Exploring the Mechanisms of the Antioxidants BHA, BHT, and TBHQ in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity from the Perspective of Network Toxicology
Abstract
:1. Introduction
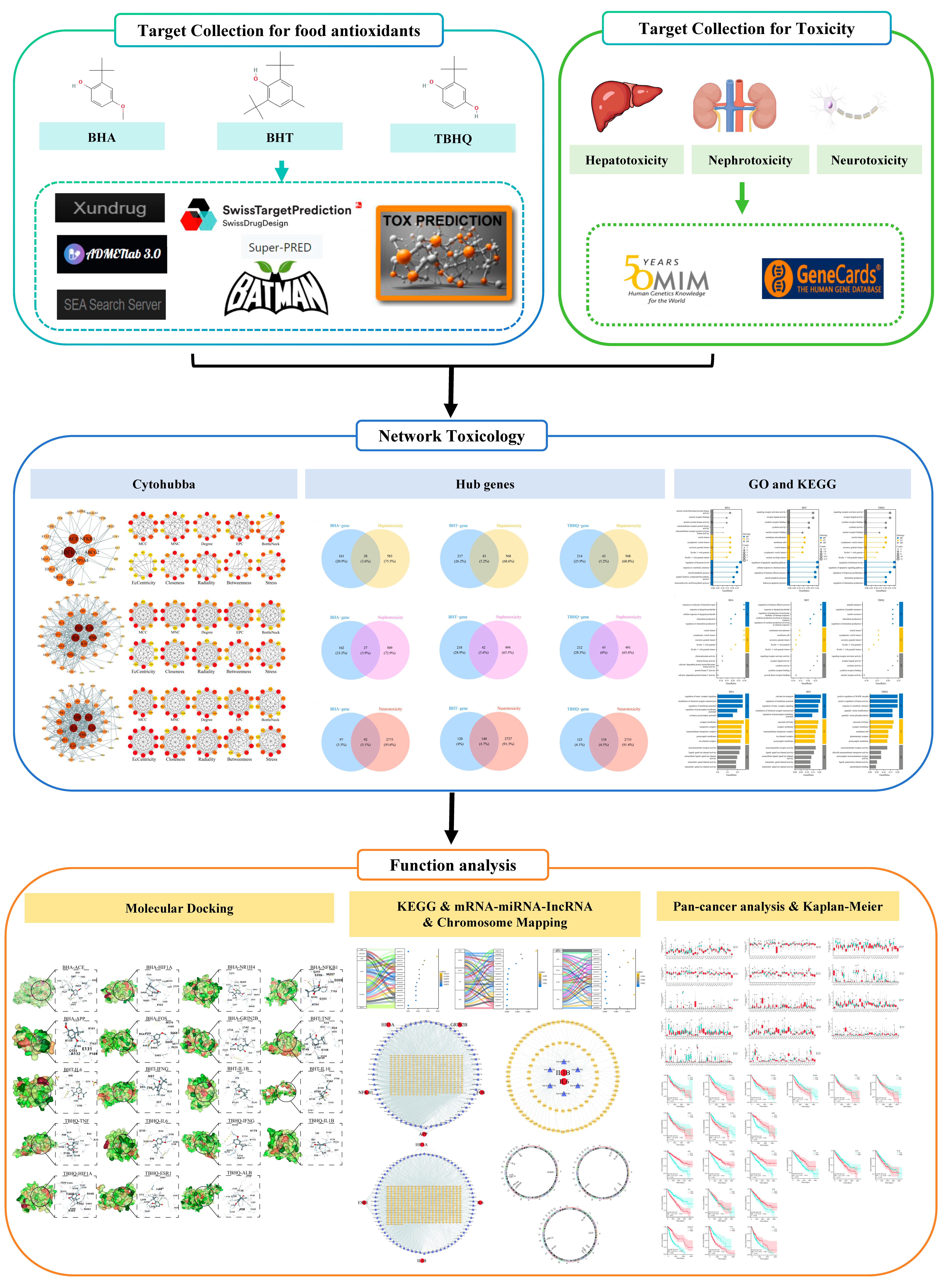
2. Materials and Methods
2.1. Preliminary Toxicity Analysis of BHA, BHT, and TBHQ
2.2. Target Collection for BHA, BHT, and TBHQ
2.3. Target Collection for Hepatotoxicity, Nephrotoxicity, and Neurotoxicity
2.4. Functional Enrichment Analysis
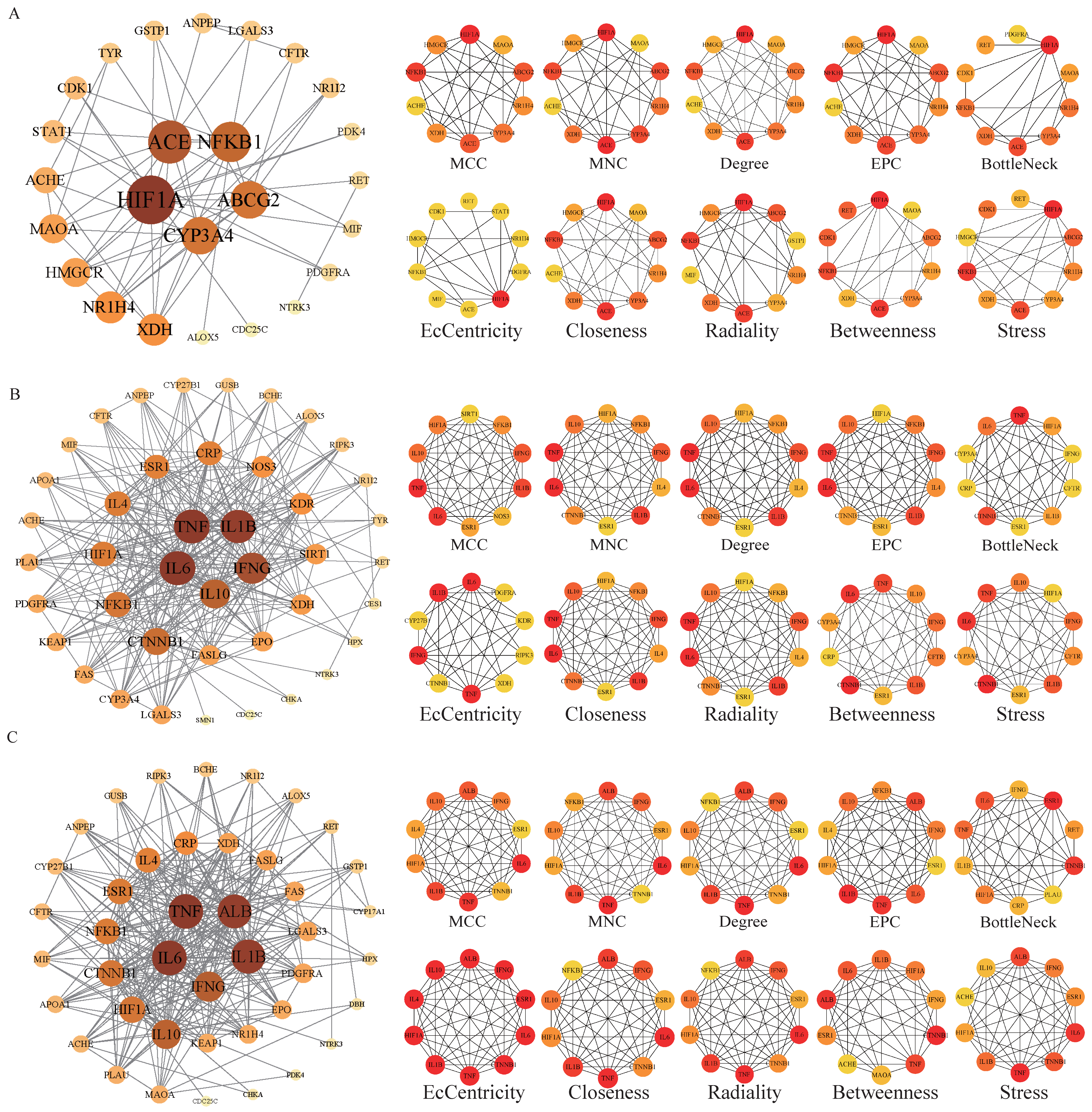
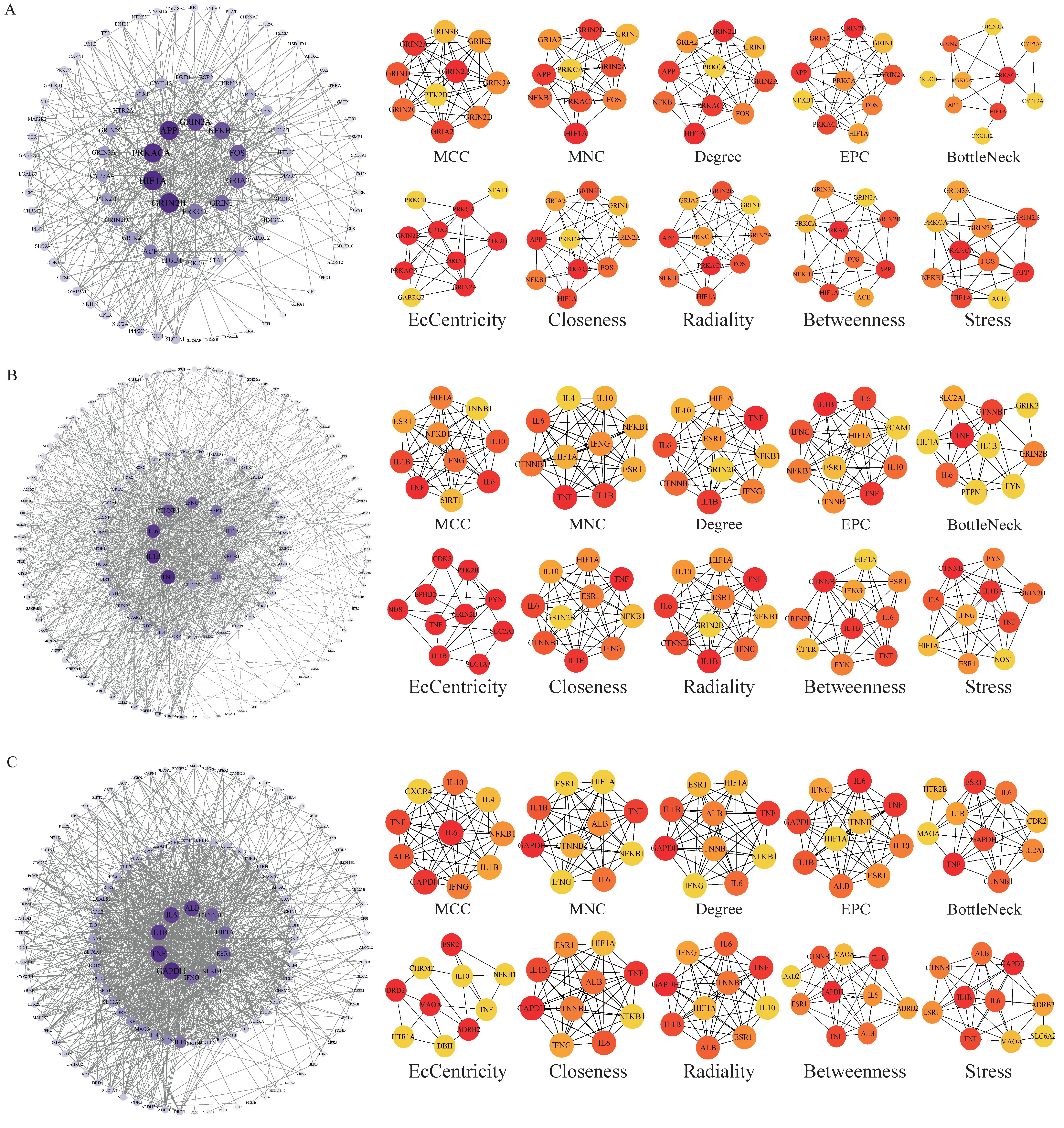
2.5. Construction of Protein Interaction Network and Screening of Core Targets
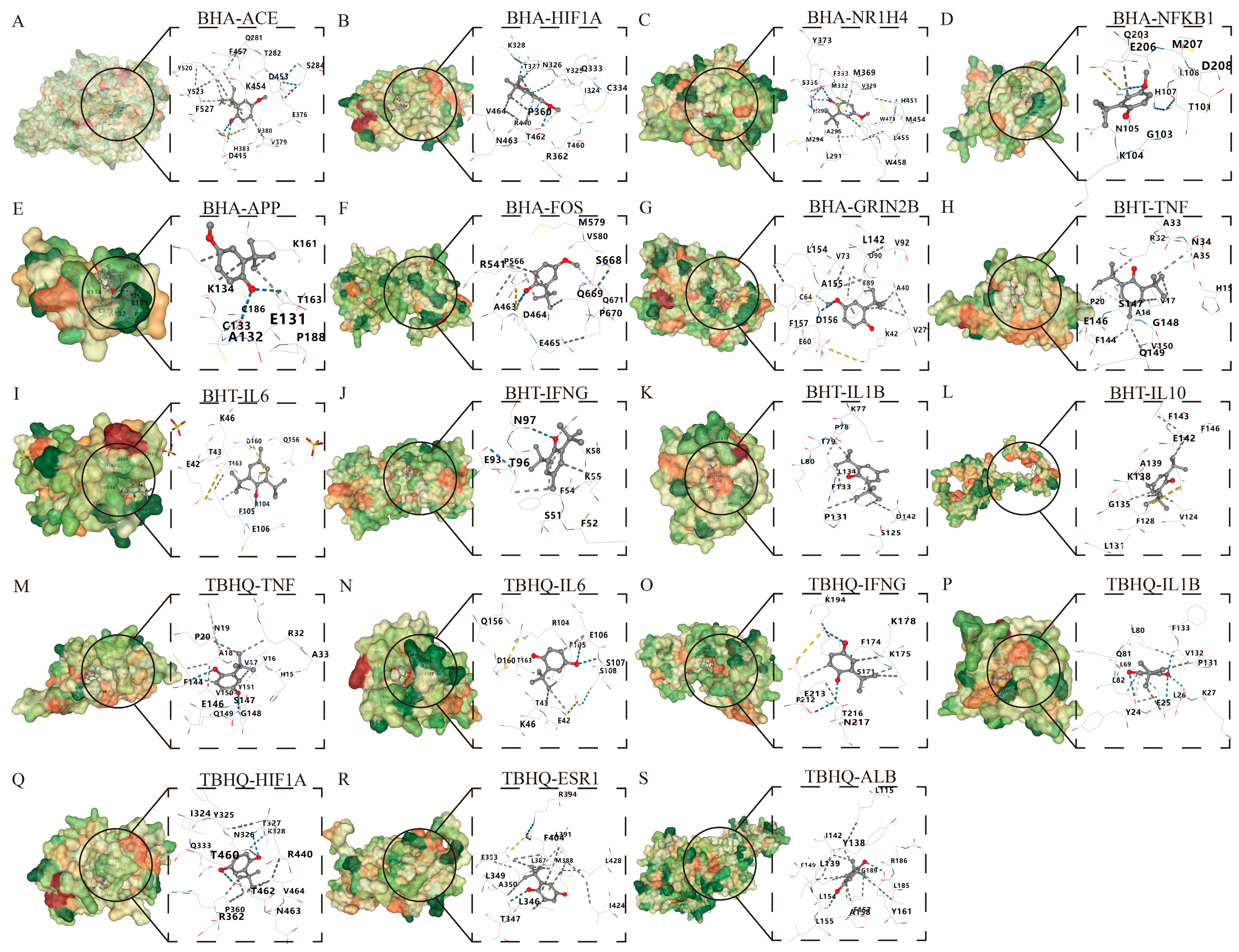
2.6. Molecular Docking of BHA, BHT, and TBHQ to Core Target Proteins During Hepatotoxicity, Nephrotoxicity, and Neurotoxicity
2.7. Functional Pathway Analysis of Core Targets
2.8. Construction of mRNA-miRNA-lncRNA Interaction Network of Core Targets
2.9. Chromosomal Localization Analysis of Core Targets
2.10. Carcinogenic Risk Analysis of Core Targets
3. Results
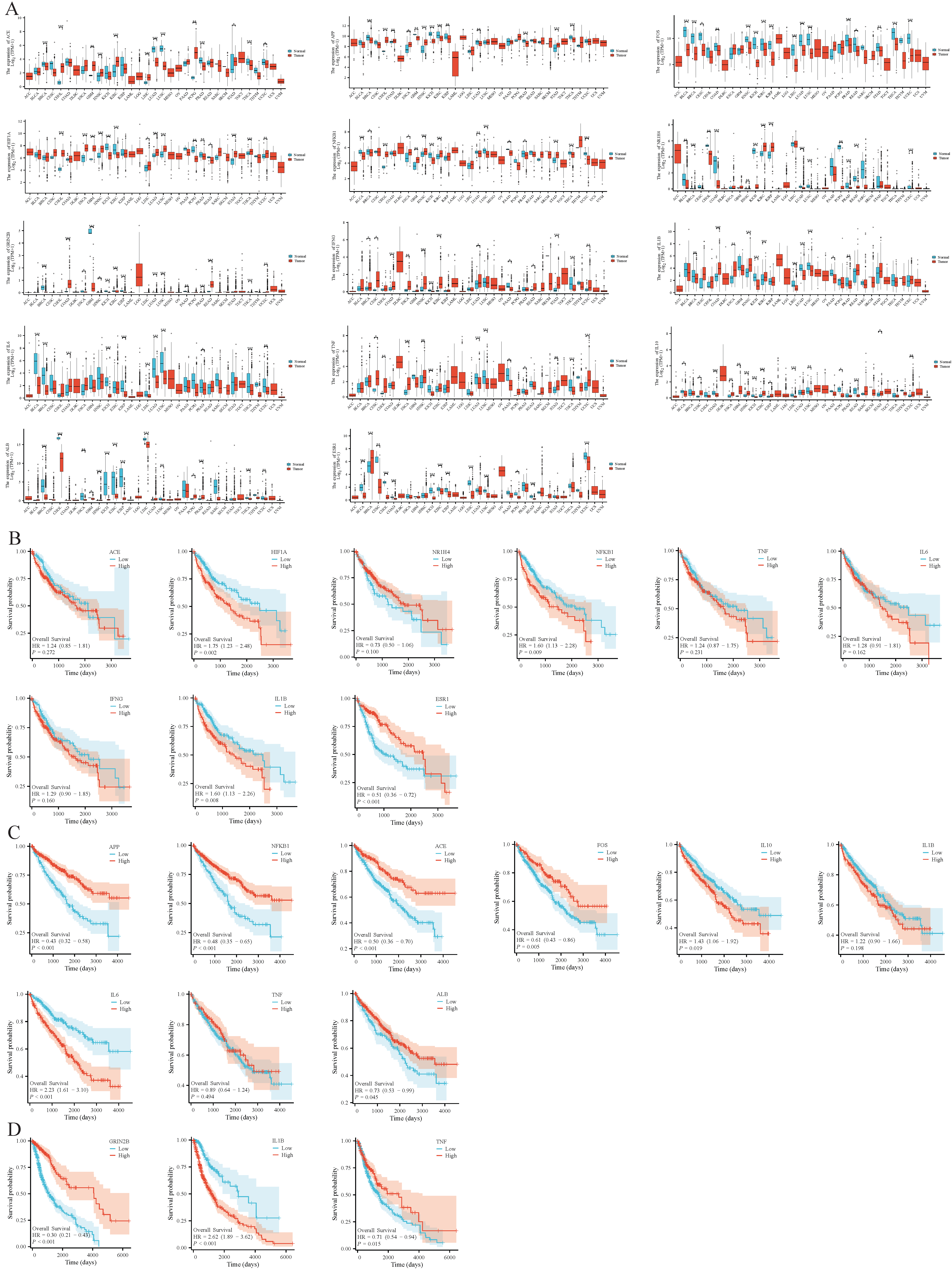
3.1. Toxicity Prediction
3.2. Potential Targets of BHT, BHT, and TBHQ for Hepatotoxicity, Nephrotoxicity, and Neurotoxicity
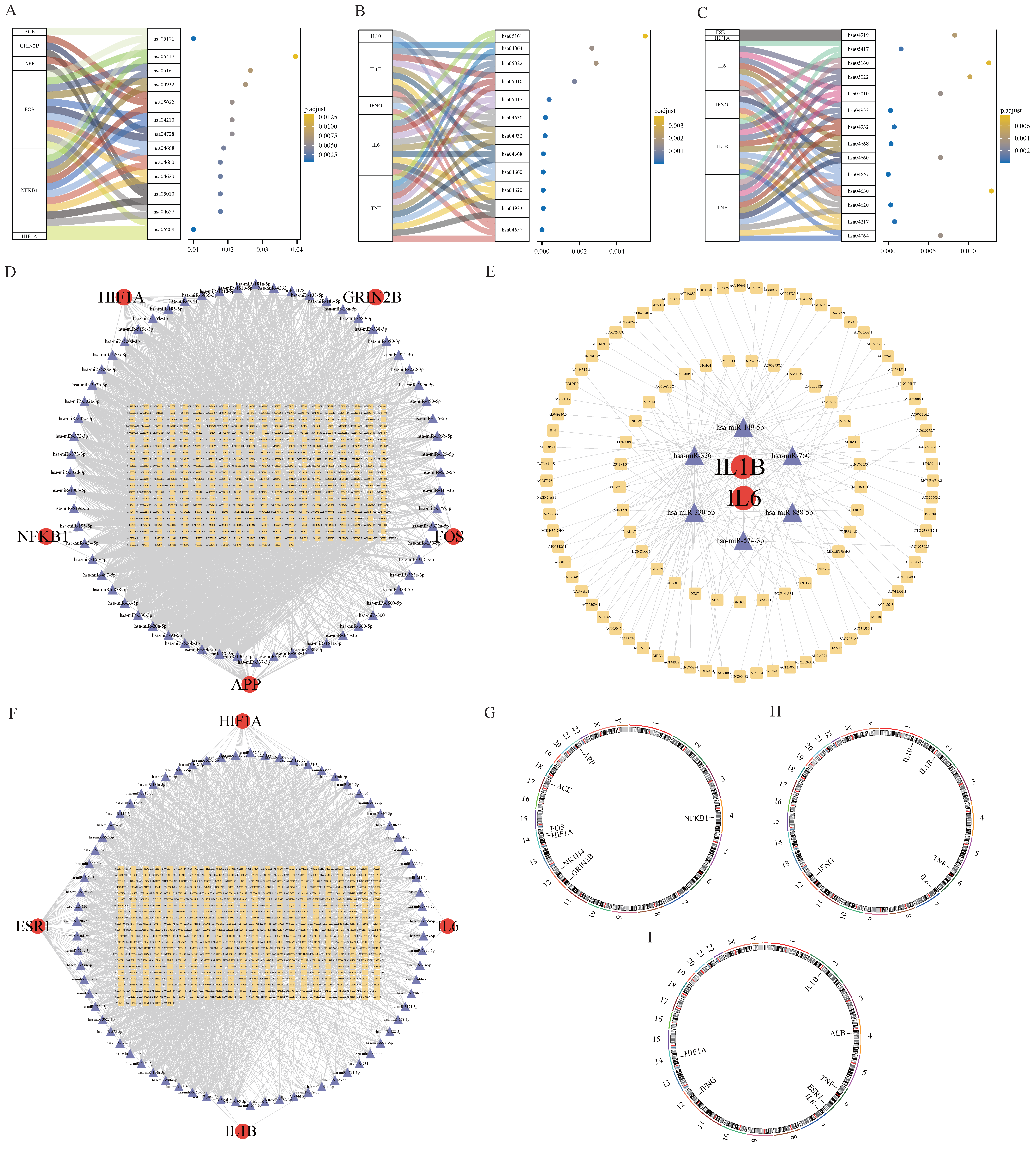
3.3. GO and KEGG Enrichment Analyses of Potential Targets
3.4. Core Target Analysis
3.5. Molecular Docking of BHA, BHT, and TBHQ with Core Target Proteins in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity
3.6. KEGG Enrichment Analysis of Core Targets
3.7. mRNA-IncmiRNA-RNA Network
3.8. Chromosome Localization
3.9. Carcinogenic Risk of Core Targets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHA | butylated hydroxyanisole |
| BHT | butylated hydroxytoluene |
| TBHQ | tert-butylhydroquinone |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| IL-17 signaling | interleukin-17 signaling |
| JAK-STAT signaling | Janus kinase-signal transducer and activator of transcription signaling |
| NF-κB signaling | nuclear factor kappa-light-chain-enhancer of activated B cells signaling |
| NAFLD | non-alcoholic fatty liver disease |
| BP | biological process |
| MF | molecular function |
| CC | cellular component |
| PPI | protein–protein interaction |
| MCC | maximum clique centrality |
| MNC | maximum neighborhood centrality |
| EC | eigenvector centrality |
