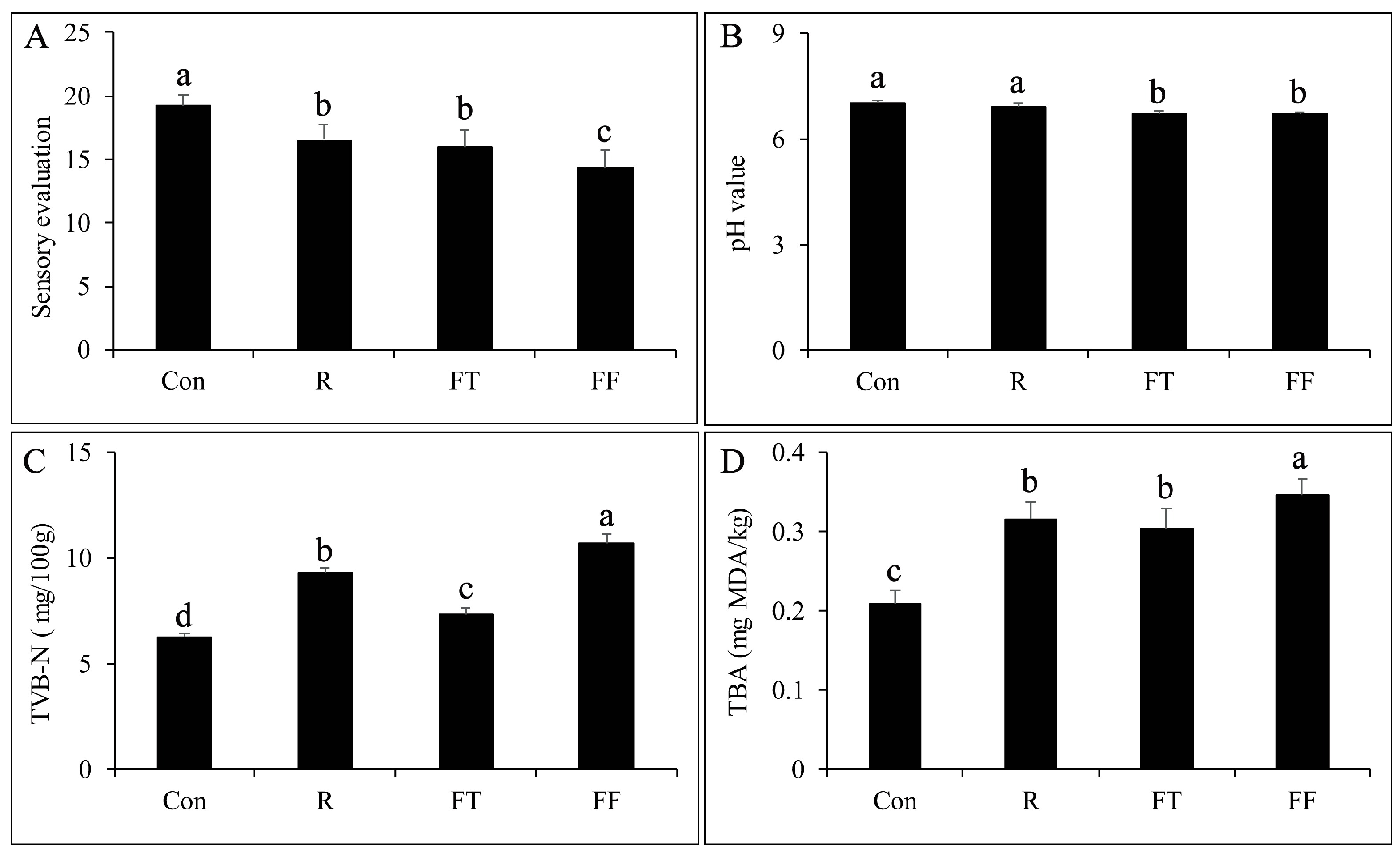
3.1. Sensory Evaluation and pH Analysis
3.2. TVB N and TBARS Analysis
3.3. Microbiome Analysis
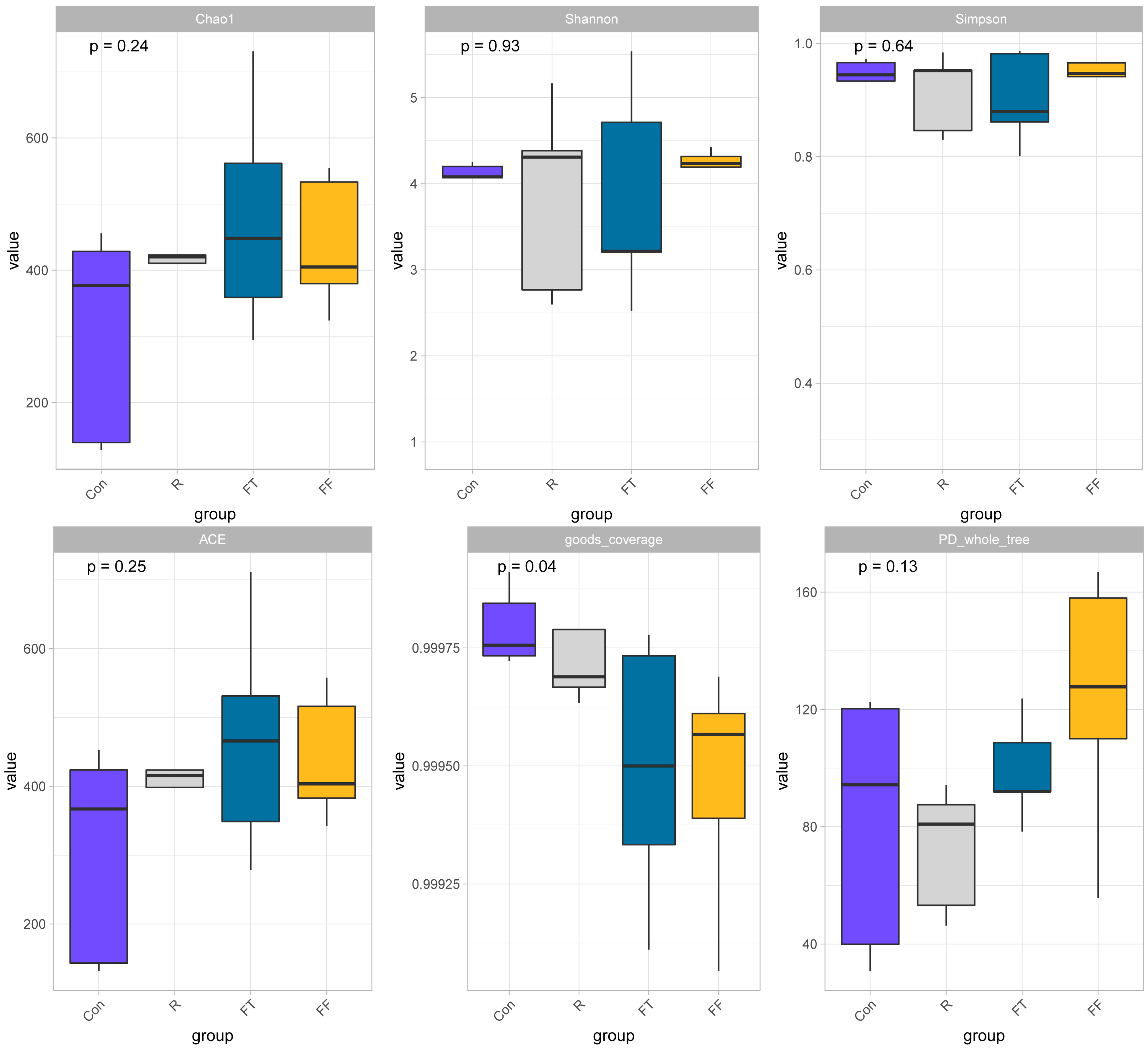
3.3.1. Microbial Diversity Analysis
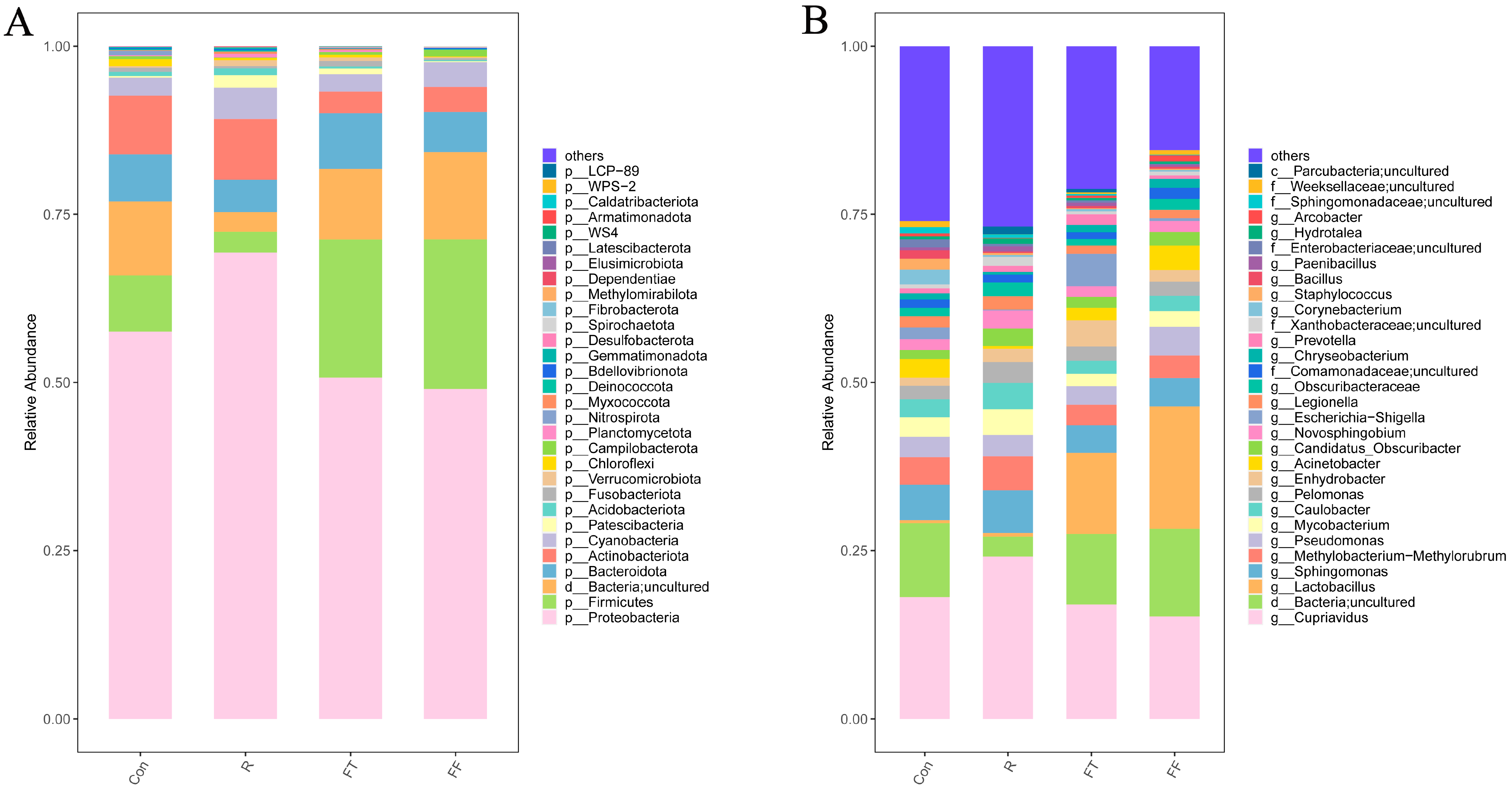
3.3.2. Changes in Microbial Community Composition
3.4. Metabolomic Analysis
3.4.1. Differential Metabolites Analysis
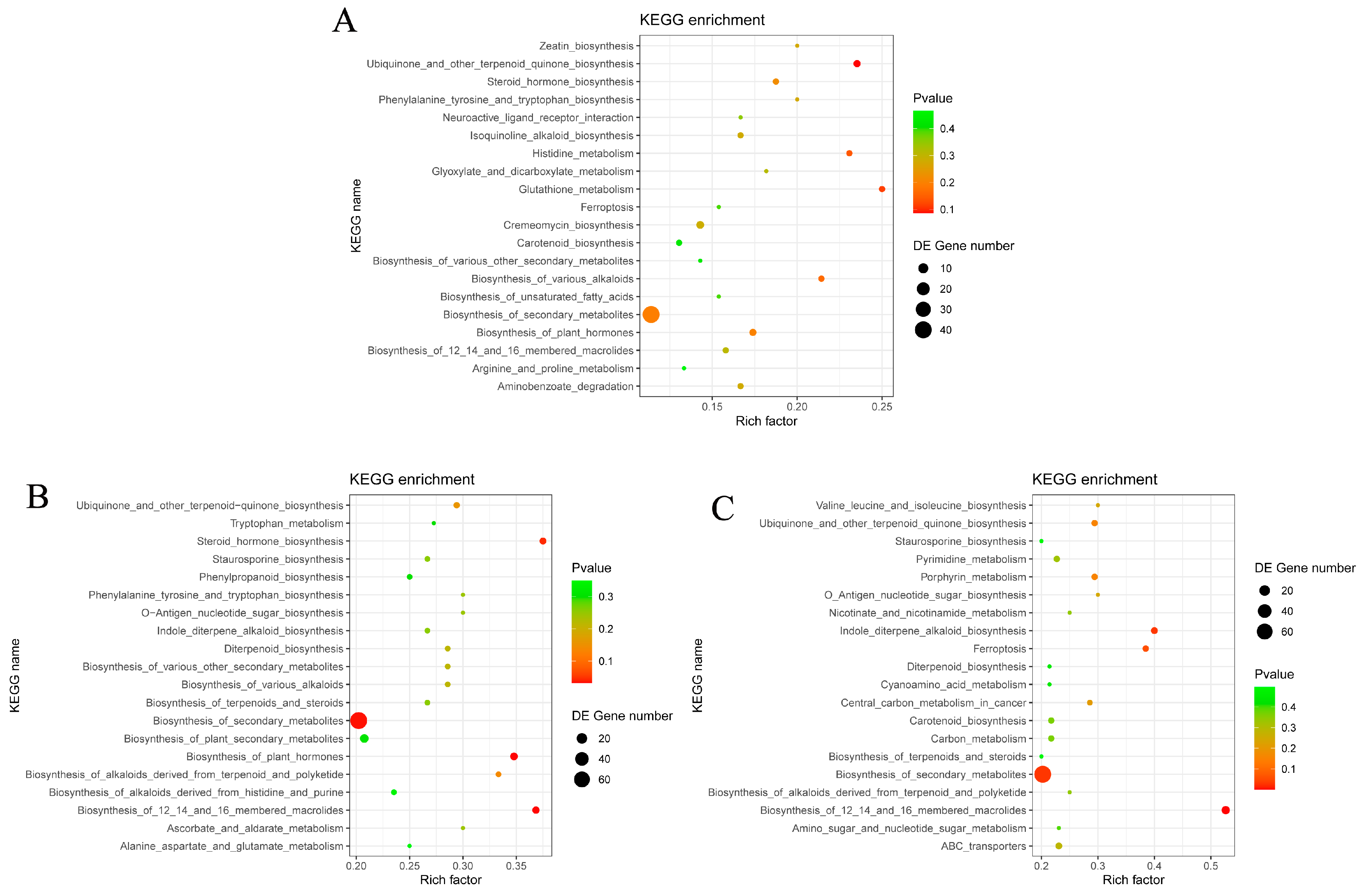
3.4.2. Metabolic Pathway Analysis
3.5. Correlations Between Major Metabolites and Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TVB-N | Total volatile basic nitrogen |
| TBARS | Thiobarbituric acid reactive substances |
| TCA | Trichloroacetic acid |
| MDA | Malonaldehyde |
| QC | Quality control |
| GS I | Ion source gas I |
| GS II | Ion source gas II |
| CUR | Curtain gas |
| CAD | Collision-activated dissociation |
| QTRAP | Triple quadrupole |
| DP | Declustering potential |
| CE | Collision energy |
| VIP | Variable importance in projection |
| RSD | Relative standard deviation |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SD | Standard deviation |
References
- Haubrock, P.J.; Copp, G.H.; Johovic, I.; Balzani, P.; Inghilesi, A.F.; Nocita, A.; Tricarico, E. North american channel catfish, Ictalurus punctatus: A neglected but potentially invasive freshwater fish species? Biol. Invasions 2021, 23, 1563–1576. [Google Scholar] [CrossRef]
- Yin, H.X.; Wan, Y.T.; Pu, J.N.; Bechtel, P.J.; Sathivel, S. Functional properties of protein fractions of channel catfish (Ictalurus punctatus) and their effects in an emulsion system. J. Food Sci. 2011, 76, E283–E290. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, C.; He, X.; Qi, C.; Gao, Y.; Liang, X. Channel catfish culture. In Aquaculture in China; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 393–403. [Google Scholar]
