A Spermidine Derivative Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Inhibiting the MAPK4/AKT Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
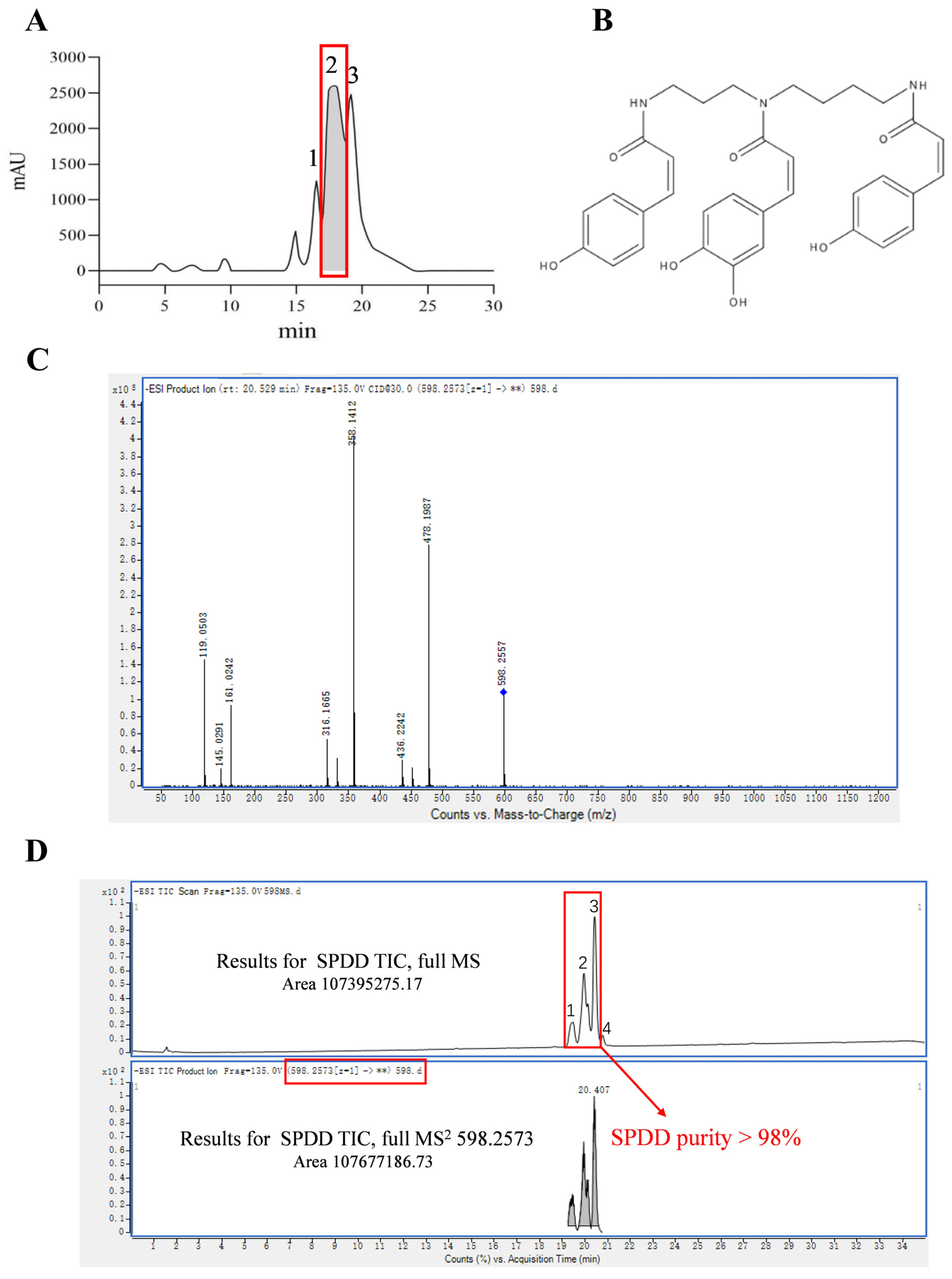
2.2. Extraction, Purification, and Identification of SPDD
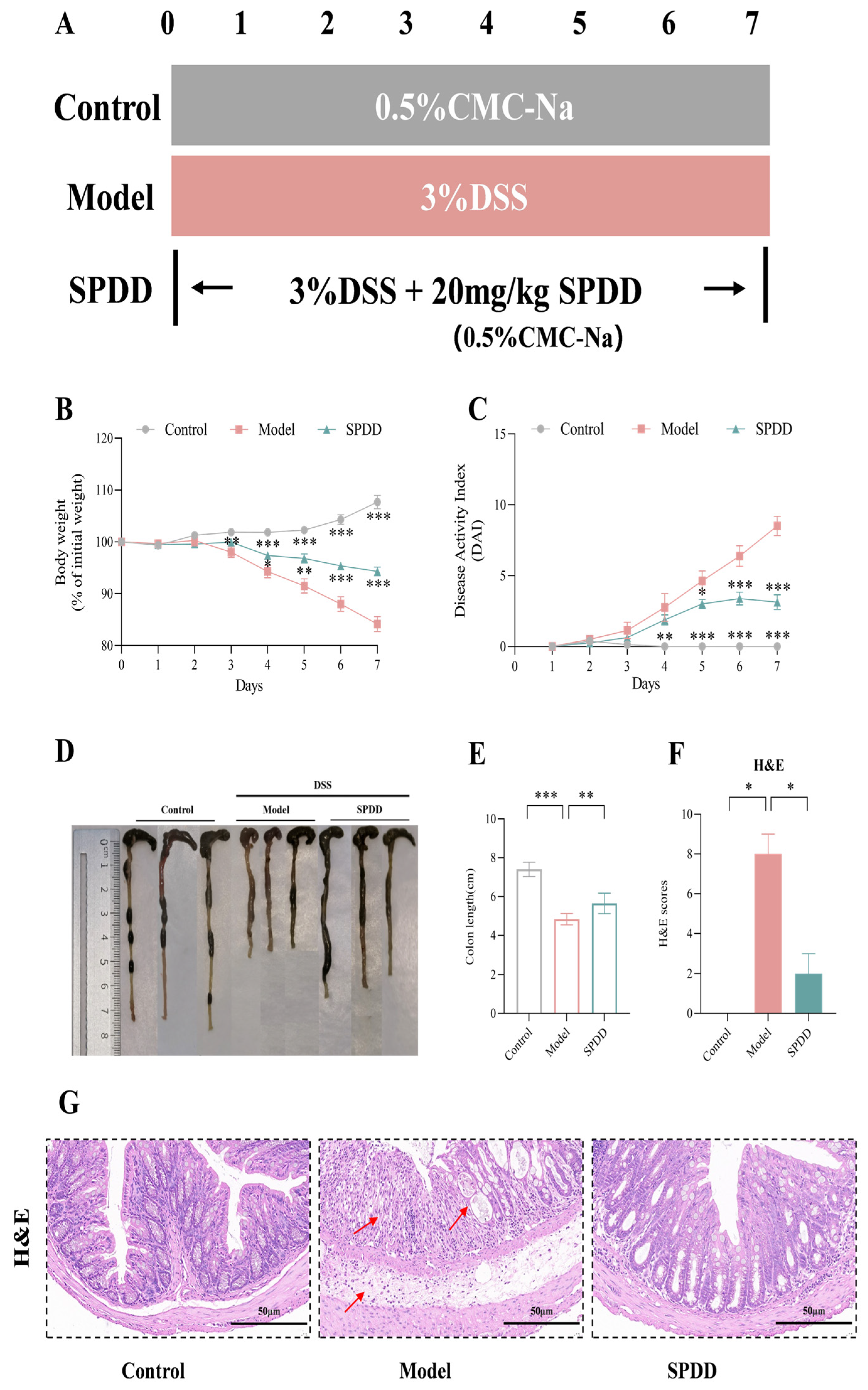
2.3. Animals and Experimental Design
2.4. DAI Scores
2.5. H and E Staining and Histological Analysis
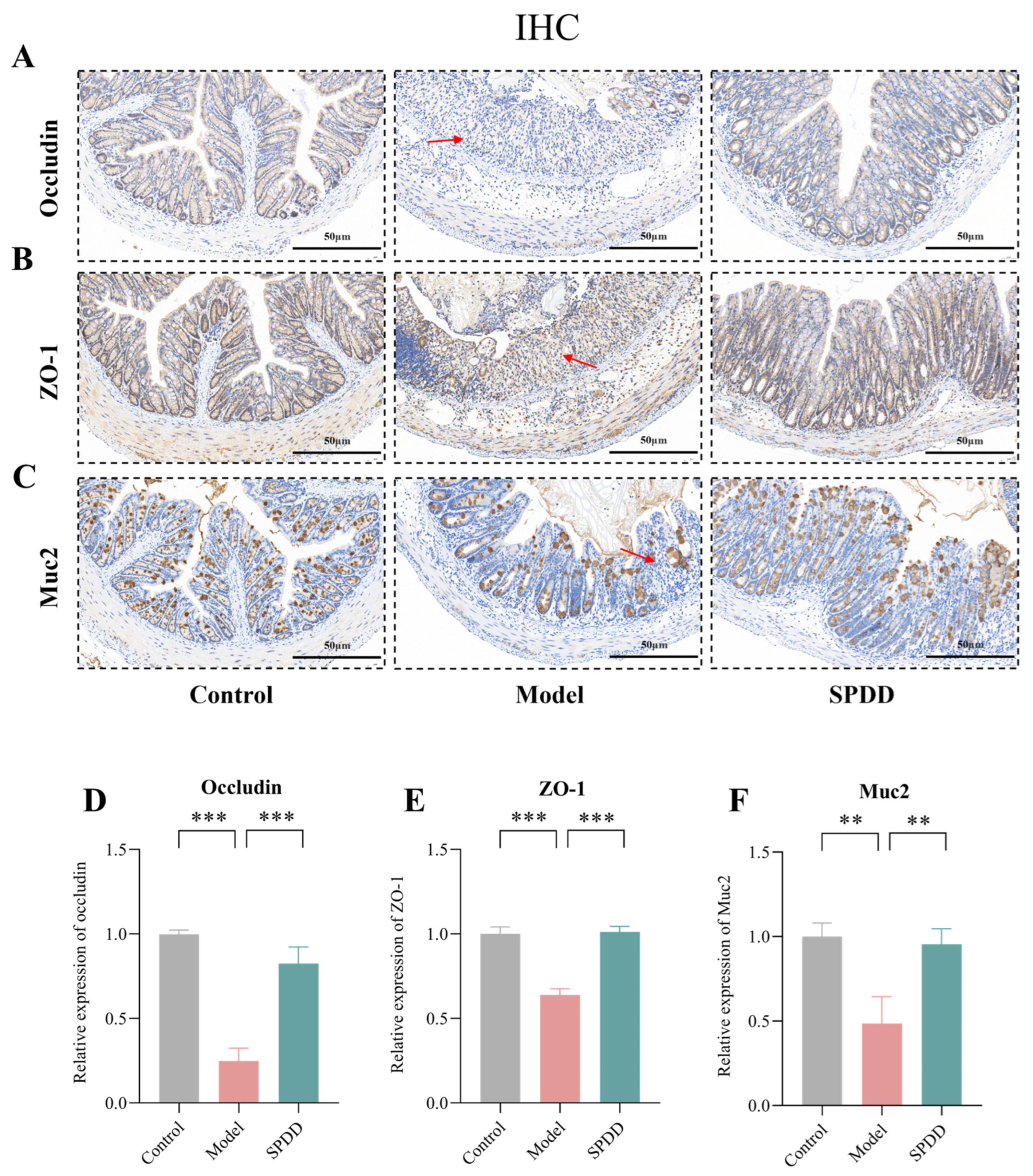
2.6. Immunohistochemistry Analysis
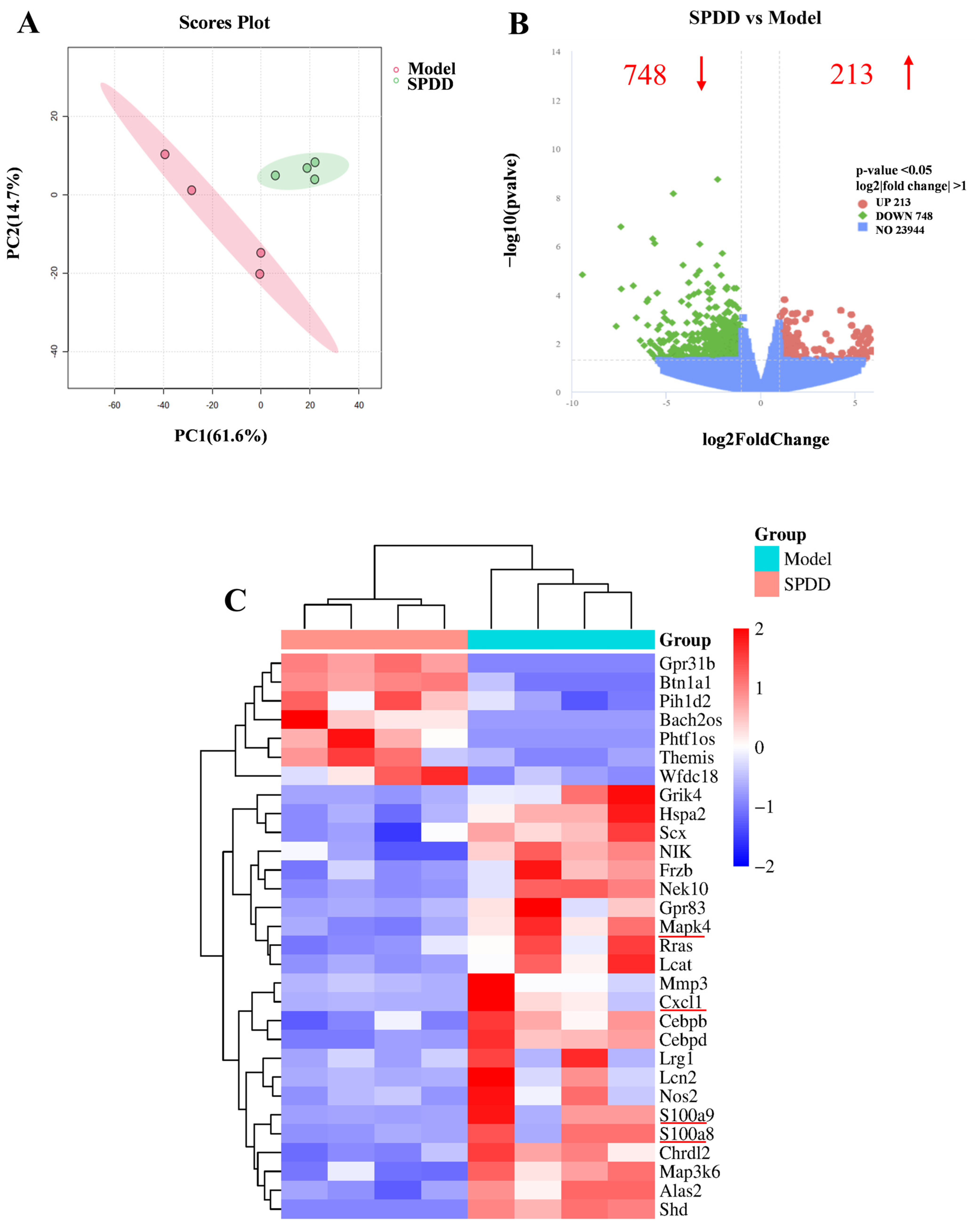
2.7. RNA-Seq Analysis
2.8. Cell Experiment
2.9. Real-Time Quantitative PCR Analysis
2.10. Western Blot Analysis
2.11. Co-Immunoprecipitation (Co-IP) Analysis
2.12. Statistical Analysis
3. Results
3.1. Purification and Identification of SPDD
3.2. SPDD Ameliorated the Weight Loss, Disease Activity Index, and Tissue Damage in Mice with Colitis
3.3. SPDD Improved Intestinal Barrier Function in Mice with Colitis
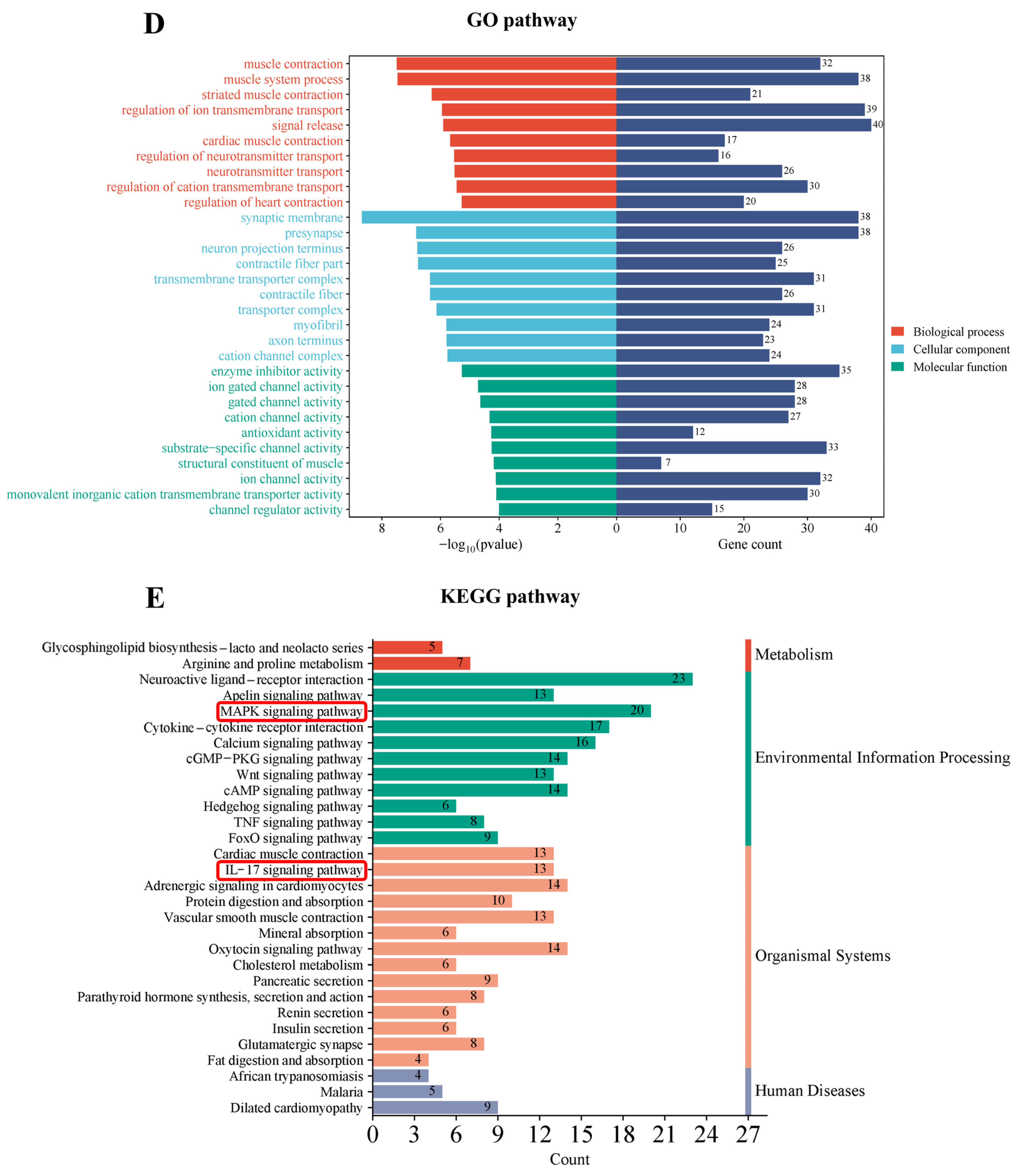
3.4. Transcriptomic Profiling and Pathway Analysis
3.5. SPDD Inhibited MAPK4/AKT Signaling in DSS-Induced Colitis
3.6. SPDD Inhibited the Interaction Between MAPK4 and AKT, Reducing the Phosphorylation Level of AKT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, K.; Mao, T.; Lu, X.; Wang, M.; Yun, Y.; Jia, Z.; Shi, L.; Jiang, H.; Li, J.; Shi, R. A potential therapeutic approach for ulcerative colitis: Targeted regulation of macrophage polarization through phytochemicals. Front. Immunol. 2023, 14, 1155077. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Finley, J.L.; Smith, T.M.; McNamara, K.M.; Singh, K.; Sierra, J.C.; et al. Protective Role of Spermidine in Colitis and Colon Carcinogenesis. Gastroenterology 2022, 162, 813–827.e818. [Google Scholar] [CrossRef]