| RCSB | Research Collaboratory for Structural Bioinformatics |
| PDB | Protein Data Bank |
| MAPK | mitogen-activated protein kinase |
| PI3K-Akt signaling | phosphoinositide 3-kinase/protein kinase B signaling |
| VEGF signaling | vascular endothelial growth factor signaling |
| FoxO signaling | Forkhead Box O signaling |
| NR1H4 | Nuclear Receptor Subfamily 1, Group H, Member 4 |
| ACE | angiotensin-converting enzyme |
| HIF1A | hypoxia-inducible factor 1-alpha |
| TNF | tumor necrosis factor |
| APP | amyloid precursor protein |
| IL1B | interleukin 1 beta |
| GRIN2B | Glutamate Receptor, Ionotropic, N-Methyl D-Aspartate 2B |
| NFKB1 | nuclear factor kappa-light-chain-enhancer of activated B cells 1 |
| IL6 | interleukin 6 |
| IFNG | interferon gamma |
| ESR1 | estrogen receptor 1 |
| FOS | FBJ osteosarcoma oncogene |
| IL10 | interleukin 10 |
| ALB | albumin |
| TNF signaling | tumor necrosis factor signaling |
| KIRC | kidney renal clear cell carcinoma |
| BRCA | breast cancer |
| LUAD | lung adenocarcinoma |
| BLCA | bladder urothelial carcinoma |
| UCEC | uterine corpus endometrial carcinoma |
| KM survival | Kaplan–Meier survival |
| ROS | reactive oxygen species |
| ERK | extracellular signal-regulated kinase |
| KEAP1 | Kelch-like ECH-associated protein 1 |
References
- Tchonkouang, R.D.; Lima, A.R.; Quintino, A.C.; Cristofoli, N.L.; Vieira, M.C. UV-C Light: A Promising Preservation Technology for Vegetable-Based Nonsolid Food Products. Foods 2023, 12, 3227. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Ranveer, R.C.; Bhagwat, P.K.; Ozogul, F.; Benjakul, S.; Pillai, S.; Annapure, U.S. Cold plasma for the preservation of aquatic food products: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4407–4425. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Leonard, E.R.; Marques, E.S.; Roy, M.A.; Conlin, S.M.; Ranjan, R.; Timme-Laragy, A.R. Dietary exposure to the food preservative tert-Butylhydroquinone (tBHQ) impairs zebrafish (Danio rerio) survival, growth, organ development, and gene expression in Nrf2a-dependent and independent ways. Food Chem. Toxicol. 2023, 176, 113788. [Google Scholar] [CrossRef]
- De Abrew, K.N.; Natoli, T.; Lester, C.C.; Wang, X.; Shobair, M.; Subramanian, A.; Daston, G.P. A New Approach Methodology (NAM) Based Assessment of Butylated hydroxytoluene (BHT) for Endocrine Disruption Potential. Toxicol. Sci. 2022, 190, 227–241. [Google Scholar] [CrossRef]
