Untargeted Screening Based on UHPLC-HRMS of Total Folates Produced by Lactic Acid Bacteria in Fermented Milk and During Yogurt Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Folate Quantification in Milk Based on Microbiological Assay
2.3. DNA Extraction and Molecular Identification of Folate-Producing Genes
2.4. Yogurt Preparation
2.5. Viability of Lactic Acid Bacteria and Folate Detection During Yogurt Shelf Life
2.6. Extraction and Semi-Quantitative Analysis of Folates Based on UHPLC-HRMS in Milk and Yogurt Samples
2.7. Statistical Analysis
3. Results
3.1. Folate Production by Microbiological Assay and LAB Strains Selection
3.2. Identification of Folate-Producing Genes
3.3. Semi-Quantification of Folate Metabolites in Milk Through a UHPLC-HRMS Approach
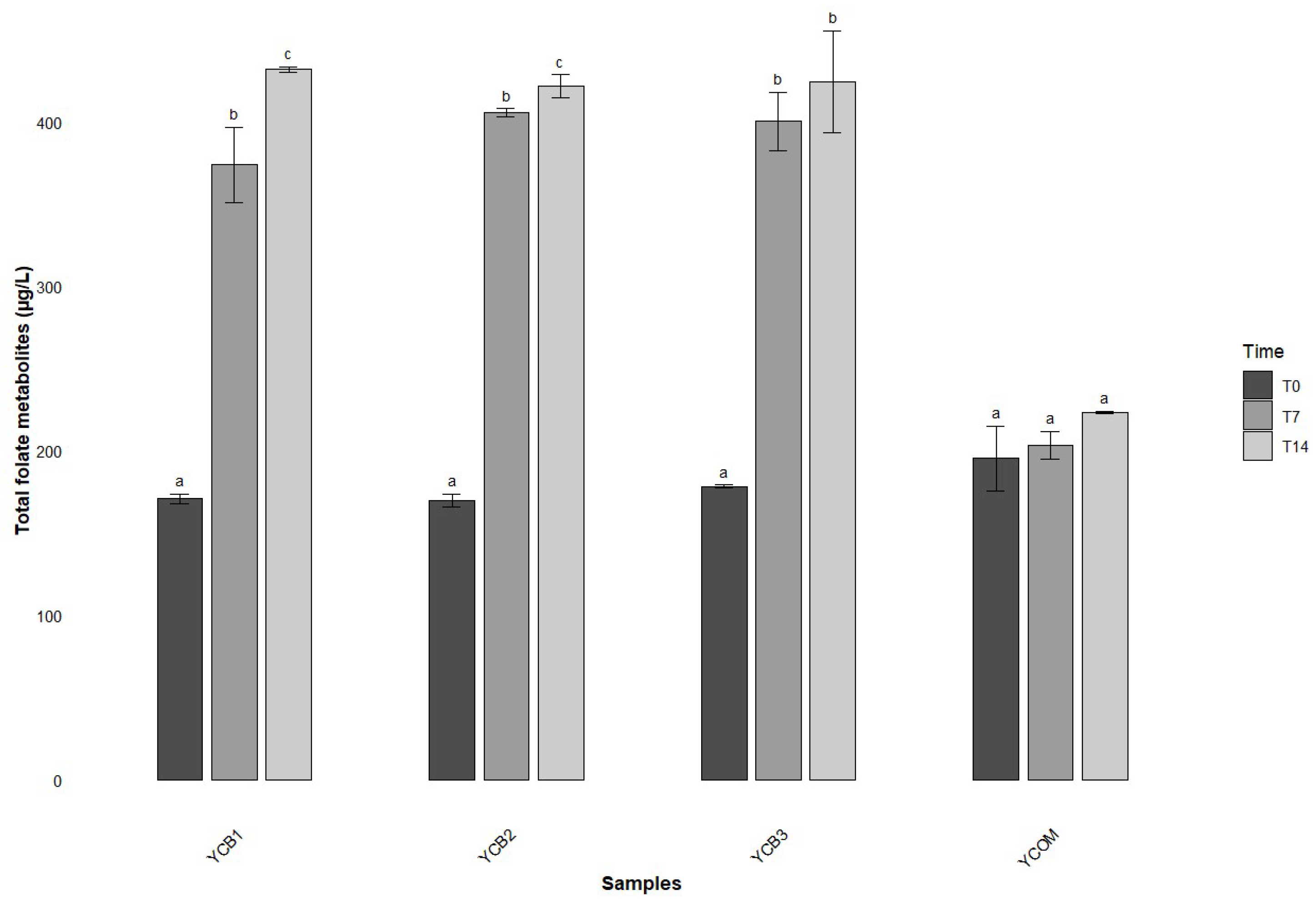
3.4. Shelf Life Study on Yogurt
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucick, A.C.C.; Gianni, K.; Todorov, S.D.; de LeBlanc, A.D.M.; LeBlanc, J.; Franco, B.D. Evaluation of the Bioavailability and Intestinal Effects of Milk Fermented by Folate Producing Lactic Acid Bacteria in a Depletion/Repletion Mice Model. J. Funct. Foods 2020, 66, 103785. [Google Scholar] [CrossRef]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Recent Update on Lactic Acid Bacteria Producing Riboflavin and Folates: Application for Food Fortification and Treatment of Intestinal Inflammation. J. Appl. Microbiol. 2021, 130, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Rad, A.H.; Khosroushahi, A.Y.; Khosravi, H.; Jafarzadeh, S. Application of Probiotics in Folate Bio-Fortification of Yoghurt. Probiotics Antimicrob. Proteins 2020, 12, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Habibi Najafi, M.B.; Edalatian Dovom, M.R. Screening of Lactic Acid Bacteria for Highly Producing Extra and Intracellular Folate and Their Potential Use in Production of Bio-Enriched Yogurt. LWT 2024, 209, 116766. [Google Scholar] [CrossRef]