- Xue, J.-C.; Yuan, S.; Meng, H.; Hou, X.-T.; Li, J.; Zhang, H.-M.; Chen, L.-L.; Zhang, C.-H.; Zhang, Q.-G. The role and mechanism of flavonoid herbal natural products in ulcerative colitis. Biomed. Pharmacother. 2023, 158, 114086. [Google Scholar] [CrossRef]
- Bamias, G.; Nyce, M.R.; De La Rue, S.A.; Cominelli, F. New concepts in the pathophysiology of inflammatory bowel disease. Ann. Intern. Med. 2005, 143, 895–904. [Google Scholar] [CrossRef]
- Hirten, R.P.; Sands, B.E. New therapeutics for ulcerative colitis. Annu. Rev. Med. 2021, 72, 199–213. [Google Scholar] [CrossRef]
- Samanta, A.K.; Torok, V.A.; Percy, N.J.; Abimosleh, S.M.; Howarth, G.S. Microbial fingerprinting detects unique bacterial communities in the faecal microbiota of rats with experimentally-induced colitis. J. Microbiol. 2012, 50, 218–225. [Google Scholar] [CrossRef]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Morón, B.; Spalinger, M.; Kasper, S.; Atrott, K.; Frey-Wagner, I.; Fried, M.; McCole, D.F.; Rogler, G.; Scharl, M. Activation of protein tyrosine phosphatase non-receptor type 2 by spermidine exerts anti-inflammatory effects in human THP-1 monocytes and in a mouse model of acute colitis. PLoS ONE 2013, 8, e73703. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Mao, X.; Hu, S.; Wang, S.; Liu, X.; Sun, J. Spermidine protects intestinal mucosal barrier function in mice colitis via the AhR/Nrf2 and AhR/STAT3 signaling pathways. Int. Immunopharmacol. 2023, 119, 110166. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Coviello, L.C.; Stein, S.L. Surgical Management of Nonpolypoid Colorectal Lesions and Strictures in Colonic Inflammatory Bowel Disease. Gastrointest. Endosc. Clin. N. Am. 2014, 24, 447–454. [Google Scholar] [CrossRef]