- Zhang, X.J.; Diao, M.; Zhang, Y.F. A review of the occurrence, metabolites and health risks of butylated hydroxyanisole (BHA). J. Sci. Food Agric. 2023, 103, 6150–6166. [Google Scholar] [CrossRef]
- Yang, X.; Song, W.; Liu, N.; Sun, Z.; Liu, R.; Liu, Q.S.; Zhou, Q.; Jiang, G. Synthetic phenolic antioxidants cause perturbation in steroidogenesis in vitro and in vivo. Environ. Sci. Technol. 2018, 52, 850–858. [Google Scholar]
- Esazadeh, K.; Ezzati Nazhad Dolatabadi, J.; Andishmand, H.; Mohammadzadeh-Aghdash, H.; Mahmoudpour, M.; Naemi Kermanshahi, M.; Roosta, Y. Cytotoxic and genotoxic effects of tert-butylhydroquinone, butylated hydroxyanisole and propyl gallate as synthetic food antioxidants. Food Sci. Nutr. 2024, 12, 7004–7016. [Google Scholar] [CrossRef]
- Peng, J.; Dai, X.; Zhang, T.; Hu, G.; Cao, H.; Guo, X.; Fan, H.; Chen, J.; Tang, W.; Yang, F. Copper as the driver of the lncRNA-TCONS-6251/miR-novel-100/TC2N axis: Unraveling ferroptosis in duck kidney. Int. J. Biol. Macromol. 2024, 282, 136797. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Ishidoh, K.; Kamemura, N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Drug Chem. Toxicol. 2021, 45, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fushimi, K.; Kouchi, H.; Mihara, K.; Miyazaki, M.; Ohe, T.; Namba, M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation 1998, 98, 794–799. [Google Scholar] [PubMed]
- Kakade, S.S.; Bote, H.K.; Pawar, P.K. Dual intervention of Boeravinone B and Chebulinic Acid mitigates BHT-Induced toxicity in HepG2 cells: Modulating apoptosis and autophagy. Sci. Rep. 2024, 14, 29595. [Google Scholar] [CrossRef]
- Li, Z.; Qi, R.; Wang, Q.; Zheng, X.; Li, P.; Liao, L.; Pan, L. Study on the detoxification and toxic effects of clam Ruditapes philippinarum exposed to 2,6-ditert-butyl-4-methylphenol (BHT): Oxidative stress, neurotoxicity, respiratory toxicity, and immunotoxicity. Environ. Res. 2025, 268, 120839. [Google Scholar] [CrossRef]
- Jyoti; Deepeka; Kaur, P.; Rana, S.; Singhal, S. Palladium-zinc ferrite varnished hydroxyapatite spherocuboids for electrochemical detection of carcinogenic food preservatives. Food Chem. 2025, 464, 141626. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, X.; Pang, X.; Huang, J.; Zhou, S.; Wu, R.; Wang, R.; Tang, Z.; Su, R. Tert-butylhydroquinone attenuates LPS-induced pyroptosis of IPEC-J2 cells via downregulating HMGB1/TLR4/NF-κB axis. J. Anim. Physiol. Anim. Nutr. 2023, 108, 194–205. [Google Scholar] [CrossRef]