- Chu, Y.; Ding, Z.; Wang, J.; Xie, J.; Ding, Y. Factors affecting the quality of frozen large yellow croaker (Pseudosciaena crocea) in cold chain logistics: Retention time and temperature fluctuation. Food Chem. X 2023, 18, 100742. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Guo, M.J.; Chen, B.H.; Zhang, C.; Li, D.P.; Xie, J. Molecular characterization of spoilage microbiota in high CO2 refrigerated large yellow croaker (Larimichthys crocea) fillets using metagenomic and metabolomic approaches. Food Biosci. 2023, 56, 103227. [Google Scholar] [CrossRef]
- Shi, L.; Yin, T.; Xiong, G.; Ding, A.; Li, X.; Wu, W.; Qiao, Y.; Liao, L.; Wang, J.; Wang, L. Microstructure and physicochemical properties: Effect of pre-chilling and storage time on the quality of channel catfish during frozen storage. LWT 2020, 130, 109606. [Google Scholar] [CrossRef]
- Suvanich, V.; Marshall, D.L. Influence of storage time and temperature on quality of catfish (Ictalurus punctatus) frames. J. Aquat. Food Product. Technol. 1998, 7, 61–74. [Google Scholar] [CrossRef]
- Xu, Y.; Song, M.; Xia, W.; Jiang, Q. Effects of freezing method on water distribution, microstructure, and taste active compounds of frozen channel catfish (Ictalurus punctatus). J. Food Process Eng. 2019, 42, e12937. [Google Scholar] [CrossRef]
- Dabadé, D.S.; Yessoufou, N.; Adido, L.; Azokpota, P.; Hounhouigan, D.J. Quality changes, potential spoilage organisms, and shelf-life prediction of brackish river prawn (Macrobrachium macrobrachion) at different storage temperatures. Int. J. Food Microbiol. 2023, 405, 110344. [Google Scholar] [CrossRef]
- Chen, B.Y.; Mei, J.; Xie, J. Effects of packaging methods and temperature variations on the quality and microbial diversity of grouper (Epinephelus Lanceolatus) during cold storage. Food Biosci. 2024, 60, 104315. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, J.; Xie, J. Exploring the correlation of microbial community diversity and succession with protein degradation and impact on the production of volatile compounds during cold storage of grouper (Epinephelus coioides). Food Chem. 2024, 460, 140469. [Google Scholar] [CrossRef]