- He, Y.; Sun, Z.; Bai, J.; Zhang, Y.; Qian, Y.; Zhao, X.; Chen, S. Citrus peel polyphenols alleviate intestinal inflammation in mice with dextran sulfate sodium-induced acute colitis. Heliyon 2023, 9, e18137. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Wang, T.; Shi, F. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis. Int. Immunopharmacol. 2017, 50, 1–5. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Zhang, J.; Wang, Y.-W.; Ding, K.; Yan, Y.; Xia, C.-Y.; Li, X.-X.; He, J.; Zhang, W.-K.; Xu, J.-K. The untapped potential of spermidine alkaloids: Sources, structures, bioactivities and syntheses. Eur. J. Med. Chem. 2022, 240, 114600. [Google Scholar] [CrossRef]
- Madeo, F.; Hofer, S.J.; Pendl, T.; Bauer, M.A.; Eisenberg, T.; Carmona-Gutierrez, D.; Kroemer, G. Nutritional aspects of spermidine. Annu. Rev. Nutr. 2020, 40, 135–159. [Google Scholar] [CrossRef]
- Wang, W.; Snooks, H.D.; Sang, S.A.-O. The Chemistry and Health Benefits of Dietary Phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef]
- Nakase, K.; Kimura, I.; Kimura, M. Effects of pollen-extract components, diamines and derivatives of feruloylputrescine on isolated bladder and urethral smooth muscles of mice. Jpn. J. Pharmacol. 1990, 53, 157–164. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Di, R.; Baibado, J.T.; Cheng, Y.-S.; Huang, Y.-Q.; Sun, H.; Cheung, H.-Y. Identification of kukoamines as the novel markers for quality assessment of Lycii Cortex. Food Res. Int. 2014, 55, 373–380. [Google Scholar] [CrossRef]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hattori, M. Inhibitory effects on HIV-1 protease of tri-p-coumaroylspermidine from Artemisia caruifolia and related amides. Chem. Pharm. Bull. 2001, 49, 915–917. [Google Scholar] [CrossRef]