- Aldaba-Muruato, L.R.; Sánchez-Barbosa, S.; Rodríguez-Purata, V.H.; Cabrera-Cruz, G.; Rosales-Domínguez, E.; Martínez-Valentín, D.; Alarcón-López, Y.A.; Aguirre-Vidal, P.; Hernández-Serda, M.A.; Cárdenas-Granados, L.A.; et al. In Vivo and In Silico Studies of the Hepatoprotective Activity of Tert-Butylhydroquinone. Int. J. Mol. Sci. 2023, 25, 475. [Google Scholar] [CrossRef]
- Sherson, D.L.; Jacobsen, I.B.; Thomsen, G.F. The antioxidant, tert-butylhydroquinone: A new cause of asthma. Occup. Med. 2023, 73, 109–111. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Wang, F.; Shi, Y.; Ren, Y.; Liu, Q.; Cao, Y.; Duan, H. Attenuation of Glomerular Injury in Diabetic Mice with Tert-Butylhydroquinone through Nuclear Factor Erythroid 2-Related Factor 2-Dependent Antioxidant Gene Activation. Am. J. Nephrol. 2011, 33, 289–297. [Google Scholar] [CrossRef]
- Deng, S.; Wu, D.; Li, L.; Li, J.; Xu, Y. TBHQ attenuates ferroptosis against 5-fluorouracil-induced intestinal epithelial cell injury and intestinal mucositis via activation of Nrf2. Cell. Mol. Biol. Lett. 2021, 26, 48. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. In Data Mining in Proteomics; Methods in Molecular Biology; Chapter 18; Humana Press: Totowa, NJ, USA, 2011; pp. 291–303. [Google Scholar]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Dweep, H.; Sticht, C.; Pandey, P.; Gretz, N. miRWalk—Database: Prediction of possible miRNA binding sites by “walking” the genes of three genomes. J. Biomed. Inform. 2011, 44, 839–847. [Google Scholar] [CrossRef]
- Yang, J.-H.; Li, J.-H.; Shao, P.; Zhou, H.; Chen, Y.-Q.; Qu, L.-H. starBase: A database for exploring microRNA–mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011, 39, D202–D209. [Google Scholar] [CrossRef]
- Zhang, H.; Meltzer, P.; Davis, S. RCircos: An R package for Circos 2D track plots. BMC Bioinform. 2013, 14, 244. [Google Scholar]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef] [PubMed]
- Van Quickelberghe, E.; De Sutter, D.; van Loo, G.; Eyckerman, S.; Gevaert, K. A protein-protein interaction map of the TNF-induced NF-κB signal transduction pathway. Sci. Data 2018, 5, 180289. [Google Scholar] [CrossRef] [PubMed]
