Evaluation of Different Drying Methods on the Quality Parameters of Acanthopanax senticosus Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Drying and Sample Extraction
2.3. Determination of Color Properties and Moisture Content
2.4. Determination of Total Polyphenols and Flavonoids
2.5. Determination of Amino Acids
2.6. Quantitative Analysis of the Main Bioactive Substances
2.7. Volatile Compound Analyses
2.8. Determination of the Antioxidant Activity
2.8.1. DPPH Radical Scavenging Capacity
2.8.2. ABTS Radical Scavenging Capacity
2.9. Statistical Analyses
3. Results
3.1. Color Properties
3.2. Determination of TPC and TFC
3.3. Quantitative Analysis of Main Active Ingredients
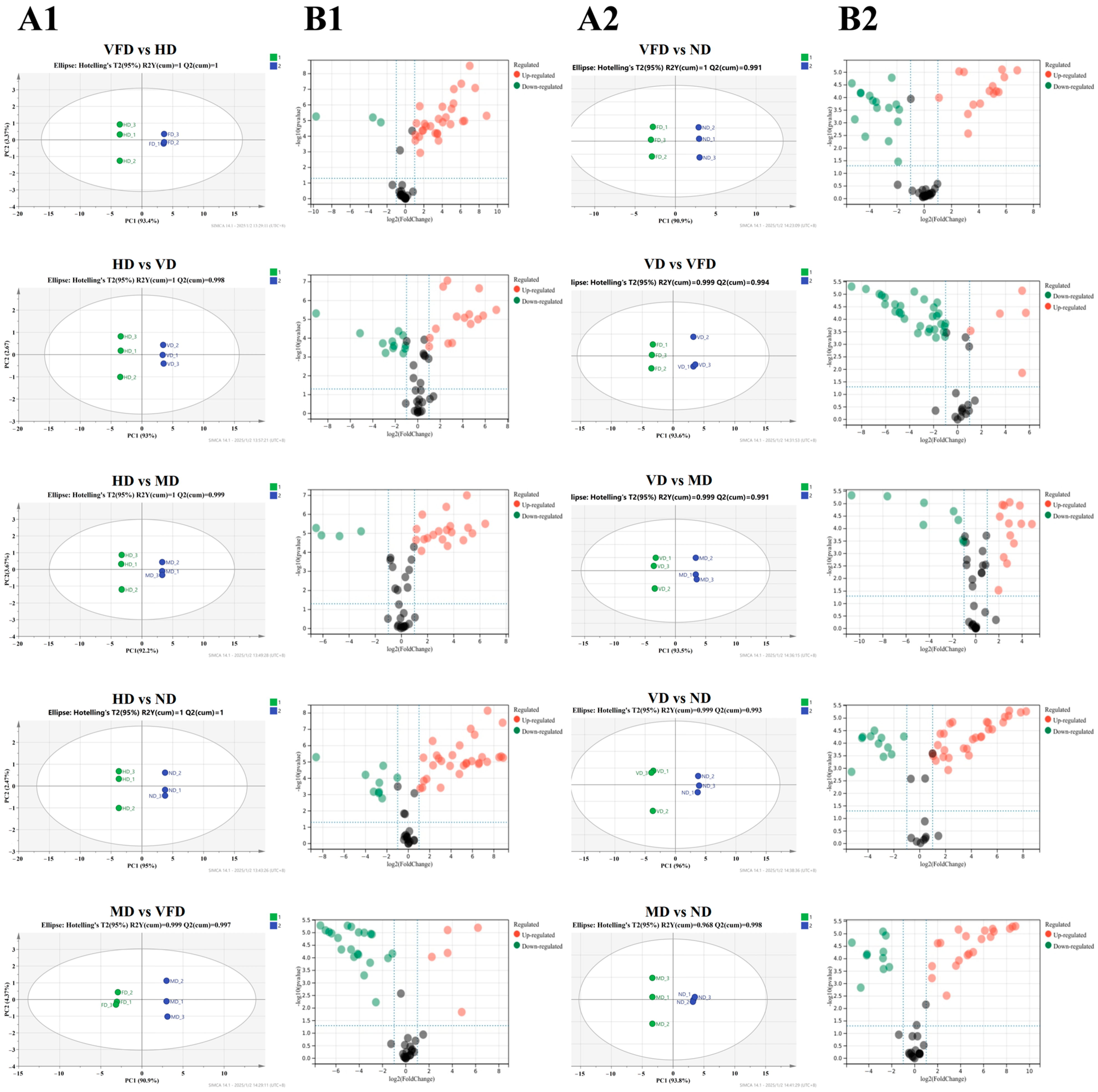
3.4. Principal Component Analysis of Main Active Ingredients
3.5. Analysis of Amino Acid Composition
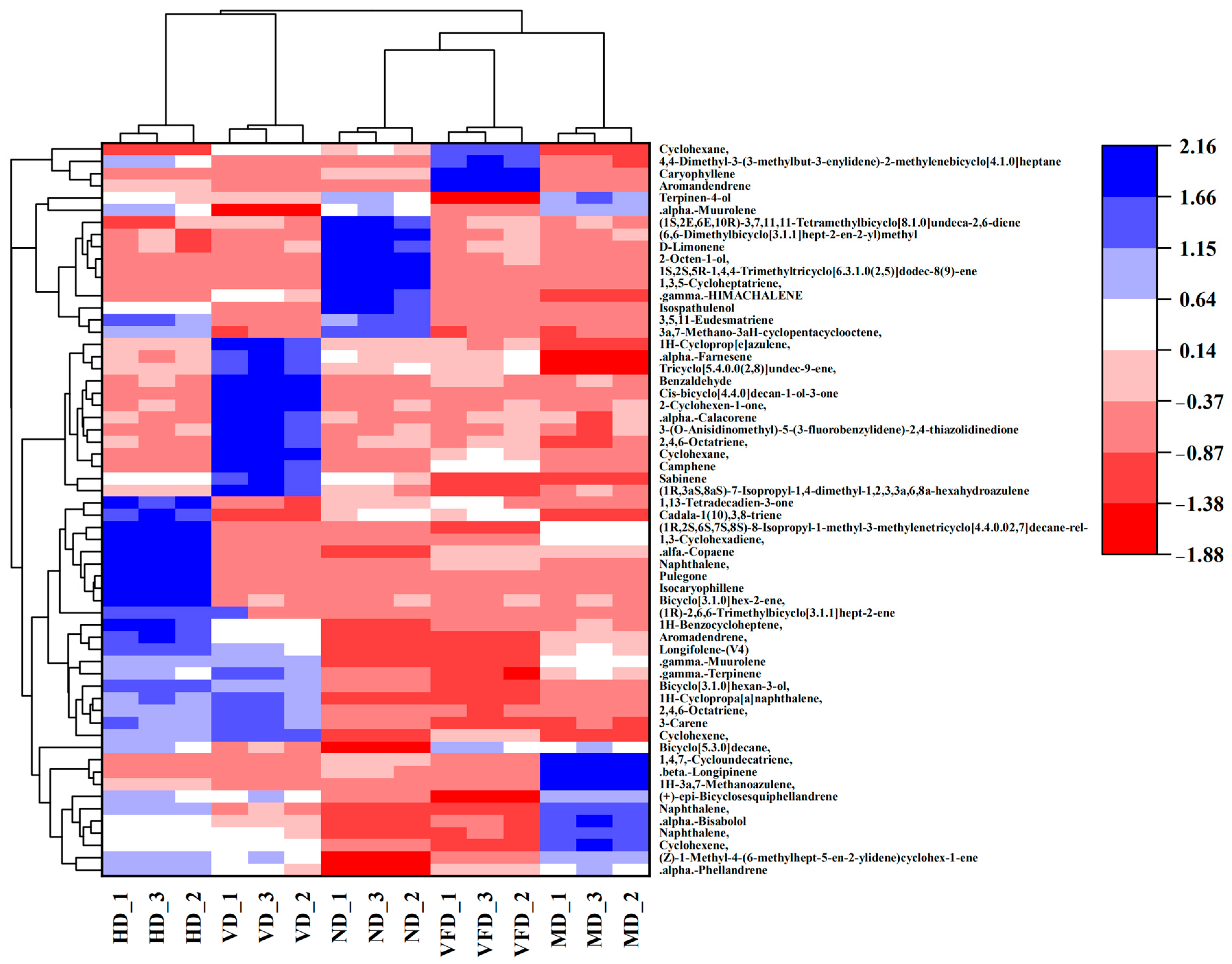
3.6. Volatile Compounds Evaluation
3.6.1. Preliminary Identification of Volatile Compounds
3.6.2. The Contribution of Volatile Compounds
3.7. Effect of Drying Treatments on the Metabolite Profiles of AS Fruit
3.8. Determination of Antioxidant Activity
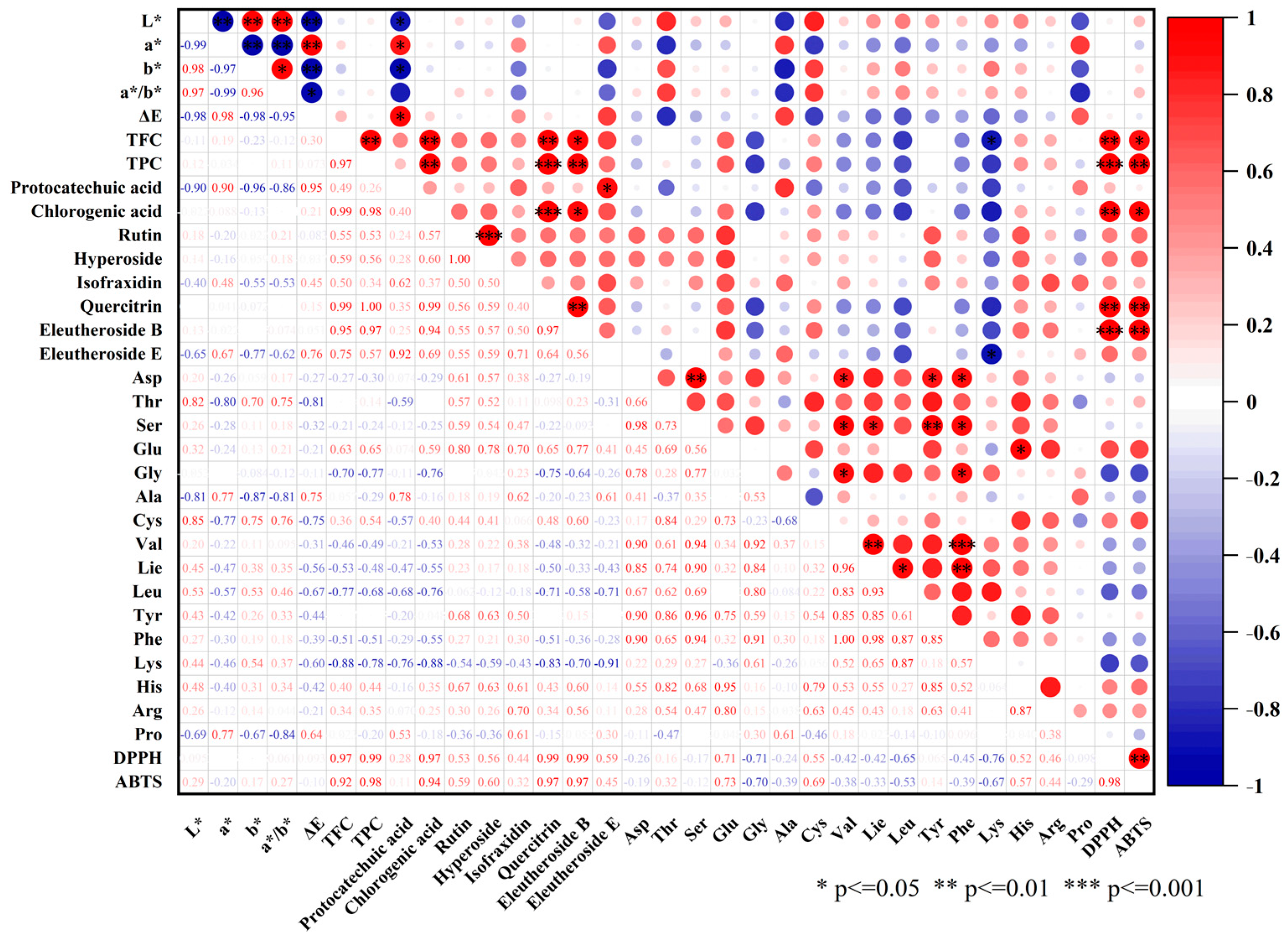
3.9. Correlation Analysis of Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity
3.9.1. Color Parameters
3.9.2. TPC, TFC, and Key Bioactive Compounds
3.9.3. Amino Acids
3.9.4. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Zhang, H.; Ding, L.; Xia, Y.; Dai, W.; Han, X.; Siqin, T.; You, X. Evaluation and Selection of Excellent Provenances of Eleutherococcus senticosus. Forests 2023, 14, 1359. [Google Scholar] [CrossRef]
- Jia, A.; Zhang, Y.; Gao, H.; Zhang, Z.; Zhang, Y.; Wang, Z.; Zhang, J.; Deng, B.; Qiu, Z.; Fu, C. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J. Ethnopharmacol. 2021, 268, 113586. [Google Scholar]
- Yan-Lin, S.; Lin-De, L.; Soon-Kwan, H. Eleutherococcus senticosus as a crude medicine: Review of biological and pharmacological effects. J. Med. Plants Res. 2011, 5, 5946–5952. [Google Scholar]
- Kim, C.; Bae, H.M.; Baik, I. Potential antiaging and hepatoprotective effects of Acanthopanax senticosus extracts in adult rat models. Rejuven. Res. 2023, 26, 51–56. [Google Scholar] [CrossRef]
- Shi, C.; Liang, Z.; Li, T.; Hao, Q.; Xiang, H.; Xie, Q. Metabolome and microbiome analyses of the anti-fatigue mechanism of Acanthopanax senticosus leaves. Food Funct. 2024, 15, 3791–3809. [Google Scholar]
- Chang, Y.; Jiang, Y.; Chen, J.; Li, S.; Wang, Y.; Chai, L.; Ma, J.; Wang, Z. Comprehensive analysis of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. fruits based on UPLC–MS/MS and GC–MS: A rapid qualitative analysis. Food Sci. Nutr. 2024, 12, 1911–1927. [Google Scholar] [PubMed]