- Mancuso, F.; Calignano, A.; Cozzolino, A.; Metafora, S.; Porta, R. Inhibition of zymosan-induced air-pouch inflammation by rat seminal vesicle protein and by its spermidine derivative. Eur. J. Pharmacol. 1996, 312, 327–332. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Deng, Z.; Li, G.; Li, H. Impact of particle size on the nutrition release and antioxidant activity of rape, buckwheat and rose bee pollens. Food Funct. 2023, 14, 1897–1908. [Google Scholar] [CrossRef]
- Ma, L.; Ni, L.; Yang, T.; Mao, P.; Huang, X.; Luo, Y.; Jiang, Z.; Hu, L.; Zhao, Y.; Fu, Z.; et al. Preventive and Therapeutic Spermidine Treatment Attenuates Acute Colitis in Mice. J. Agric. Food Chem. 2021, 69, 1864–1876. [Google Scholar] [CrossRef]
- Kihara, N.; De la Fuente, S.; Fujino, K.; Takahashi, T.; Pappas, T.; Mantyh, C. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003, 52, 713–719. [Google Scholar] [CrossRef]
- Wlodarska, M.; Thaiss, C.A.; Nowarski, R.; Henao-Mejia, J.; Zhang, J.-P.; Brown, E.M.; Frankel, G.; Levy, M.; Katz, M.N.; Philbrick, W.M.; et al. NLRP6 Inflammasome Orchestrates the Colonic Host-Microbial Interface by Regulating Goblet Cell Mucus Secretion. Cell 2014, 156, 1045–1059. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, X.; Zhang, K.; Wu, H.; Dong, Y.; Zhou, F.; Cheng, B.; Li, L.; Xu, W.; Su, J.; et al. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J. Ethnopharmacol. 2022, 289, 115001. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.; Zheng, X.; Li, K.; Zhang, Y.; Pang, X.; Gao, J.; Lou, Z. Linderae Radix extract attenuates ulcerative colitis by inhibiting the JAK/STAT signaling pathway. Phytomedicine 2024, 132, 155868. [Google Scholar] [CrossRef]
- He, Y.; Sun, Y.A.-O.; Li, J.A.-O.; Peng, X.; Li, W.; Gao, Y.A.-O.; Wang, J.; Ni, X.; Pan, L.; Deng, Z.A.-O. Effects of Human Milk Fat Substitutes on Lipid Metabolism in First-Weaned Rats. J. Agric. Food Chem. 2023, 71, 13906–13919. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wei, Y.; Wang, Y.A.-O.; Wei, X.A.-O. Amelioration Effects and Regulatory Mechanisms of Different Tea Active Ingredients on DSS-Induced Colitis. J. Agric. Food Chem. 2023, 71, 16604–16617. [Google Scholar] [CrossRef]
