Dual-Mode Quantitative Immunochromatographic Assay for Highly Sensitive On-Site Detection of Ciprofloxacin in Fish Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of PVP@Pd NPs and PVP@Pd NPs-mAb
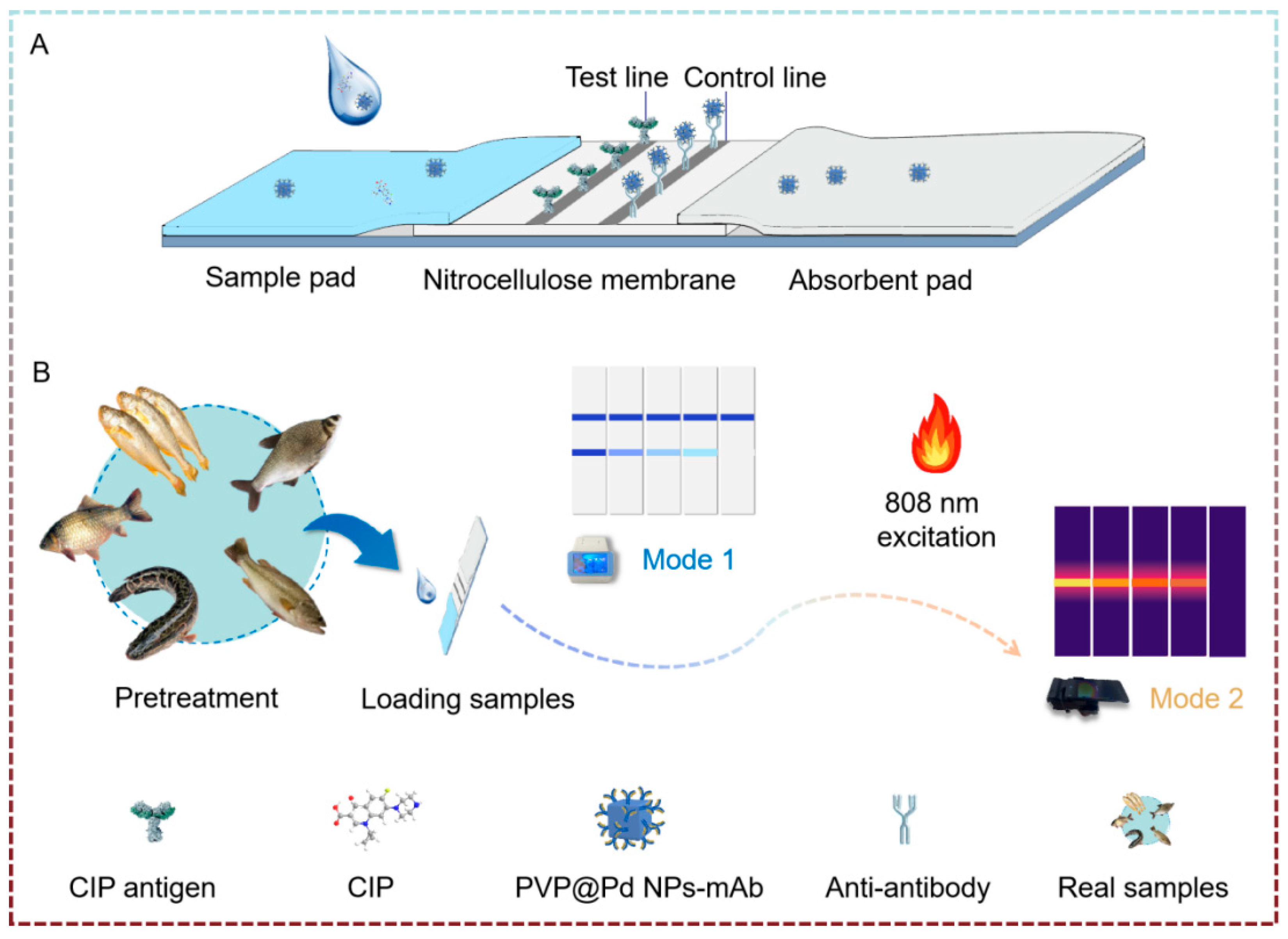
2.3. Procedure of Dual-Mode Quantitative Immunochromatographic Assay
2.4. Application in Real Sample
2.5. Instrument Validation
3. Results
3.1. Principle of Dual-Mode Quantitative Immunochromatographic Assay
3.2. Characterization of PVP@Pd NPs and PVP@Pd NPs-mAb
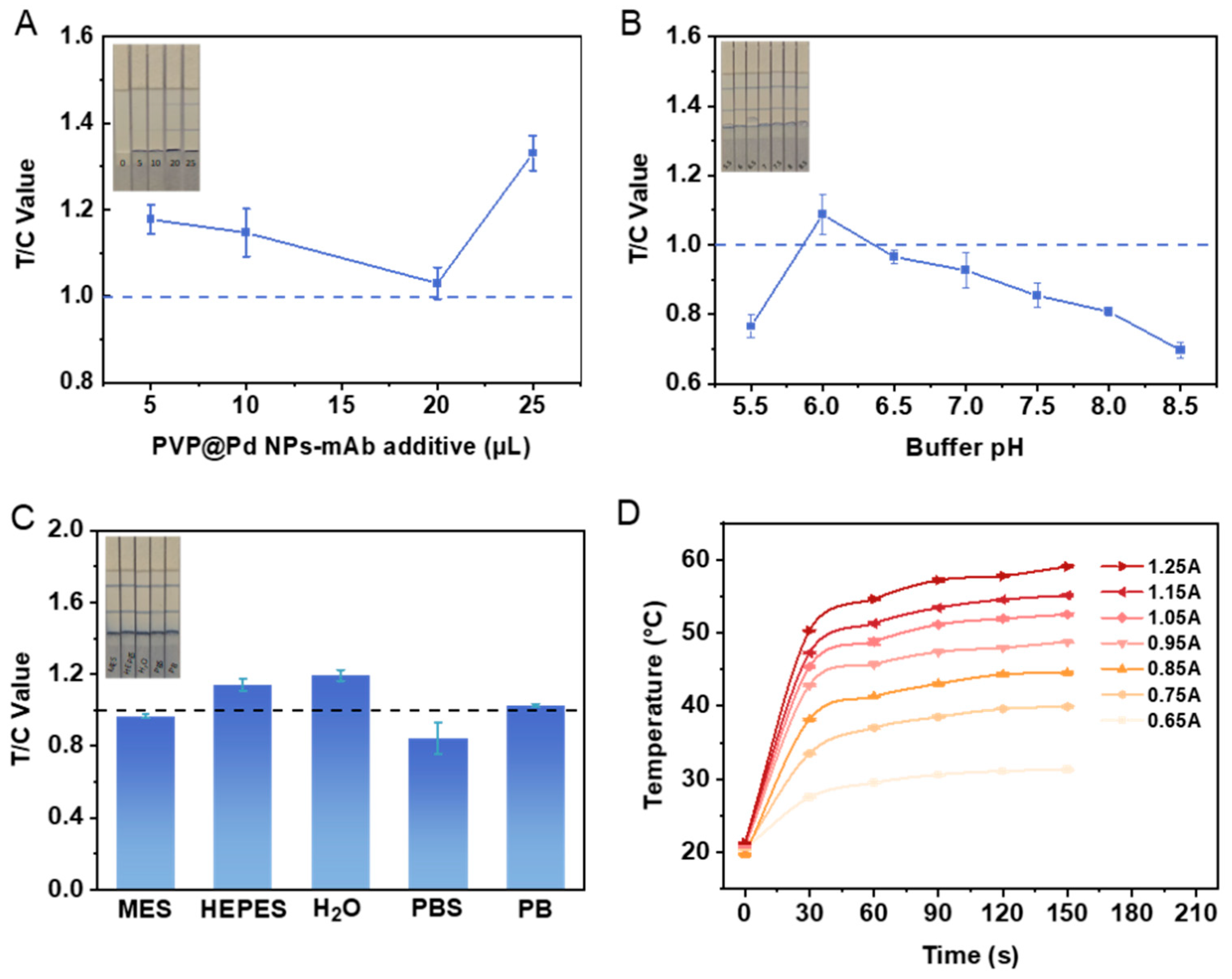
3.3. Optimization of Experimental Conditions
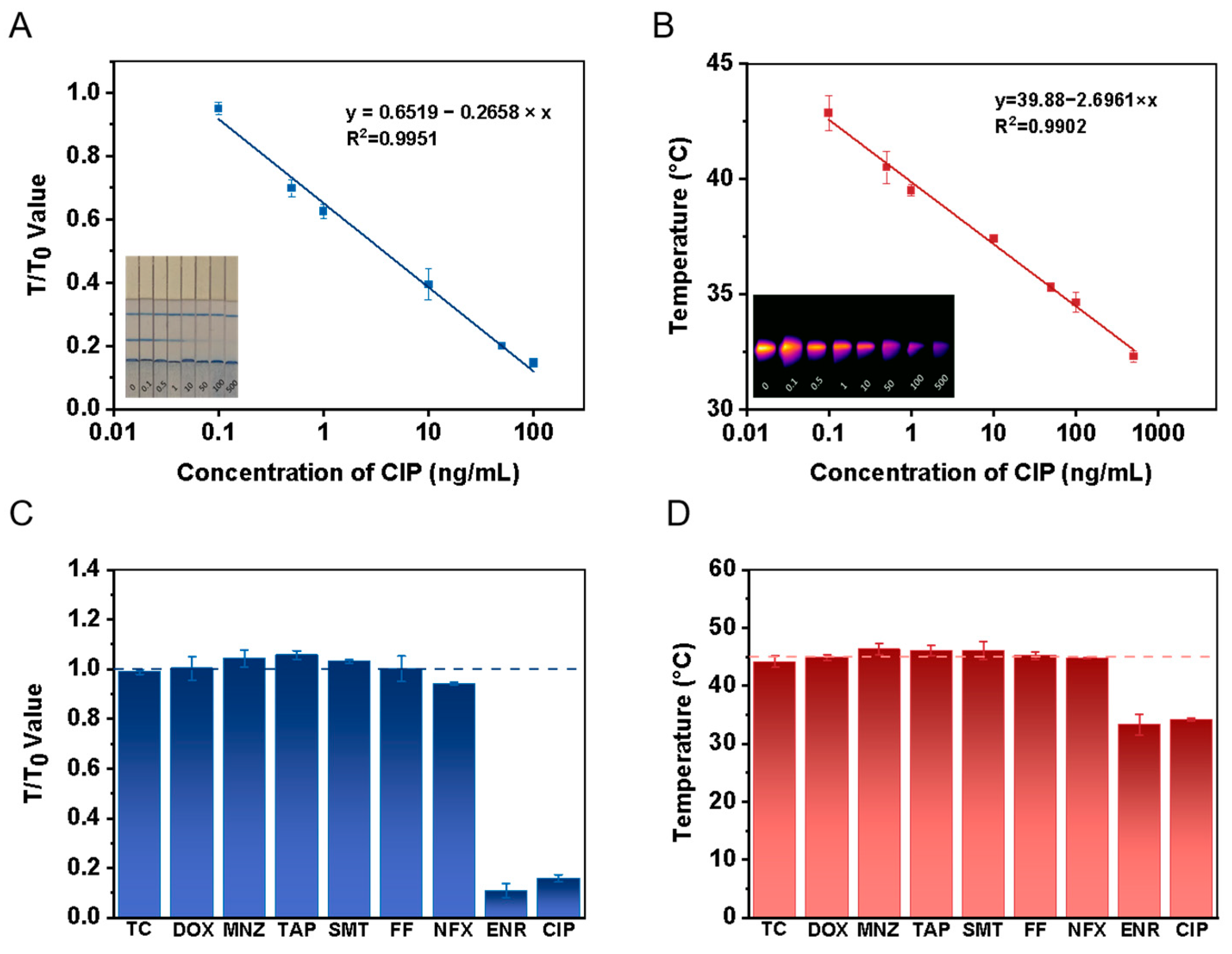
3.4. Performance of Dual-Mode Quantitative Immunochromatographic Assay
3.5. Practical Application Results for Real Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Bambeke, F.; Michot, J.M.; Van Eldere, J.; Tulkens, P.M. Quinolones in 2005: An update. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2005, 11, 256–280. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, H. Quinolone Antibiotics: Resistance and Therapy. Infect. Drug Resist. 2023, 16, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Ojkic, N.; Lilja, E.; Direito, S.; Dawson, A.; Allen, R.J.; Waclaw, B. A Roadblock-and-Kill Mechanism of Action Model for the DNA-Targeting Antibiotic Ciprofloxacin. Antimicrob. Agents Chemother. 2020, 64, e02487-19. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Duan, Y.P.; Zhang, Z.B.; Gao, Y.F.; Dai, C.M.; Tu, Y.J.; Gao, J. Comprehensive evaluation of antibiotics pollution the Yangtze River basin, China: Emission, multimedia fate and risk assessment. J. Hazard. Mater. 2024, 465, 133247. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S. Antibiotic resistance in environment of animal farms. Sheng Wu Gong Cheng Xue Bao. Chin. J. Biotechnol. 2018, 34, 1234–1245. [Google Scholar] [CrossRef]
- Xie, R.; Huo, S.Y.; Li, Y.R.; Chen, L.G.; Zhang, F.Y.; Wu, X.J. Molecular epidemiological survey on quinolone resistance genotype and phenotype of Escherichia coli in septicemic broilers in Hebei, China. Poult. Sci. 2014, 93, 335–339. [Google Scholar] [CrossRef]
- Góchez, D.; Raicek, M.; Ferreira, J.P.; Jeannin, M.; Moulin, G.; Erlacher-Vindel, E. OIE Annual Report on Antimicrobial Agents Intended for Use in Animals: Methods Used. Front. Vet. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020–2021: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023, 23, 706–718. [Google Scholar] [CrossRef]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef]
- Atta, A.H.; Atta, S.A.; Nasr, S.M.; Mouneir, S.M. Current perspective on veterinary drug and chemical residues in food of animal origin. Environ. Sci. Pollut. Res. 2022, 29, 15282–15302. [Google Scholar] [CrossRef]