- Vardanyan, A.; Ghalachyan, L.; Tadevosyan, A.; Gasparyan, T.; Sardaryan, A.; Daryadar, M. Antiradical activity and bioactive substances of Eleutherococcus senticosus (Rupr. and Maxim) grown under hydroponic and soil conditions in Ararat Valley and Dilijan Forest zone. Bioact. Compd. Health Dis. 2024, 7, 467–475. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, A.; Sun, H.; Zhang, Y.; Meng, X.; Yan, G.; Liu, L.; Wang, X. High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and characterization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms. J. Sep. Sci. 2017, 40, 2178–2187. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Cho, M.L.; Kim, D.-B.; Shin, G.-H.; Lee, J.-H.; Lee, J.S.; Park, S.-O.; Lee, S.-J.; Shin, H.M.; Lee, O.-H. The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281–13295. [Google Scholar]
- Jiang, Y.; Wang, M.-H. Different solvent fractions of Acanthopanax senticosus harms exert antioxidant and anti-inflammatory activities and inhibit the human Kv1. 3 channel. J. Med. Food 2015, 18, 468–475. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, S.; Hwang, G.; Jeon, C.; Kang, K. Effect of extract of Acanthopanax senticosus fruit on breast cancer cells. J. Intern. Korean Med. 2022, 43, 529–541. [Google Scholar]
- Cong, D.; Wang, H.; Gao, X.; Zheng, Y.; Zhang, C. Antifatigue Effect of Acanthopanax Senticosus Fruits. J. Jilin Univ. (Med. Ed.) 2010, 36, 891–894. [Google Scholar]
- Song, C.; Li, S.; Duan, F.; Liu, M.; Shan, S.; Ju, T.; Zhang, Y.; Lu, W. The therapeutic effect of acanthopanax senticosus components on radiation-induced brain injury based on the pharmacokinetics and neurotransmitters. Molecules 2022, 27, 1106. [Google Scholar] [CrossRef]
- Ahmed, N.; Singh, J.; Chauhan, H.; Anjum, P.G.A.; Kour, H. Different drying methods: Their applications and recent advances. Int. J. Food Nutr. Saf. 2013, 4, 34–42. [Google Scholar]
- Müller, J.; Heindl, A. Drying of medicinal plants. In Medicinal and Aromatic Plants; Springer: Berlin/Heidelberg, Germany, 2006; pp. 237–252. [Google Scholar]
- Zhang, X.; Guan, L.; Zhu, L.; Wang, K.; Gao, Y.; Li, J.; Yan, S.; Ji, N.; Zhou, Y.; Yao, X. A review of the extraction and purification methods, biological activities, and applications of active compounds in Acanthopanax senticosus. Front. Nutr. 2024, 11, 1391601. [Google Scholar]
- Hasan, M.U.; Malik, A.U.; Ali, S.; Imtiaz, A.; Munir, A.; Amjad, W.; Anwar, R. Modern drying techniques in fruits and vegetables to overcome postharvest losses: A review. J. Food Process. Preserv. 2019, 43, e14280. [Google Scholar]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar]
- Sari, K.R.P.; Ikawati, Z.; Danarti, R.; Hertiani, T. Micro-titer plate assay for measurement of total phenolic and total flavonoid contents in medicinal plant extracts. Arab. J. Chem. 2023, 16, 105003. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, S. Analysis of anthocyanin, flavonoids, and phenolic acids in tropical bignay berries. Int. J. Fruit Sci. 2008, 8, 15–34. [Google Scholar] [CrossRef]