- Sha, Z.; Shang, H.; Miao, Y.; Huang, J.; Niu, X.; Chen, R.; Peng, D.; Wei, K.; Zhu, R. Polysaccharides from Pinus massoniana pollen improve intestinal mucosal immunity in chickens. Poult. Sci. 2021, 100, 507–516. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; Jiao, X.; Zhang, J.; Zhang, C.; Wang, Z. Oxysophocarpine suppresses TRAF6 level to ameliorate oxidative stress and inflammatory factors secretion in mice with dextran sulphate sodium (DSS) induced-ulcerative colitis. Microb. Pathog. 2023, 182, 106244. [Google Scholar] [CrossRef]
- Mao, N.; Yu, Y.; He, J.; Yang, Y.; Liu, Z.; Lu, Y.; Wang, D. Matrine Ameliorates DSS-Induced Colitis by Suppressing Inflammation, Modulating Oxidative Stress and Remodeling the Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 6613. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.A.-O. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Wang, W.; Shen, T.; Dong, B.; Creighton, C.J.; Meng, Y.; Zhou, W.; Shi, Q.; Zhou, H.; Zhang, Y.; Moore, D.D.; et al. MAPK4 overexpression promotes tumor progression via noncanonical activation of AKT/mTOR signaling. J. Clin. Investig. 2019, 129, 1015–1029. [Google Scholar] [CrossRef]
- Kunisada, M.; Hosaka, C.; Takemori, C.; Nakano, E.; Nishigori, C. CXCL1 Inhibition Regulates UVB-Induced Skin Inflammation and Tumorigenesis in Xpa-Deficient Mice. J. Investig. Dermatol. 2017, 137, 1975–1983. [Google Scholar] [CrossRef]
- Shou, C.; Sun, Y.; Zhang, Q.; Zhang, W.; Yan, Q.; Xu, T.; Li, H. S100A9 Inhibition Mitigates Acute Pancreatitis by Suppressing RAGE Expression and Subsequently Ameliorating Inflammation. Inflammation 2024, 1–12. [Google Scholar] [CrossRef]