- Peng, L.; You, J.; Wang, L.; Shi, L.; Liao, T.; Huang, Q.L.; Xiong, S.B.; Yin, T. Insight into the mechanism on texture change of Wuchang bream muscle during live transportation using a UPLC-QTOF-MS based metabolomics method. Food Chem. 2023, 398, 133796. [Google Scholar] [PubMed]
- Chen, B.; Xu, T.; Yan, Q.; Karsli, B.; Li, D.; Xie, J. Effect of temperature fluctuations on large yellow croaker fillets (Larimichthys crocea) in cold chain logistics: A microbiological and metabolomic analysis. J. Food Eng. 2025, 386, 112290. [Google Scholar]
- Fei, L.; Ma, Z.; Yue, A.; Cui, P.; Qiu, Y.; Lyu, F.; Zhang, J. Effect of low-voltage electrostatic field-assisted partial freezing on large yellow croaker protein properties and metabolomic analysis during storage. J. Sci. Food Agric. 2024, 104, 2359–2371. [Google Scholar] [PubMed]
- Jääskeläinen, E.; Jakobsen, L.M.A.; Hultman, J.; Eggers, N.; Bertram, H.C.; Björkroth, J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int. J. Food Microbiol. 2019, 293, 44–52. [Google Scholar] [PubMed]
- Shen, S.-K.; Chen, Y.-W.; Dong, X.-P.; Liu, F.-J.; Cai, W.-Q.; Wei, J.-L.; Bai, F.; Shi, Y.-G.; Li, P.; Wang, Y.-R. Changes in food quality and microbial composition of Russian sturgeon (Acipenser gueldenstaedti) fillets treated with low temperature vacuum heating method during storage at 4 °C. Food Res. Int. 2020, 138, 109665. [Google Scholar]
- Parlapani, F.F.; Boziaris, I.S. Monitoring of spoilage and determination of microbial communities based on 16S rRNA gene sequence analysis of whole sea bream stored at various temperatures. LWT Food Sci. Technol. 2016, 66, 553–559. [Google Scholar]
- Du, G.; Gai, Y.; Zhou, H.; Fu, S.; Zhang, D. Assessment of spoilage microbiota of rainbow trout (Oncorhynchus mykiss) during storage by 16S rDNA sequencing. J. Food Qual. 2022, 2022, 5367984. [Google Scholar] [CrossRef]