- Jin, W.; Fan, X.; Jiang, C.; Liu, Y.; Zhu, K.; Miao, X.; Jiang, P. Characterization of non-volatile and volatile flavor profiles of Coregonus peled meat cooked by different methods. Food Chem. X 2023, 17, 100584. [Google Scholar]
- Kumar, N.; Ahmad, A.; Singh, S.; Pant, D.; Prasad, A.; Rastogi, S. Phytochemical analysis and antioxidant activity of Trigonella foenum-graecum seeds. J. Pharmacogn. Phytochem. 2021, 10, 23–26. [Google Scholar]
- Hussen, E.M.; Endalew, S.A. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement. Med. Ther. 2023, 23, 146. [Google Scholar] [CrossRef]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-oxidant and anti-enzymatic activities of sea buckthorn (Hippophaë rhamnoides L.) fruits modulated by chemical components. Antioxidants 2019, 8, 618. [Google Scholar] [PubMed]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, X.; Wang, S. Comparative analyses of three pretreatments on color of kiwifruits during hot air drying. Int. J. Agric. Biol. Eng. 2020, 13, 228–234. [Google Scholar] [CrossRef]
- Eshak, N.S. Quality attributes of some vegetables and fruits preserved by sun and oven drying methods. Alex. Sci. Exch. J. 2018, 39, 707–721. [Google Scholar] [CrossRef]
- Guclu, G.; Polat, S.; Kelebek, H.; Capanoglu, E.; Selli, S. Elucidation of the impact of four different drying methods on the phenolics, volatiles, and color properties of the peels of four types of citrus fruits. J. Sci. Food Agric. 2022, 102, 6036–6046. [Google Scholar] [CrossRef]
- Zia, M.P.; Alibas, I. Influence of the drying methods on color, vitamin C, anthocyanin, phenolic compounds, antioxidant activity, and in vitro bioaccessibility of blueberry fruits. Food Biosci. 2021, 42, 101179. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Albanese, D.; Cinquanta, L.; Cuccurullo, G.; Di Matteo, M. Effects of microwave and hot-air drying methods on colour, β-carotene and radical scavenging activity of apricots. Int. J. Food Sci. Technol. 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Tan, S.; Wang, W.; Wang, X.; Li, W.; Zhao, X. Effects of two drying methods on the stability and antioxidant activity of phenolic compounds in mulberry fruits. Int. Food Res. J. 2021, 28, 83. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, Y.; Ma, Y.; Zhang, M. Different thermal drying methods affect the phenolic profiles, their bioaccessibility and antioxidant activity in Rhodomyrtus tomentosa (Ait.) Hassk berries. LWT Food Sci. Technol. 2017, 79, 260–266. [Google Scholar] [CrossRef]
- Kohout, J. Modified Arrhenius equation in materials science, chemistry and biology. Molecules 2021, 26, 7162. [Google Scholar] [CrossRef] [PubMed]
- An, N.-n.; Zhao, S.-y.; Li, D.; Wang, Y.; Wang, L.-j. Hot-air flow rolling dry-blanching pretreatment improves the drying quality of Acanthopanax sessiliflorus by increasing the drying rate and inactivating enzymes. Foods 2022, 11, 3186. [Google Scholar] [CrossRef]