- Gao, B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J. Gastroenterol. Hepatol. 2012, 27, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Q.; Wang, G.; Wang, H.; Liu, H. The effects of blunt snout bream (Megalobrama amblycephala) IL-6 trans-signaling on immunity and iron metabolism via JAK/STAT3 pathway. Dev. Comp. Immunol. 2022, 131, 104372. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.-T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Jones, S.A.; Fraser, D.J.; Fielding, C.A.; Jones, G.W. Interleukin-6 in renal disease and therapy. Nephrol. Dial. Transplant. 2015, 30, 564–574. [Google Scholar]
- Shan, C.; Zhang, C.; Zhang, C. The Role of IL-6 in Neurodegenerative Disorders. Neurochem. Res. 2024, 49, 834–846. [Google Scholar] [CrossRef]
- Kim, K.S.; Jung, H.; Shin, I.K.; Choi, B.R.; Kim, D.H. Induction of interleukin-1 beta (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J. Med. Virol. 2015, 87, 1104–1112. [Google Scholar]
- Luo, Y.; Cheng, J.; Fu, Y.; Zhang, M.; Gou, M.; Li, J.; Li, X.; Bai, J.; Zhou, Y.; Zhang, L.; et al. D-allose Inhibits TLR4/PI3K/AKT Signaling to Attenuate Neuroinflammation and Neuronal Apoptosis by Inhibiting Gal-3 Following Ischemic Stroke. Biol. Proced. Online 2023, 25, 30. [Google Scholar] [CrossRef]
- Lu, G.; Wen, Z.; Yu, L.; Wang, C.; Gao, Y. HIF1A overexpression caused by etomidate activates PGK1-induced oxidative stress in postoperative cognitive dysfunction. Brain Res. 2024, 1841, 149069. [Google Scholar] [CrossRef]
- Pekgöz, S.; Asci, H.; Erzurumlu, Y.; Savran, M.; Ilhan, I.; Hasseyid, N.; Ciris, M. Nebivolol alleviates liver damage caused by methotrexate via AKT1/Hif1α/eNOS signaling. Drug Chem. Toxicol. 2022, 45, 2153–2159. [Google Scholar] [PubMed]
- Maroni, P.; Bendinelli, P.; Matteucci, E.; Desiderio, M.A. The therapeutic effect of miR-125b is enhanced by the prostaglandin endoperoxide synthase 2/cyclooxygenase 2 blockade and hampers ETS1 in the context of the microenvironment of bone metastasis. Cell Death Dis. 2018, 9, 472. [Google Scholar] [CrossRef]
- Verret, V.; Namur, J.; Ghegediban, S.H.; Wassef, M.; Moine, L.; Bonneau, M.; Pelage, J.-P.; Laurent, A. Toxicity of doxorubicin on pig liver after chemoembolization with doxorubicin-loaded microspheres: A pilot DNA-microarrays and histology study. Cardiovasc. Interv. Radiol. 2013, 36, 204–212. [Google Scholar]