- Meucci, A.; Rossetti, L.; Zago, M.; Monti, L.; Giraffa, G.; Carminati, D.; Tidona, F. Folates Biosynthesis by Streptococcus Thermophilus during Growth in Milk. Food Microbiol. 2018, 69, 116–122. [Google Scholar] [CrossRef]
- Gangadharan, D.; Sivaramakrishnan, S.; Pandey, A.; Madhavan Nampoothiri, K. Folate-Producing Lactic Acid Bacteria from Cow’s Milk with Probiotic Characteristics. Int. J. Dairy Technol. 2010, 63, 339–348. [Google Scholar] [CrossRef]
- Padalino, M.; Perez-Conesa, D.; López-Nicolás, R.; Frontela-Saseta, C.; Ros-Berruezo, G. Effect of Fructooligosaccharides and Galactooligosaccharides on the Folate Production of Some Folate-Producing Bacteria in Media Cultures or Milk. Int. Dairy J. 2012, 27, 27–33. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic Acid Bacteria Producing B-Group Vitamins: A Great Potential for Functional Cereals Products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Greppi, A.; Hemery, Y.; Berrazaga, I.; Almaksour, Z.; Humblot, C. Ability of Lactobacilli Isolated from Traditional Cereal-Based Fermented Food to Produce Folate in Culture Media under Different Growth Conditions. LWT 2017, 86, 277–284. [Google Scholar] [CrossRef]
- Laiño, J.E.; LeBlanc, J.G.; de Giori, G.S. Production of Natural Folates by Lactic Acid Bacteria Starter Cultures Isolated from Artisanal Argentinean Yogurts. Can. J. Microbiol. 2012, 58, 581–588. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Serrano-Amatriain, C.; Ledesma-Amaro, R.; Jiménez, A. Formation of Folates by Microorganisms: Towards the Biotechnological Production of This Vitamin. Appl. Microbiol. Biotechnol. 2018, 102, 8613–8620. [Google Scholar] [CrossRef] [PubMed]