- Mbondo, N.N.; Owino, W.O.; Ambuko, J.; Sila, D.N. Effect of drying methods on the retention of bioactive compounds in African eggplant. Food Sci. Nutr. 2018, 6, 814–823. [Google Scholar] [CrossRef]
- Moon, J.H.; Chung, D.H.; Pan, C.-H.; Yoon, W.B. Determination of principal components for characterizing the drying of sea cucumbers (Stichopus japonicus Selenka) using far infrared radiation drying and hot air drying. J. Aquat. Food Prod. Technol. 2017, 26, 1221–1232. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sinha, B.K.; Chattopadhyay, A.K.; Mukherjee, S.; Sinha, B.K.; Chattopadhyay, A.K. Principal Component Analysis. Stat. Methods Soc. Sci. Res. 2018, 95–102. [Google Scholar]
- Lee, H.J.; Cho, I.H.; Lee, K.E.; Kim, Y.-S. The compositions of volatiles and aroma-active compounds in dried omija fruits (Schisandra chinensis Baillon) according to the cultivation areas. J. Agric. Food Chem. 2011, 59, 8338–8346. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [PubMed]
- Gökçe, G.F.; Özbay, M. The Effect of Different Amino Acids on the Development of Cucumber (Cucumis sativus L.) Plant Species Growing in Düzce Region. J. Anatol. Environ. Anim. Sci. 2019, 4, 575–580. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Lech, K.; Tkacz, K.; Nowicka, P. Influence of different drying methods on the quality of Japanese quince fruit. LWT 2019, 114, 108416. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Gunata, Z. Aroma compounds in fresh and dried mango fruit (Mangifera indica L. cv. Kent): Impact of drying on volatile composition. Int. J. Food Sci. Technol. 2016, 51, 789–800. [Google Scholar]
- Liu, H.; Yu, Y.; Zou, B.; Yu, Y.; Yang, J.; Xu, Y.; Chen, X.; Yang, F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar]
- González-Bonilla, S.M.; Marín-Arroyo, M.R. Characterization and classification of lulo (Solanum quitoense Lam.) fruits by ripening stage using partial least squares discriminant analysis (PLS-DA). Agron. Colomb. 2022, 40, 419–428. [Google Scholar]
- Xiao, O.; Li, M.; Chen, D.; Chen, J.; Simal-Gandara, J.; Dai, X.; Kong, Z. The dissipation, processing factors, metabolites, and risk assessment of pesticides in honeysuckle from field to table. J. Hazard. Mater. 2022, 431, 128519. [Google Scholar]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Ouyang, H.-J.; Liu, Y.-J.; Yuan, Y.; Jingwei; Zhang, L.; Li, J.-H. HS-SPME-GC-MS coupled with OPLS-DA to analyze the effects of extraction methods on volatile aroma compounds of avocado oil. J. South. Agric. 2021, 52, 779–788. [Google Scholar]
- Kumar, N.; Hoque, M.A.; Sugimoto, M. Robust volcano plot: Identification of differential metabolites in the presence of outliers. BMC Bioinform. 2018, 19, 128. [Google Scholar]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [PubMed]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M. Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. [Google Scholar]