- Singh, P.; Ananthakrishnan, A.; Ahuja, V. Pivot to Asia: Inflammatory bowel disease burden. Intest. Res. 2017, 15, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114.e2105. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Rajput, R.; Gupta, G.; Ydyrys, A.; Kulbayeva, M.; Abdull Razis, A.F.; et al. Spermidine as a promising anticancer agent: Recent advances and newer insights on its molecular mechanisms. Front. Chem. 2023, 11, 1164477. [Google Scholar] [CrossRef]
- Pietrocola, F.; Castoldi, F.; Kepp, O.A.-O.; Carmona-Gutierrez, D.; Madeo, F.; Kroemer, G.A.-O. Spermidine reduces cancer-related mortality in humans. Autophagy 2018, 359, eaan2788. [Google Scholar] [CrossRef]
- Ekhtiar, M.; Ghasemi-Dehnoo, M.; Mirzaei, Y.; Azadegan-Dehkordi, F.; Amini-Khoei, H.; Lorigooini, Z.; Samiei-Sefat, A.; Bagheri, N. The coumaric acid and syringic acid ameliorate acetic acid-induced ulcerative colitis in rats via modulator of Nrf2/HO-1 and pro-inflammatory cytokines. Int. Immunopharmacol. 2023, 120, 110309. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Shen, P.; Li, S.; Lu, X.; Liu, J.; Cao, Y.; Liu, B.; Fu, Y.; Zhang, N. Administration of geniposide ameliorates dextran sulfate sodium-induced colitis in mice via inhibition of inflammation and mucosal damage. Int. Immunopharmacol. 2017, 49, 168–177. [Google Scholar] [CrossRef]
- Zucker, S.D.; Vogel, M.E.; Kindel, T.L.; Smith, D.L.; Idelman, G.; Avissar, U.; Kakarlapudi, G.; Masnovi, M.E. Bilirubin prevents acute DSS-induced colitis by inhibiting leukocyte infiltration and suppressing upregulation of inducible nitric oxide synthase. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G841–G854. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, K.; Xu, J.; Ma, Z.; Zhao, J.; Li, L.; Zhao, C.; Yang, L.; Li, F.; Liu, Y. Study of the mechanism of acupuncture and moxibustion in protecting the intestinal mucosal barrier in DSS-induced UC rats based on the IL-9/IL-9R pathway. J. Acupunct. Tuina Sci. 2024, 22, 91–103. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Zhu, L.; Ma, S.; Luo, Y.; Liang, H.; Liu, Q.; Chen, J.; Guli, S.; Chen, X. Orchestration of MUC2—The key regulatory target of gut barrier and homeostasis: A review. Int. J. Biol. Macromol. 2023, 236, 123862. [Google Scholar] [CrossRef]
- Mennillo, E.; Yang, X.; Paszek, M.; Auwerx, J.; Benner, C.; Chen, S. NCoR1 Protects Mice From Dextran Sodium Sulfate–Induced Colitis by Guarding Colonic Crypt Cells From Luminal Insult. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yao, Y.; Liu, Y.; Zhang, J.; Mao, L. The mechanism of traditional medicine in alleviating ulcerative colitis: Regulating intestinal barrier function. Front. Pharmacol. 2023, 14, 1228969. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, D.; Cai, Q.; Shen, T.; Dong, B.; Lewis, M.T.; Wang, R.; Meng, Y.; Zhou, W.; Yi, P.; et al. MAPK4 promotes triple negative breast cancer growth and reduces tumor sensitivity to PI3K blockade. Nat. Commun. 2022, 13, 245. [Google Scholar] [CrossRef]
- Ren, J.A.-O.; Zheng, S.; Zhang, L.; Liu, J.; Cao, H.; Wu, S.; Xu, Y.; Sun, J. MAPK4 predicts poor prognosis and facilitates the proliferation and migration of glioma through the AKT/mTOR pathway. Cancer Med. 2022, 12, 11624–11640. [Google Scholar] [CrossRef]
- Shen, T.; Wang, W.; Zhou, W.; Coleman, I.; Cai, Q.; Dong, B.; Ittmann, M.M.; Creighton, C.J.; Bian, Y.; Meng, Y.; et al. MAPK4 promotes prostate cancer by concerted activation of androgen receptor and AKT. J. Clin. Investig. 2021, 131, e135465. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Li, F.; Wang, D.; Li, H.; He, X.; Zhang, J. Methane attenuates lung ischemia-reperfusion injury via regulating PI3K-AKT-NFκB signaling pathway. J. Recept. Signal Transduct. 2020, 40, 209–217. [Google Scholar] [CrossRef]