- Zeydanli, M.N.; Yüksekdağ, Z.; Acar, Ç. Folate Production in Lactic Acid Bacteria and Determination of Folate Derivatives by HPLC Laktik Asit Bakterilerinde Folat Üretimi ve HPLC Ile Folat Türevlerinin Tayini. Pamukkale Univ. J. Eng. Sci. 2025. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.M.; Guyot, J.P.; Humblot, C. Lactic Acid Fermentation as a Tool for Increasing the Folate Content of Foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- Arcot, J.; Shrestha, A. Folate: Methods of Analysis. Trends Food Sci. Technol. 2005, 16, 253–266. [Google Scholar] [CrossRef]
- Iyer, R.; Tomar, S.K. Folate: A Functional Food Constituent. J. Food Sci. 2009, 74, R114–R122. [Google Scholar] [CrossRef]
- Van Haandel, L.; Stobaugh, J.F. Folate Determination in Human Health: UPLC-MS/MS Is the Emerging Methodology of Choice. Bioanalysis 2013, 5, 3023–3031. [Google Scholar] [CrossRef]
- Öncü-Kaya, E.M. Determination of Folic Acid by Ultra-High Performance Liquid Chromatography in Certain Malt-Based Beverages after Solid-Phase Extraction. Celal Bayar Üniversitesi Fen. Bilimleri Dergisi 2017, 13, 623–630. [Google Scholar] [CrossRef]
- Ložnjak Švarc, P.; Oveland, E.; Strandler, H.S.; Kariluoto, S.; Campos-Giménez, E.; Ivarsen, E.; Malaviole, I.; Motta, C.; Rychlik, M.; Striegel, L.; et al. Collaborative Study: Quantification of Total Folate in Food Using an Efficient Single-Enzyme Extraction Combined with LC-MS/MS. Food Chem. 2020, 333, 127447. [Google Scholar] [CrossRef]
- Nygren-Babol, L.; Jägerstad, M. Folate-Binding Protein in Milk: A Review of Biochemistry, Physiology, and Analytical Methods. Crit. Rev. Food Sci. Nutr. 2012, 52, 410–425. [Google Scholar] [CrossRef]