- Chen, J.; Kong, Q.; Sun, Z.T.; Liu, J.Y. Freshness analysis based on lipidomics for farmed Atlantic salmon (Salmo salar L.) stored at different times. Food Chem. 2022, 373, 131564. [Google Scholar]
- Chen, Y.S.; Ning, Q.; Wang, S.N.; Chen, Y.; Mo, X.Y.; Liang, P.; Pang, J.; Dong, X.P. Effects of cold treatments on lipidomics profiles of large yellow croaker (Larimichthys crocea) fillets by UPLC-Q-Exactive Orbitrap MS analysis. J. Food Compos. Anal. 2022, 109, 104481. [Google Scholar]
- GB 5009.228-2016; Determination of Volatile Base Nitrogen in Food Safety National Standards. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Siu, G.M.; Draper, H.H. A survey of the malonaldehyde content of retail meats and fish. J. Food Sci. 1978, 43, 1147–1149. [Google Scholar]
- Zhang, T.; Wang, N.F.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of fungal communities in the marine sediments of Kongsfjorden, Svalbard (High Arctic). Sci. Rep. 2015, 5, 14524. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, D.; Qin, N.; Hong, H.; Luo, Y. Comparative studies of quality changes in white and dark muscles from common carp (Cyprinus carpio) during refrigerated (4 °C) storage. Int. J. Food Sci. Technol. 2016, 51, 1130–1139. [Google Scholar] [CrossRef]
- Huang, Q.; Jiao, X.; Yan, B.; Zhang, N.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Changes in physicochemical properties of silver carp (Hypophthalmichthys molitrix) surimi during chilled storage: The roles of spoilage bacteria. Food Chem. 2022, 387, 132847. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. Physicochemical and structural changes of myofibrillar proteins in muscle foods during thawing: Occurrence, consequences, evidence, and implications. Compr. Rev. Food Sci. F 2023, 22, 3444–3477. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Cai, W.-Q.; Shang, S.; Miao, X.-Q.; Dong, X.-P.; Zhou, D.-Y.; Jiang, P.-F. Comparative analysis of the flavor profile and microbial diversity of high white salmon (Coregonus peled) caviar at different storage temperatures. LWT 2022, 169, 114068. [Google Scholar] [CrossRef]