- Yu, A.; Wang, S.; Xing, Y.; Han, M.; Shao, K. 7,8-Dihydroxyflavone alleviates apoptosis and inflammation induced by retinal ischemia-reperfusion injury via activating TrkB/Akt/NF-kB signaling pathway. Int. J. Med Sci. 2022, 19, 13–24. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Li, W.; Liu, L.; Shen, G.; Zhu, Y.; Tu, Q. Syringic acid (SA) inhibits the IL-1β-induced inflammation in mice chondrocytes and ameliorates the progression of osteoarthritis via the PTEN/AKT/NF-κB pathway. J. Funct. Foods 2023, 107, 105683. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A comprehensive review of the classification, sources, biosynthesis, and biological properties of hydroxybenzoic and hydroxycinnamic acids. Phytochem. Rev. 2023, 1–30. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Deng, Z.; Li, H.; Jiang, Z. A Spermidine Derivative Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Inhibiting the MAPK4/AKT Signaling Pathway. Foods 2025, 14, 1110. https://doi.org/10.3390/foods14071110
Zhang Y, Deng Z, Li H, Jiang Z. A Spermidine Derivative Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Inhibiting the MAPK4/AKT Signaling Pathway. Foods. 2025; 14(7):1110. https://doi.org/10.3390/foods14071110
Chicago/Turabian StyleZhang, Yuxin, Zeyuan Deng, Hongyan Li, and Zeyin Jiang. 2025. "A Spermidine Derivative Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Inhibiting the MAPK4/AKT Signaling Pathway" Foods 14, no. 7: 1110. https://doi.org/10.3390/foods14071110
APA StyleZhang, Y., Deng, Z., Li, H., & Jiang, Z. (2025). A Spermidine Derivative Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Inhibiting the MAPK4/AKT Signaling Pathway. Foods, 14(7), 1110. https://doi.org/10.3390/foods14071110