- Hugenschmidt, S.; Schwenninger, S.M.; Gnehm, N.; Lacroix, C. Screening of a Natural Biodiversity of Lactic and Propionic Acid Bacteria for Folate and Vitamin B12 Production in Supplemented Whey Permeate. Int. Dairy J. 2010, 20, 852–857. [Google Scholar] [CrossRef]
- Horne, D.W.; Patterson, D. Lactobacillus Casei Microbiological Assay of Folic Acid Derivatives in 96-Well Microtiter Plates. Clin. Chem. 1988, 34, 2357–2359. [Google Scholar] [CrossRef] [PubMed]
- Laiño, J.E.; Juarez del Valle, M.; Savoy de Giori, G.; LeBlanc, J.G.J. Development of a High Folate Concentration Yogurt Naturally Bio-Enriched Using Selected Lactic Acid Bacteria. LWT 2013, 54, 1–5. [Google Scholar] [CrossRef]
- Sarv, V.; Kerner, K.; Rimantas Venskutonis, P.; Rocchetti, G.; Paolo Becchi, P.; Lucini, L.; Tänavots, A.; Bhat, R. Untargeted Metabolomics and Conventional Quality Characterization of Rowanberry Pomace Ingredients in Meatballs. Food Chem. X 2023, 19, 100761. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Ghilardelli, F.; Masoero, F.; Gallo, A. Screening of Regulated and Emerging Mycotoxins in Bulk Milk Samples by High-Resolution Mass Spectrometry. Foods 2021, 10, 2025. [Google Scholar] [CrossRef]
- Holasova, M.; Roubal, P.; Ústav Mlékárenský, V. Possibility of Increasing Natural Folate Content in Fermented Milk Products by Fermentation and Fruit Component Addition. Czech J. Food Sci. 2005, 23, 196–201. [Google Scholar] [CrossRef]
- Rao, D.R.; Reddy, A.V.; Pulusani, S.R.; Cornwell, P.E. Biosynthesis and Utilization of Folic Acid and Vitamin B12 by Lactic Cultures in Skim Milk. J. Dairy. Sci. 1984, 67, 1169–1174. [Google Scholar] [CrossRef]
- Iyer, R.; Tomar, S.K.; Mohanty, A.K.; Singh, P.; Singh, R. Bioprospecting of Strains of Streptococcus Thermophilus from Indian Fermented Milk Products for Folate Production. Dairy Sci. Technol. 2011, 91, 237–246. [Google Scholar] [CrossRef]