- Shah, P.; Modi, H. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E. A review on the effect of drying on antioxidant potential of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Fidrianny, I.; Elviana, D.; Ruslan, K. In vitro antioxidant activities in various beans extracts of five legumes from West of Java-Indonesia using DPPH and ABTS methods. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 470–476. [Google Scholar]
- Minuye, M.; Yenasew, A.; Belew, S. Effect of drying method on the nutritional and antioxidant properties of mango, avocado, and tomato. J. Hortic. Res. 2024, 32, 43–50. [Google Scholar]
- Yadav, S. Correlation analysis in biological studies. J. Pract. Cardiovasc. Sci. 2018, 4, 116–121. [Google Scholar]
- Pandey, S. Principles of correlation and regression analysis. J. Pract. Cardiovasc. Sci. 2020, 6, 7–11. [Google Scholar]
- Akcicek, A.; Avci, E.; Tekin-Cakmak, Z.H.; Kasapoglu, M.Z.; Sagdic, O.; Karasu, S. Influence of different drying techniques on the drying kinetics, total bioactive compounds, anthocyanin profile, color, and microstructural properties of blueberry fruit. ACS Omega 2023, 8, 41603–41611. [Google Scholar]
- Turkmen, F.; Karasu, S.; Karadag, A. Effects of different drying methods and temperature on the drying behavior and quality attributes of cherry laurel fruit. Processes 2020, 8, 761. [Google Scholar] [CrossRef]
- Ozay-Arancioglu, I.; Bekiroglu, H.; Karadag, A.; Saroglu, O.; Tekin-Çakmak, Z.H.; Karasu, S. Effect of different drying methods on the bioactive, microstructural, and in-vitro bioaccessibility of bioactive compounds of the pomegranate arils. Food Sci. Technol. 2021, 42, e06221. [Google Scholar] [CrossRef]
- Munir, A.; Anwar, A.; Sarfraz, A.; Qin, H.; Boran, H. Effect of Freeze-Drying on Apple Pomace and Pomegranate Peel Powders Used as a Source of Bioactive Ingredients for the Development of Functional Yogurt. J. Food Qual. 2022, 2022, 9. [Google Scholar]
- Holland, C.K.; Jez, J.M. Structural Biology of Aromatic Amino Acid Biosynthesis in Plants: The Prephenate Branch Point. FASEB J. 2016, 30, 1137. [Google Scholar] [CrossRef]
- Velisek, J.; Cejpek, K. Biosynthesis of food constituents: Amino acids: 2. The alanine-valine-leucine, serine-cysteine-glycine, and aromatic and heterocyclic amino acids groups. Czech J. Food Sci. 2006, 24, 45. [Google Scholar] [CrossRef]
- Sayre, J.M. Microbial Diversity and Carbon Dynamics in a Forage Crop System. Ph.D. Thesis, University of California, Davis, CA, USA, 2023. [Google Scholar]
- Pan, S.; Fan, M.; Liu, Z.; Li, X.; Wang, H. Serine, glycine and one-carbon metabolism in cancer. Int. J. Oncol. 2020, 58, 158–170. [Google Scholar] [CrossRef]
- Polivanova, O.; Cherednichenko, M.Y. Regulation and metabolic engineering of the general phenylpropanoid pathway in response to stress in plants. Probl. Biol. Med. Pharm. Chem. 2023, 26, 3–9. [Google Scholar] [CrossRef]