- Li, Z.J.; Jin, Y.Y.; Wang, X.Y.; Xu, L.D.; Teng, L.Y.; Fu, K.; Li, B.L.; Li, Y.L.; Huang, Y.; Ma, N.; et al. Health Risk Assessment of Antibiotic Pollutants in Large Yellow Croakers from Zhejiang Aquaculture Sites. Foods 2024, 13, 31. [Google Scholar] [CrossRef]
- Fang, L.; Pan, S.; Lu, B.; Zhao, Y.; Chen, X.; Jin, M. Contamination status and the health risk evaluation of dietary exposure of quinolone and tetracycline antibiotics in animal derived foods in Ningbo City from 2018 to 2020. Wei sheng yan jiu. J. Hyg. Res. 2022, 51, 113–117. [Google Scholar] [CrossRef]
- Hayes, A.; Murray, L.M.; Stanton, I.C.; Zhang, L.H.; Snape, J.; Gaze, W.H.; Murray, A.K. Predicting selection for antimicrobial resistance in UK wastewater and aquatic environments: Ciprofloxacin poses a significant risk. Environ. Int. 2022, 169, 107488. [Google Scholar] [CrossRef] [PubMed]
- LeMaire, S.A.; Zhang, L.; Luo, W.; Ren, P.P.; Azares, A.R.; Wang, Y.D.; Zhang, C.; Coselli, J.S.; Shen, Y.H. Effect of Ciprofloxacin on Susceptibility to Aortic Dissection and Rupture in Mice. JAMA Surg. 2018, 153, e181804. [Google Scholar] [CrossRef]
- Noergaard, M.; Gotfredsen, D.R.; Sorensen, A.M.S.; Andersen, J.T. Ciprofloxacin exposure and adverse pregnancy outcomes: A Danish nationwide cohort study. Bjog-Int. J. Obstet. Gynaecol. 2022, 129, 1503–1511. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Cabello, F.; Good, C.M.; Lunestad, B.T. Veterinary drug use in United States net pen Salmon aquaculture: Implications for drug use policy. Aquaculture 2020, 518, 734820. [Google Scholar] [CrossRef]