- Leblanc, J.G.; Laiño, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-Group Vitamin Production by Lactic Acid Bacteria—Current Knowledge and Potential Applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Laiño, J.E.; Juárez del Valle, M.; Hébert, E.M.; Savoy de Giori, G.; LeBlanc, J.G. Folate Production and Fol Genes Expression by the Dairy Starter Culture Streptococcus Thermophilus CRL803 in Free and Controlled PH Batch Fermentations. LWT—Food Sci. Technol. 2017, 85, 146–150. [Google Scholar] [CrossRef]
- Zayed, A.; Bustami, R.; Alabsi, W.; El-Elimat, T. Development and Validation of a Rapid High-Performance Liquid Chromatography–Tandem Mass Spectrometric Method for Determination of Folic Acid in Human Plasma. Pharmaceuticals 2018, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Ringling, C.; Rychlik, M. Origins of the Difference between Food Folate Analysis Results Obtained by LC–MS/MS and Microbiological Assays. Anal. Bioanal. Chem. 2017, 409, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Mahara, F.A.; Nuraida, L.; Lioe, H.N.; Nurjanah, S. Hypothetical Regulation of Folate Biosynthesis and Strategies for Folate Overproduction in Lactic Acid Bacteria. Prev. Nutr. Food Sci. 2023, 28, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Nantel, G.; Tontisirin, K. Human Vitamin and Mineral Requirements, Report of a Joint FAO/WHO Expert Consultation; Library Catalogue World Food Programme: Bangkok, Thailand, 2001; 286. [Google Scholar]
- Homayouni Rad, A.; Yari Khosroushahi, A.; Khalili, M.; Jafarzadeh, S. Folate Bio-Fortification of Yoghurt and Fermented Milk: A Review. Dairy Sci. Technol. 2016, 96, 427–441. [Google Scholar] [CrossRef]
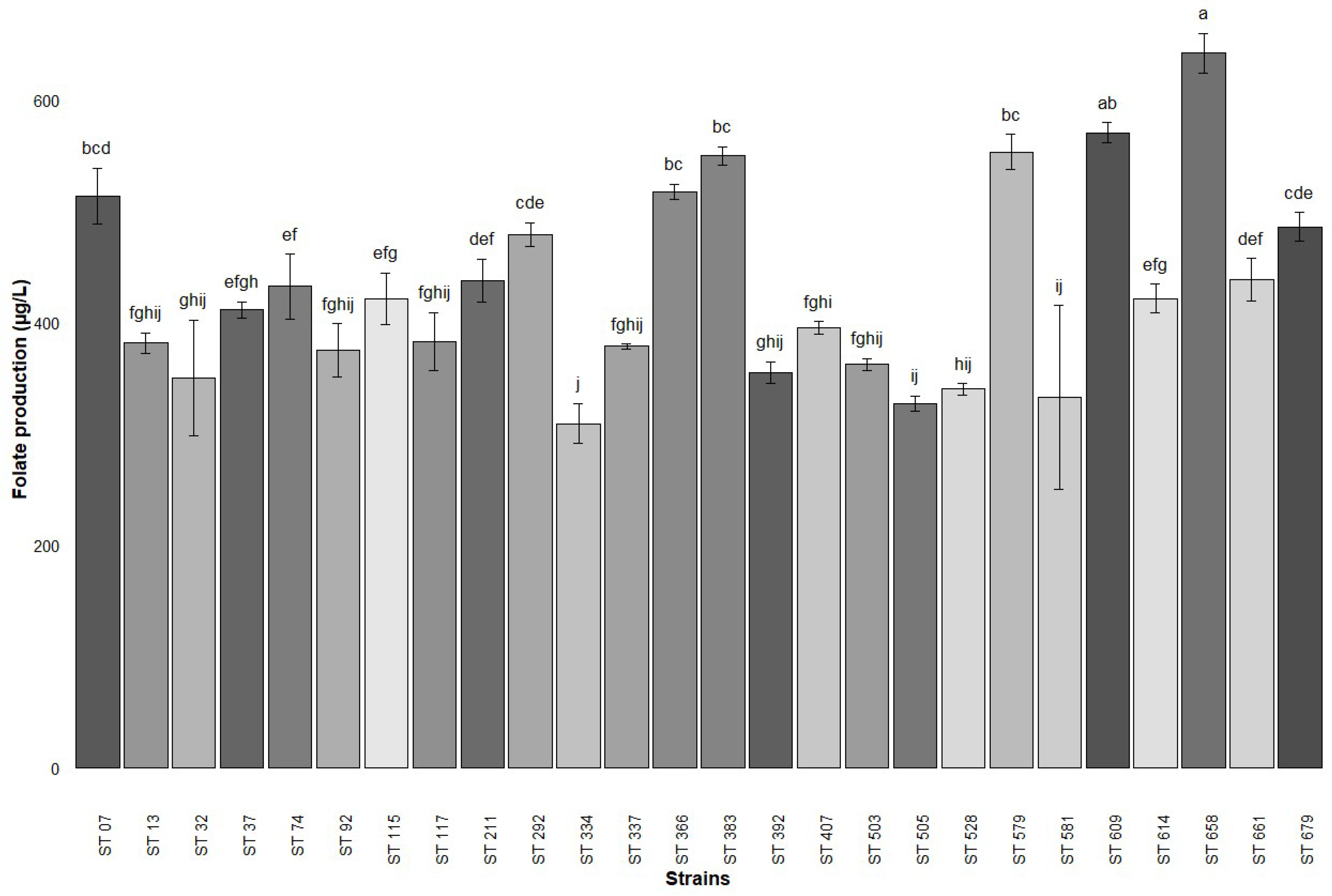
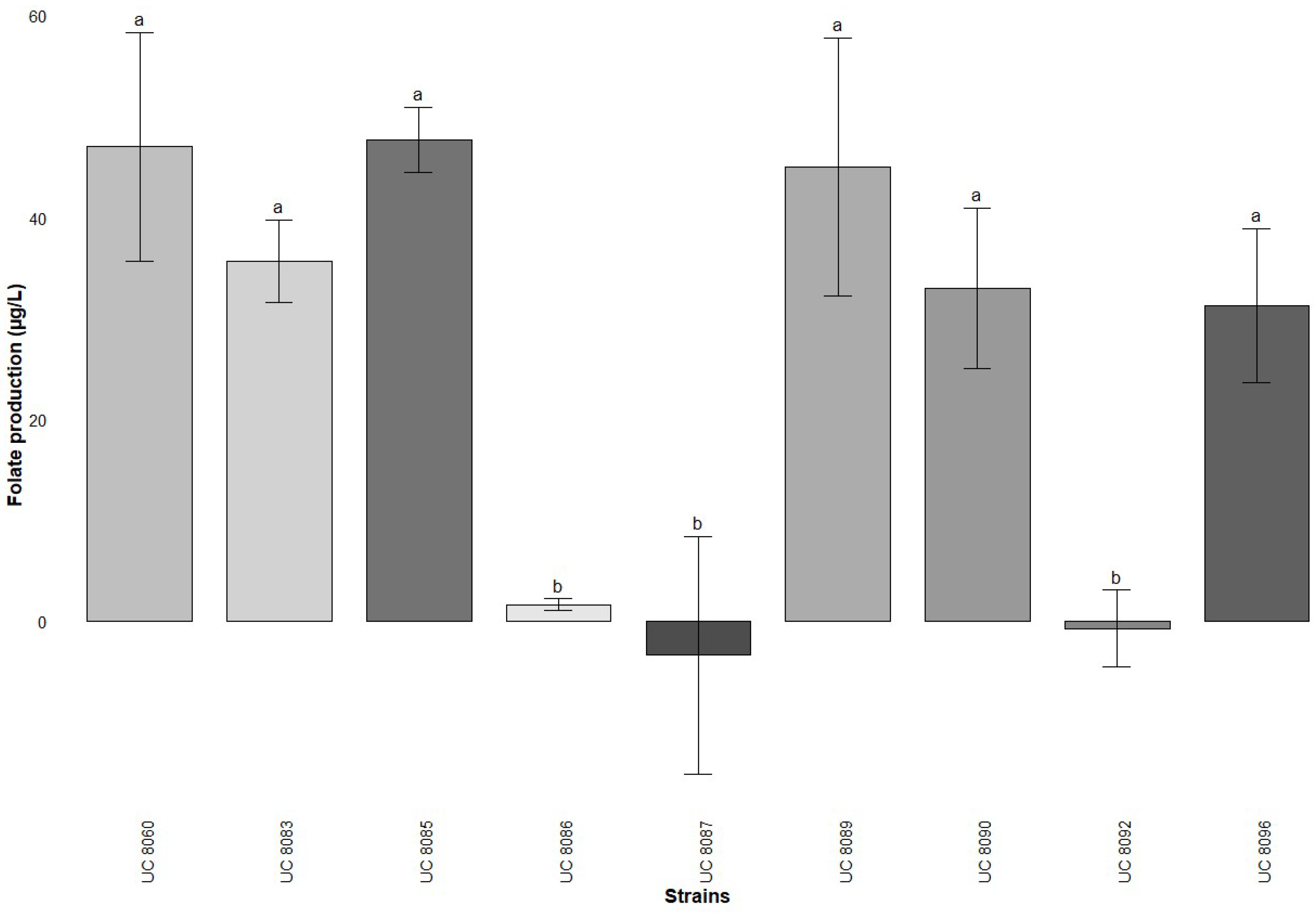
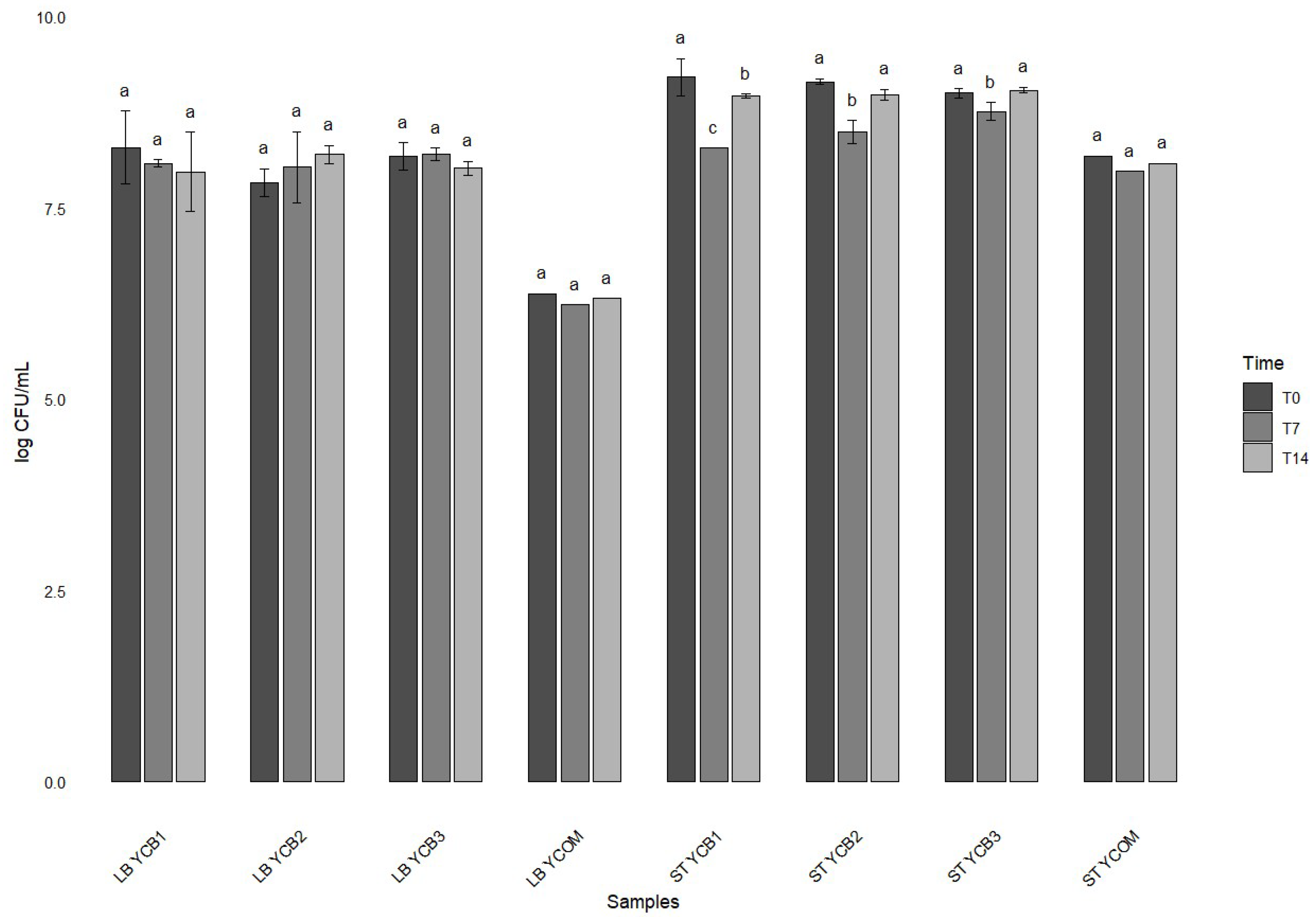