- Wojdyło, A.; Lech, K.; Nowicka, P. Effects of different drying methods on the retention of bioactive compounds, on-line antioxidant capacity and color of the novel snack from red-fleshed apples. Molecules 2020, 25, 5521. [Google Scholar] [CrossRef]
- Dadhaneeya, H.; Kesavan, R.K.; Inbaraj, B.S.; Sharma, M.; Kamma, S.; Nayak, P.K.; Sridhar, K. Impact of different drying methods on the phenolic composition, in vitro antioxidant activity, and quality attributes of dragon fruit slices and pulp. Foods 2023, 12, 1387. [Google Scholar] [CrossRef]
- Lu, H.; Peng, S.; Xu, N.; Shang, X.; Liu, J.; Xu, Z.; Jiang, N.; Dong, H.; Wang, R.; Dong, H. Exploring the effects of different drying methods on related differential metabolites of Pleurotus citrinopileatus singer based on untargeted metabolomics. Plants 2024, 13, 1594. [Google Scholar] [CrossRef]
- Peries, C.M.; Navarthne, S.; Wijesinghe, J.; Henagamage, A.P.; Coorey, R. Effect of different drying methods on antioxidant activity and availability of phytochemicals in leaves of Costus speciosus, Coccinia grandis and Gymnema sylvestre. Asian J. Eng. Appl. Technol. 2022, 11, 29–35. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Saensouk, S. Taxonomy, phytochemical and bioactive compounds and potential use as material with different drying methods of Alpinia latilabris Ridl. new record from Thailand. Not. Bot. Horti Agrobot. 2022, 50, 12619. [Google Scholar] [CrossRef]
- Wongklom, A. Effect of drying methods on antioxidant capacity, total phenolic and flavonoid contents of Phakwan (Sauropus androgynus (L.) Merr.) powder. Creat. Sci. 2018, 10, 96–103. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Nicolescu, A.; Bunea, C.I.; Mocan, A. Total flavonoid content revised: An overview of past, present, and future determinations in phytochemical analysis. Anal. Biochem. 2025, 700, 115794. [Google Scholar] [CrossRef]
- Zitouni, H.; Fauconnier, M.L.; Hssaini, L.; Ouaabou, R.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Haddou, L.A.; Messaoudi, Z.; Hanine, H. Phenols, volatile compounds, organic acids and antioxidant activity of strawberry tree (Arbutus unedo L.) fruits belonging to five genotypes growing in morocco. Int. J. Fruit Sci. 2022, 22, 414–437. [Google Scholar] [CrossRef]
- Tufekci, S.; Özkal, S.G. The Optimization of Hybrid (Microwave-Conventional) Drying of Sweet Potato Using Response Surface Methodology (RSM). Foods 2023, 12, 3003. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, S. Modeling and optimization of spray drying process parameters using artificial neural network and genetic algorithm for the production of probiotic (Lactiplantibacillus plantarum) finger millet milk powder. J. Food Process Eng. 2024, 47, e14505. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Morais, S.F.; Kulozik, U. Storage stability of dried raspberry foam as a snack product: Effect of foam structure and microwave-assisted freeze drying on the stability of plant bioactives and ascorbic acid. J. Food Eng. 2020, 270, 109779. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, X.; Li, Y.; Sun, X.; Wang, D.; Wang, W. Response of bioactive phytochemicals in vegetables and fruits to environmental factors. Eur. J. Nutr. Food Saf. 2019, 9, 233–247. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Q.; Li, Z.; Chen, S.; Liu, Y.; Qi, P.; Wei, Y.; Zheng, Y. Planting locations with higher temperature produce more bioactive compounds and antioxidant capacities of wheat. Agronomy 2019, 9, 538. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Su, J.; Chu, X. Acanthopanax senticosus total flavonoids alleviate lipopolysaccharide-induced intestinal inflammation and modulate the gut microbiota in mice. Biosci. Rep. 2022, 42, BSR20212670. [Google Scholar] [CrossRef]
- Oh, E.; Kim, Y.; Park, S.-y.; Lim, Y.; Shin, J.-y.; Kim, J.Y.; Kim, J.-H.; Rhee, M.-Y.; Kwon, O. The fruit of Acanthopanax senticosus Harms improves arterial stiffness and blood pressure: A randomized, placebo-controlled trial. Nutr. Res. Pract. 2020, 14, 322–333. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Liu, R.; Zhang, X.; Xue, J.; Li, Y.; Zhang, R.; Li, X.; Wang, X.; Wang, X. Antioxidant and Antiapoptotic Properties of n-Butanol Fraction the Acanthopanax senticosus Extracts in H2O2-RAW264. 7 Cells and CCl4-Induced Liver Injury in Mice. Evid.-Based Complement. Altern. Med. 2023, 2023, 9190198. [Google Scholar] [CrossRef]


| Sample Code | HD | VD | ND | MD | VFD | Fresh Fruit |
|---|---|---|---|---|---|---|
| L* | 22.66 ± 0.56 f | 30.36 ± 0.26 c | 26.92 ± 0.18 e | 27.41 ± 0.22 d | 33.56 ± 0.23 a | 32.01 ± 0.16 b |
| a* | 4.91 ± 0.10 a | 1.25 ± 0.10 d | 3.17 ± 0.15 b | 2.18 ± 0.14 c | 0.52 ± 0.02 e | −1.12 ± 0.04 f |
| b* | −7.11 ± 0.03 d | −4.37 ± 0.19 c | −6.42 ± 0.08 d | −6.38 ± 0.05 d | −3.33 ± 0.21 b | −2.45 ± 0.11 a |
| a*/b* | −0.69 ± 0.01 f | −0.29 ± 0.03 c | −0.49 ± 0.02 e | −0.34 ± 0.02 b | −0.16 ± 0.01 d | 0.46 ± 0.02 a |
| ΔE | 11.11 ± 0.50 a | 2.53 ± 0.20 d | 6.77 ± 0.26 b | 6.14 ± 0.24 c | 1.88 ± 0.33 e | 0.00 |
| MC% | 9.48 ± 0.02 c | 8.54 ± 0.12 d | 14.97 ± 0.12 b | 6.63 ± 0.18 e | 5.91 ± 0.14 f | 82.95 ± 0.28 a |
| Sample Code | HD | VD | ND | MD | VFD |
|---|---|---|---|---|---|
| Protocatechuic acid (mg/100 g) | 5.4 ± 0.3 a | 1.8 ± 0.2 d | 3.5 ± 0.1 b | 3.6 ± 0.1 b | 2.5 ± 0.2 c |
| Chlorogenic acid (mg/100 g) | 294 ± 2 d | 71.6 ± 0.2 e | 499 ± 1 b | 486 ± 8 c | 601 ± 6 a |
| Rutin (mg/100 g) | 15.5 ± 0.3 b | 6.7 ± 0.6 d | 6.9 ± 0.3 d | 13.9 ± 0.4 c | 23.3 ± 0.6 a |
| Hyperoside (mg/100 g) | 72.0 ± 1.5 b | 40.0 ± 0.4 e | 42.3 ± 1.6 d | 68.1 ± 3.7 c | 96.6 ± 1.2 a |
| Isofraxidin (mg/100 g) | 2.32 ± 0.03 a | 0.52 ± 0.03 d | 1.28 ± 0.03 c | 0.49 ± 0.02 d | 1.67 ± 0.05 b |
| Quercitrin (mg/100 g) | 238 ± 5 d | 66.9 ± 1.3 e | 434 ± 4 b | 351 ± 5 c | 535 ± 6 a |