- Pecoraro, A.; Crescenzi, L.; Varricchi, G.; Marone, G.; Spadaro, G. Heterogeneity of Liver Disease in Common Variable Immunodeficiency Disorders. Front. Immunol. 2020, 11, 338. [Google Scholar] [CrossRef]
- Park, K.-H.; Lee, H.; Lee, J.H.; Yon, D.K.; Choi, Y.-I.; Chung, H.-J.; Jung, J.; Jeong, N.Y. Unique and Shared Molecular Mechanisms of Alcoholic and Non-Alcoholic Liver Cirrhosis Identified Through Transcriptomics Data Integration. OMICS A J. Integr. Biol. 2024, 28, 537–547. [Google Scholar] [CrossRef]
- Kim, Y.C.; Mungunsukh, O.; Day, R.M. Erythropoietin regulation by angiotensin II. Vitam. Horm. 2017, 105, 57–77. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef]
- Gao, H.; Liu, L.; Zhao, Y.; Hara, H.; Chen, P.; Xu, J.; Tang, J.; Wei, L.; Li, Z.; Cooper, D.K.C.; et al. Human IL-6, IL-17, IL-1β, and TNF-α differently regulate the expression of pro-inflammatory related genes, tissue factor, and swine leukocyte antigen class I in porcine aortic endothelial cells. Xenotransplantation 2017, 24, e12291. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xu, K.H.; Liu, S.; Xiao, W.J. Protective Effect and Mechanism of L-Theanine on Acute Alcoholic Liver Injury in Mice. Mol. Nutr. Food Res. 2024, 68, e2400766. [Google Scholar] [CrossRef]
- Schmidt, T.; Luebbe, J.; Kilian, C.; Riedel, J.-H.; Hiekmann, S.; Asada, N.; Ginsberg, P.; Robben, L.; Song, N.; Kaffke, A.; et al. IL-17 Receptor C Signaling Controls CD4+ TH17 Immune Responses and Tissue Injury in Immune-Mediated Kidney Diseases. J. Am. Soc. Nephrol. 2021, 32, 3081–3098. [Google Scholar] [CrossRef]
- Kannan, G.; Paul, B.M.; Thangaraj, P. Stimulation, regulation, and inflammaging interventions of natural compounds on nuclear factor kappa B (NF-kB) pathway: A comprehensive review. Inflammopharmacology 2025, 33, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, Z.; Karin, M. NF-κB: A double-edged sword controlling inflammation. Biomedicines 2022, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Kaplan, M.H. STAT Transcription Factors in T Cell Control of Health and Disease. Int. Rev. Cell Mol. Biol. 2017, 331, 123–180. [Google Scholar]