- Yang, F.; Jing, D.; Yu, D.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Differential roles of ice crystal, endogenous proteolytic activities and oxidation in softening of obscure pufferfish (Takifugu obscurus) fillets during frozen storage. Food Chem. 2019, 278, 452–459. [Google Scholar] [CrossRef]
- Tan, K.R.; Lim, L.; Peng, Y.; Cheong, K.L. Effects of food processing on the lipid nutritional quality of commercially important fish and shellfish. Food Chem. X 2023, 20, 101034. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, K.S.; Ma, L.Z.; Huo, N.R.; Yang, H.; Hao, J.M. Sensory, physicochemical, and microbiological changes in vacuum packed channel catfish (Clarias lazera) patties during controlled freezing-point storage. Food Sci. Biotechnol. 2015, 24, 1249–1256. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Michailidou, S.; Anagnostopoulos, D.A.; Koromilas, S.; Kios, K.; Pasentsis, K.; Psomopoulos, F.; Argiriou, A.; Haroutounian, S.A.; Boziaris, I.S. Bacterial communities and potential spoilage markers of whole blue crab (Callinectes sapidus) stored under commercial simulated conditions. Food Microbiol. 2019, 82, 325–333. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Growth kinetics and spoilage potential of co-culturing Acinetobacter johnsonii and Pseudomonas fluorescens from Bigeye tuna (Thunnus obesus) during refrigerated storage. Curr. Microbiol. 2020, 77, 1637–1646. [Google Scholar] [PubMed]
- Yang, Z.; Liu, S.; Lv, J.; Sun, Z.; Xu, W.; Ji, C.; Liang, H.; Li, S.; Yu, C.; Lin, X. Microbial succession and the changes of flavor and aroma in Chouguiyu, a traditional Chinese fermented fish. Food Biosci. 2020, 37, 100725. [Google Scholar]
- Yang, S.-P.; Xie, J.; Qian, Y.-F. Determination of spoilage microbiota of pacific white shrimp during ambient and cold storage using next-generation sequencing and culture-dependent method. J. Food Sci. 2017, 82, 1178–1183. [Google Scholar] [PubMed]