| Eleutheroside B (mg/100 g) | 65.9 ± 1.1 c | 21.0 ± 0.3 d | 124 ± 1 b | 69.2 ± 3.8 c | 162 ± 2 a |
| Eleutheroside E (mg/100 g) | 20.5 ± 0.5 a | 11.8 ± 0.2 d | 16.4 ± 0.4 c | 17.3 ± 0.7 b | 16.4 ± 0.2 c |
| Amino Acid (mg/g) | HD | VD | ND | MD | VFD |
|---|---|---|---|---|---|
| Asp | 0.54 ± 0.02 b | 0.48 ± 0.01 c | 0.14 ± 0.01 e | 0.31 ± 0.01 d | 0.57 ± 0.02 a |
| Thr | 0.95 ± 0.03 c | 1.83 ± 0.06 b | 0.72 ± 0.02 d | 0.92 ± 0.03 cd | 3.54 ± 0.11 a |
| Ser | 0.62 ± 0.02 b | 0.55 ± 0.02 c | 0.17 ± 0.01 e | 0.26 ± 0.01 d | 0.71 ± 0.02 a |
| Glu | 0.58 ± 0.02 b | 0.31 ± 0.01 ce | 0.49 ± 0.02 c | 0.37 ± 0.01 d | 0.90 ± 0.03 a |
| Gly | 0.073 ± 0.006 ab | 0.080 ± 0.002 a | 0.024 ± 0.006 cd | 0.027 ± 0.011 cd | 0.050 ± 0.002 bc |
| Ala | 0.88 ± 0.03 a | 0.37 ± 0.01 b | 0.29 ± 0.01 bc | 0.37 ± 0.02 b | 0.28 ± 0.01 bc |
| Cys | 0.082 ± 0.011 c | 0.090 ± 0.003 bc | 0.094 ± 0.005 b | 0.088 ± 0.007 b c | 0.110 ± 0.003 a |
| Val | 0.34 ± 0.01 b | 0.36 ± 0.01 a | 0.13 ± 0.02 c | 0.12 ± 0.01 c | 0.34 ± 0.01 b |
| Met | n.d. | n.d. | n.d. | n.d. | 0.040 ± 0.001 a |
| Ile | 0.19 ± 0.01 c | 0.28 ± 0.01 a | 0.064 ± 0.005 d | 0.060 ± 0.002 d | 0.26 ± 0.01 b |
| Leu | 0.18 ± 0.01 c | 0.45 ± 0.01 a | 0.074 ± 0.012 e | 0.090 ± 0.003 d | 0.29 ± 0.01 b |
| Tyr | 0.18 ± 0.01 b | 0.17 ± 0.01 bc | 0.13 ± 0.01 cd | 0.13 ± 0.01 cd | 0.22 ± 0.01 a |
| Phe | 0.31 ± 0.01 c | 0.38 ± 0.01 a | 0.023 ± 0.006 d | 0.030 ± 0.001 d | 0.34 ± 0.01 b |
| Lys | 0.026 ± 0.006 cd | 0.21 ± 0.01 a | 0.054 ± 0.005 bc | 0.010 ± 0.000 de | 0.060 ± 0.002 b |
| His | 0.090 ± 0.003 b | 0.060 ± 0.002 d | 0.074 ± 0.005 c | 0.030 ± 0.001 e | 0.180 ± 0.005 a |
| Arg | 2.63 ± 0.08 c | 2.07 ± 0.08 d | 2.90 ± 0.09 b | 1.07 ± 0.03 e | 3.61 ± 0.11 a |
| Pro | 0.29 ± 0.01 a | 0.16 ± 0.01 c | 0.25 ± 0.01 b | 0.050 ± 0.002 e | 0.090 ± 0.003 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; He, Z.; Song, X.; Zhang, X.; Xiao, Y.; Yu, J.; Yang, M.; Tang, Z. Evaluation of Different Drying Methods on the Quality Parameters of Acanthopanax senticosus Fruits. Foods 2025, 14, 1100. https://doi.org/10.3390/foods14071100
Zhao C, He Z, Song X, Zhang X, Xiao Y, Yu J, Yang M, Tang Z. Evaluation of Different Drying Methods on the Quality Parameters of Acanthopanax senticosus Fruits. Foods. 2025; 14(7):1100. https://doi.org/10.3390/foods14071100
Chicago/Turabian StyleZhao, Chunbo, Zhiqiang He, Xiaoqian Song, Xiaoning Zhang, Yu Xiao, Jia Yu, Minghui Yang, and Zhonghua Tang. 2025. "Evaluation of Different Drying Methods on the Quality Parameters of Acanthopanax senticosus Fruits" Foods 14, no. 7: 1100. https://doi.org/10.3390/foods14071100
APA StyleZhao, C., He, Z., Song, X., Zhang, X., Xiao, Y., Yu, J., Yang, M., & Tang, Z. (2025). Evaluation of Different Drying Methods on the Quality Parameters of Acanthopanax senticosus Fruits. Foods, 14(7), 1100. https://doi.org/10.3390/foods14071100