| Bacterial Strain | ID | Isolation Source | |
|---|---|---|---|
| 1 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8060 | Matsoni, Georgia |
| 2 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8083 | Ayran, Turkey |
| 3 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8085 | Ayran, Turkey |
| 4 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8086 | Ayran, Turkey |
| 5 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8087 | Ayran, Turkey |
| 6 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8089 | Ayran, Turkey |
| 7 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8090 | Ayran, Turkey |
| 8 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8092 | Ayran, Turkey |
| 9 | Lactobacillus delbrueckii subsp. bulgaricus | UC 8096 | Ayran, Turkey |
| 10 | Streptococcus thermophilus | ST 07 | Hard cheese, Italy |
| 11 | Streptococcus thermophilus | ST 13 | Hard cheese, Italy |
| 12 | Streptococcus thermophilus | ST 32 | Fermented milk |
| 13 | Streptococcus thermophilus | ST 37 | Fermented milk |
| 14 | Streptococcus thermophilus | ST 74 | Fermented milk |
| 15 | Streptococcus thermophilus | ST 92 | Fermented milk |
| 16 | Streptococcus thermophilus | ST 115 | Fermented milk |
| 17 | Streptococcus thermophilus | ST 117 | Fermented milk |
| 18 | Streptococcus thermophilus | ST 211 | Fermented milk |
| 19 | Streptococcus thermophilus | ST 292 | Ayran, Turkey |
| 20 | Streptococcus thermophilus | ST 298 | Fermented milk |
| 21 | Streptococcus thermophilus | ST 334 | Fermented milk |
| 22 | Streptococcus thermophilus | ST 337 | Fermented milk |
| 23 | Streptococcus thermophilus | ST 366 | Ayran, Turkey |
| 24 | Streptococcus thermophilus | ST 383 | Ayran, Turkey |
| 25 | Streptococcus thermophilus | ST 392 | Fermented milk |