- Wu, X.; Su, Y.-C. Effects of frozen storage on survival of Staphylococcus aureus and enterotoxin production in precooked tuna meat. J. Food Sci. 2014, 79, M1554–M1559. [Google Scholar]
- Snyder, A.B.; Martin, N.; Wiedmann, M. Microbial food spoilage: Impact, causative agents and control strategies. Nat. Rev. Microbiol. 2024, 22, 528–542. [Google Scholar]
- Raposo, A.; Pérez, E.; de Faria, C.T.; Ferrús, M.A.; Carrascosa, C. Food spoilage by Pseudomonas spp.—An overview. In Foodborne Pathogens and Antibiotic Resistance. Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 41–71. [Google Scholar]
- Li, Q.; Zhang, L.; Luo, Y. Changes in microbial communities and quality attributes of white muscle and dark muscle from common carp (Cyprinus carpio) during chilled and freeze-chilled storage. Food Microbiol. 2018, 73, 237–244. [Google Scholar]
- Mendes, R. Technological processing of fresh gilthead seabream (Sparus aurata): A review of quality changes. Food Rev. Int. 2019, 35, 20–53. [Google Scholar]
- Li, B.; Liu, S.; Chen, X.; Su, Y.; Pan, N.; Liao, D.; Qiao, K.; Chen, Y.; Liu, Z. Dynamic changes in the microbial composition and spoilage characteristics of refrigerated large yellow croaker (Larimichthys crocea) during storage. Foods 2023, 12, 3994. [Google Scholar] [CrossRef]
- Yan, H.B.; Jiao, L.; Fang, C.A.D.; Benjakul, S.; Zhang, B. Chemical and LC–MS-based lipidomics analyses revealed changes in lipid profiles in hairtail (Trichiurus haumela) muscle during chilled storage. Food Res. Int. 2022, 159, 111600. [Google Scholar]
- Wang, X.-Y.; Xie, J.; Chen, X.-J. Differences in lipid composition of Bigeye tuna (Thunnus obesus) during storage at 0 °C and 4 °C. Food Res. Int. 2021, 143, 110233. [Google Scholar]
- Bienias, K.; Fiedorowicz, A.; Sadowska, A.; Prokopiuk, S.; Car, H. Regulation of sphingomyelin metabolism. Pharmacol. Rep. 2016, 68, 570–581. [Google Scholar]