- Prater, M.R.; Laudermilch, C.L.; Holladay, S.D. Does Immune Stimulation or Antioxidant Therapy Reduce MNU-induced Placental Damage Via Activation of Jak-STAT and NFκB Signaling Pathways? Placenta 2007, 28, 566–570. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef]


| Database and Software | Database Link and Software Version |
|---|---|
| Public Chemical Database, Pubchem | https://pubchem.ncbi.nlm.nih.gov/ |
| Swiss Target Prediction | http://www.swisstargetprediction.ch/ |
| Universal Protein Resource, UniProt | https://www.uniprot.org/ |
| GeneCards-The Human Gene Database | https://www.genecards.org/ |
| Protein Data Bank, PDB | http://www.rcsb.org/ |
| Xiantao Academic | https://www.xiantaozi.com/ |
| ProTox 3.0—Prediction of Toxicity of Chemicals, ProTox 3.0 | https://tox.charite.de/protox3/index.php?site=home |
| ADMETlab, ADMETlab 3.0 | https://admetlab3.scbdd.com/ |
| Xundrug Database | https://xundrug.cn/ |
| microRNA Database, miRDB | http://mirdb.org |
| starBase: A Database for MicroRNA-Target Interaction, starBase | https://rnasysu.com/encori/ |
| miRWalk: Database for miRNA Target Prediction and Functional Annotations, miRWalk | http://mirwalk.umm.uni-heidelberg.de |
| SuperPred | https://prediction.charite.de/ |
| Similarity ensemble approach, SEA | https://sea.bkslab.org/ |
| Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine, BATMAN-TCM | http://bionet.ncpsb.org.cn/batman-tcm/index.php/Home/document/index |
| Online Mendelian Inheritance in Man, OMIM | https://www.omim.org/ |
| Cavity-detection guided Blind Docking 2, CB-dock2 | https://cadd.labshare.cn/cb-dock2/php/index.php |
| Gencode database | https://www.gencodegenes.org/ |
| Cytoscape | Cytoscape Consortium (San Diego, CA, USA), 3.9.0 |
| RStudio | Public Benefit Corporation (Boston, MA, USA), 4.3.3 |
| Pymol | Schrödinger, Limited Liability Company (New York, NY, USA), 2.6 |
| No. | Compound Name | Predicted LGC50 | Predicted LC50DM | Predicted LC50FM | Predicted LD50 | ADI | Maximum Dosage |
|---|---|---|---|---|---|---|---|
| 1 | Butylated Hydroxyanisole (BHA) | 4.252 | 5.75 | 5.083 | 880 mg/kg | ≤1 mg/kg/d | ≤200 mg/kg (EFSA) |
| 2 | Butylated Hydroxy Toluene (BHT) | 3.844 | 5.293 | 5.296 | 650 mg/kg | ≤0.25 mg/kg/d | ≤200 mg/kg (EFSA) |
| 3 | Tert-Butylhydroquinone (TBHQ) | 4.524 | 5.276 | 4.955 | 700 mg/kg | ≤0.7 mg/kg/d | ≤200 mg/kg (FDA) |
| No. | PubChem CID | Compound Name | IUPAC Name | Abbreviation | MW | MF | Canonical SMILES | Structure |