| 26 | Streptococcus thermophilus | ST 407 | Ayran, Turkey |
| 27 | Streptococcus thermophilus | ST 503 | Hard cheese, Italy |
| 28 | Streptococcus thermophilus | ST 505 | Fermented milk |
| 29 | Streptococcus thermophilus | ST 528 | Fermented milk |
| 30 | Streptococcus thermophilus | ST 579 | Fermented milk |
| 31 | Streptococcus thermophilus | ST 581 | Ayran, Turkey |
| 32 | Streptococcus thermophilus | ST 609 | Matsoni, Georgia |
| 33 | Streptococcus thermophilus | ST 614 | Fermented milk |
| 34 | Streptococcus thermophilus | ST 658 | Matsoni, Georgia |
| 35 | Streptococcus thermophilus | ST 661 | Fermented milk |
| 36 | Streptococcus thermophilus | ST 679 | Ayran, Turkey |
| Genes | Primers | Sequence (5′ → 3′) | Size (bp) |
|---|---|---|---|
| fol A | folAf | AGCTACGTTTGGGCAGAAGA | 489 bp |
| folAr | CGGTGGGCTTCACTCTTTAC | ||
| fol C | folCf | GTATTTTGCCGAACAGCGGG | 1338 bp |
| folCr | TCAACAAATGCGCTGATGCC | ||
| fol K | folKf | GTATTTTGCCGAACAGCGGG | 1074 bp |
| folKr | GAAAGTTCGCGCTGCTGATT | ||
| fol P | folPf | ACATTTAGCGGCAACGTCAC | 1101 bp |
| folPr | CTTTTTCAAGCCCAACGCCT |
| Name of the Blend | S. thermophilus | L. delbrueckii subsp. bulgaricus | Ratio | % Inoculated |
|---|---|---|---|---|
| YCB1 | ST 07 | UC 8085 | 2:1 | 8:4% |
| YCB2 | ST 292 | UC 8085 | 2:1 | 8:4% |
| YCB3 | ST 658 | UC 8085 | 2:1 | 8:4% |
| Strain | Total Folate Metabolites (µg/L) |
|---|---|
| ST 07 | 211.02 ± 4.20 d |
| ST 292 | 140.83 ± 28.10 c |
| ST 366 | 128.82 ± 1.90 bc |
| ST 407 | 71.60 ± 12.30 a |
| ST 503 | 132.68 ± 3.20 bc |
| ST 581 | 58.14 ± 5.04 a |
| ST 609 | 72.34 ± 2.50 a |
| ST 658 | 227.99 ± 9.30 d |
| ST 679 | 89.02 ± 9.50 ab |
| UC 8085 | 398.57 ± 51.5 e |
| UC 8089 | 100.12 ± 1.5 abc |
| Blend | Time (Days) | Total Folate Metabolites (µg/L) | Folate Intake (µg/125 mL) |
|---|---|---|---|