- Chu, Y.; Mei, J.; Xie, J. Exploring the effects of lipid oxidation and free fatty acids on the development of volatile compounds in grouper during cold storage based on multivariate analysis. Food Chem. X 2023, 20, 100968. [Google Scholar] [PubMed]
- Das, J.; Mishra, H.N. A comprehensive review of the spoilage of shrimp and advances in various indicators/sensors for shrimp spoilage monitoring. Food Res. Int. 2023, 173, 113270. [Google Scholar] [PubMed]
- Bicas, J.L.; Rodriguez-Amaya, D.B. Chapter 9—Generation of process-derived flavors and off-flavors. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 385–451. [Google Scholar]
- Shen, X.; Niu, X.; Yang, Y.; Yang, D.; Li, J.; Yu, F.; Sun, X.; Meng, X. Widely targeted metabolomics combined with E-tongue and E-nose reveal dynamic changes of tender coconut water in responses to the infection of Ceratocystis paradoxa. Food Chem. 2024, 439, 138035. [Google Scholar] [PubMed]
- Wang, X.-Y.; Yan, J.; Xie, J. Applications of genomics, metabolomics, fourier transform infrared in the evaluation of spoilage targets of Shewanella putrefaciens from spoiled bigeye tuna. J. Agr. Food Chem. 2023, 71, 9558–9568. [Google Scholar]
- Savvi, S.; Warner, D.F.; Kana, B.D.; McKinney, J.D.; Mizrahi, V.; Dawes, S.S. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: Implications for propionate metabolism during growth on fatty acids. J. Bacteriol. 2008, 190, 3886–3895. [Google Scholar]
- Ghosh, S.; Bandyopadhyay, S.; Smith, D.M.; Adak, S.; Semenkovich, C.F.; Nagy, L.; Wolfgang, M.J.; O’Connor, T.J. Legionella pneumophila usurps host cell lipids for vacuole expansion and bacterial growth. PLoS Pathog. 2024, 20, e1011996. [Google Scholar]



| Points | Color | Odor | Appearance | Texture |
|---|---|---|---|---|
| 5 | the color is bright and normal | it has a strong inherent aroma and no rancidity | the muscle texture is very clear and the tissue is firm | the muscle was elastic and the depression disappeared immediately after pressing |