- Gouvêa, R.; Dos, S.F.F.; Aquino, M.; Pereira, V.L.D. Fluoroquinolones in Industrial Poultry Production, Bacterial Resistance and Food Residues: A Review. Braz. J. Poult. Sci. 2015, 17, 734820. [Google Scholar] [CrossRef]
- Zawack, K.; Li, M.; Booth, J.G.; Love, W.; Lanzas, C.; Gröhm, Y.T. Monitoring Antimicrobial Resistance in the Food Supply Chain and Its Implications for FDA Policy Initiatives. Antimicrob. Agents Chemother. 2016, 60, 5302–5311. [Google Scholar] [CrossRef]
- GB 31650-2019; National Health Commission of the People’s Republic of China. National Food Safety Standard—Maximum Residue Limits for Veterinary Drugs in Foods. China National Standards: Beijing, China, 2019.
- Nessim, C.; El Hamd, M.; Obaydo, R.; Saleh, S.; Lotfy, H. KPI-based standards benchmarking for the preference of different analytical approaches developed for simultaneous determination of ciprofloxacin and hydrocortisone: A SWOT case study. Rev. Anal. Chem. 2024, 43. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, B.; Yu, M.; Han, J.; Wang, Y.; Tan, Z.J.; Yan, Y.S. Simultaneous separation/enrichment and detection of trace ciprofloxacin and lomefloxacin in food samples using thermosensitive