| YCB1 | 0 | 170.68 ± 2.79 a | 21.34 a |
| 7 | 373.83 ± 23.13 b | 46.73 b | |
| 14 | 432.08 ± 1.63 c | 54.01 c | |
| YCB2 | 0 | 169.53 ± 3.96 a | 21.19 a |
| 7 | 405.70 ± 2.46 b | 50.71 b | |
| 14 | 421.74 ± 6.96 c | 52.72 c | |
| YCB3 | 0 | 178.23 ± 0.78 a | 22.28 a |
| 7 | 400.30 ± 17.96 b | 50.04 b | |
| 14 | 424.41 ± 30.97 b | 53.05 b | |
| YCOM | 0 | 195.35 ± 16.69 a | 24.42 a |
| 7 | 203.31 ± 8.5 a | 25.41 a | |
| 14 | 223.23 ± 0.62 a | 27.90 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzetti, M.; Cerri, C.; Morandi, S.; Rocchetti, G.; Mussio, C.; Barbieri, F.; Tabanelli, G.; Bassi, D. Untargeted Screening Based on UHPLC-HRMS of Total Folates Produced by Lactic Acid Bacteria in Fermented Milk and During Yogurt Shelf Life. Foods 2025, 14, 1112. https://doi.org/10.3390/foods14071112
Bozzetti M, Cerri C, Morandi S, Rocchetti G, Mussio C, Barbieri F, Tabanelli G, Bassi D. Untargeted Screening Based on UHPLC-HRMS of Total Folates Produced by Lactic Acid Bacteria in Fermented Milk and During Yogurt Shelf Life. Foods. 2025; 14(7):1112. https://doi.org/10.3390/foods14071112
Chicago/Turabian StyleBozzetti, Marianna, Carolina Cerri, Sara Morandi, Gabriele Rocchetti, Chiara Mussio, Federica Barbieri, Giulia Tabanelli, and Daniela Bassi. 2025. "Untargeted Screening Based on UHPLC-HRMS of Total Folates Produced by Lactic Acid Bacteria in Fermented Milk and During Yogurt Shelf Life" Foods 14, no. 7: 1112. https://doi.org/10.3390/foods14071112
APA StyleBozzetti, M., Cerri, C., Morandi, S., Rocchetti, G., Mussio, C., Barbieri, F., Tabanelli, G., & Bassi, D. (2025). Untargeted Screening Based on UHPLC-HRMS of Total Folates Produced by Lactic Acid Bacteria in Fermented Milk and During Yogurt Shelf Life. Foods, 14(7), 1112. https://doi.org/10.3390/foods14071112