| 4 | the color is normal | it has an inherent aroma and no rancidity | the muscle texture is clear and the tissue is firm | the muscles were elastic and the depression disappeared quickly after pressing |
| 3 | the color is dull | the inherent aroma is light and slightly fishy | the muscle lines are slightly clear and the tissue is not tight | the muscles were elastic and the depression disappeared very slowly after pressing |
| 2 | the color is very dull | the inherent aroma disappeared and there was a strong fishy smell | the muscle structure is partly loose | the muscles were less elastic and the depression disappeared very slowly after pressing |
| 1 | the color is dull and grey | it has a strong fishy smell | the muscle structure is loose | the muscle is inelastic and the depression barely changes after pressing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Zhou, S.; Lian, K.; Chen, S. Integrated Metabolomic and Microbial Analysis of Quality Dynamics in Channel Catfish (Ictalurus punctatus) Under Refrigerated and Frozen Storage. Foods 2025, 14, 1089. https://doi.org/10.3390/foods14071089
Xia L, Zhou S, Lian K, Chen S. Integrated Metabolomic and Microbial Analysis of Quality Dynamics in Channel Catfish (Ictalurus punctatus) Under Refrigerated and Frozen Storage. Foods. 2025; 14(7):1089. https://doi.org/10.3390/foods14071089
Chicago/Turabian StyleXia, Liwei, Shun Zhou, Kaiqi Lian, and Shengao Chen. 2025. "Integrated Metabolomic and Microbial Analysis of Quality Dynamics in Channel Catfish (Ictalurus punctatus) Under Refrigerated and Frozen Storage" Foods 14, no. 7: 1089. https://doi.org/10.3390/foods14071089
APA StyleXia, L., Zhou, S., Lian, K., & Chen, S. (2025). Integrated Metabolomic and Microbial Analysis of Quality Dynamics in Channel Catfish (Ictalurus punctatus) Under Refrigerated and Frozen Storage. Foods, 14(7), 1089. https://doi.org/10.3390/foods14071089