|---|---|---|---|---|---|---|---|---|
| 1 | CID 8456 | Butylated Hydroxyanisole | 2-tert-butyl-4-methoxyphenol | BHA | 180.24 | C11H16O2 | CC(C)(C)C1=C(C=CC(=C1)OC)O |  |
| 2 | CID 31404 | Butylated Hydroxy Toluene | 2,6-ditert-butyl-4-methylphenol | BHT | 220.35 | C15H24O | CC1=CC(=C(C(=C1)C(C)(C)C)O)C(C)(C)C | 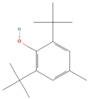 |
| 3 | CID 16043 | Tert-Butylhydroquinone | 2-tert-butylbenzene-1,4-diol | TBHQ | 166.22 | C10H14O2 | CC(C)(C)C1=C(C=CC(=C1)O)O |  |
| Compound | Affinity (kcal/mol) | |||
|---|---|---|---|---|
| Target | CID 8456 | CID 31404 | CID 16043 | |
| ACE | −6 | -- | -- | |
| HIF1A | −5.7 | -- | −5.5 | |
| NR1H4 | −6.3 | -- | -- | |
| NFKB1 | −5 | -- | -- | |
| APP | −4.3 | -- | -- | |
| FOS | −5.7 | -- | -- | |
| GRIN2B | −6.1 | -- | -- | |
| TNF | -- | −5.5 | −5.2 | |
| IL6 | -- | −5.4 | −5.5 | |
| IFNG | -- | −5.1 | −5 | |
| IL1B | -- | −5.3 | −5 | |
| IL10 | -- | −6.7 | -- | |
| ESR1 | -- | -- | −6 | |
| ALB | -- | -- | −7.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Li, Z.; Li, X.; Yang, L.; Bu, Z.; Wu, Y.; Li, Y.; Zhang, S.; Meng, X. Exploring the Mechanisms of the Antioxidants BHA, BHT, and TBHQ in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity from the Perspective of Network Toxicology. Foods 2025, 14, 1095. https://doi.org/10.3390/foods14071095
Ren J, Li Z, Li X, Yang L, Bu Z, Wu Y, Li Y, Zhang S, Meng X. Exploring the Mechanisms of the Antioxidants BHA, BHT, and TBHQ in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity from the Perspective of Network Toxicology. Foods. 2025; 14(7):1095. https://doi.org/10.3390/foods14071095
Chicago/Turabian StyleRen, Jing, Ziang Li, Xiaofen Li, Lin Yang, Zhulin Bu, Yuhui Wu, Yuting Li, Shuosheng Zhang, and Xianglong Meng. 2025. "Exploring the Mechanisms of the Antioxidants BHA, BHT, and TBHQ in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity from the Perspective of Network Toxicology" Foods 14, no. 7: 1095. https://doi.org/10.3390/foods14071095
APA StyleRen, J., Li, Z., Li, X., Yang, L., Bu, Z., Wu, Y., Li, Y., Zhang, S., & Meng, X. (2025). Exploring the Mechanisms of the Antioxidants BHA, BHT, and TBHQ in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity from the Perspective of Network Toxicology. Foods, 14(7), 1095. https://doi.org/10.3390/foods14071095