smart polymers aqueous two-phase flotation system combined with HPLC. Food Chem. 2016, 210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gezahegn, T.; Tegegne, B.; Zewge, F.; Chandravanshi, B.S. Salting-out assisted liquid-liquid extraction for the determination of ciprofloxacin residues in water samples by high performance liquid chromatography-diode array detector. BMC Chem. 2019, 13, 28. [Google Scholar] [CrossRef]
- Brandi, J.; Siragusa, G.; Robotti, E.; Marengo, E.; Cecconi, D. “Analysis of veterinary drugs and pesticides in food using liquid chromatography-mass spectrometry”. Trac Trends Anal. Chem. 2024, 179, 117888. [Google Scholar] [CrossRef]
- Zhang, B.; Lang, Y.H.; Guo, B.W.; Cao, Z.Y.; Cheng, J.; Cai, D.F.; Shentu, X.; Yu, X.P. Indirect Competitive Enzyme-Linked Immunosorbent Assay Based on Broad-Spectrum Antibody for Simultaneous Determination of Thirteen Fluoroquinolone Antibiotics in Rana catesbeianus. Foods 2023, 12, 2530. [Google Scholar] [CrossRef]
- Ajala, O.J.; Tijani, J.O.; Salau, R.B.; Abdulkareem, A.S.; Aremu, O.S. A review of emerging micro-pollutants in hospital wastewater: Environmental fate and remediation options. Results Eng. 2022, 16, 100671. [Google Scholar] [CrossRef]
- Hird, S.J.; Lau, B.P.Y.; Schuhmacher, R.; Krska, R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. Trac-Trends Anal. Chem. 2014, 59, 59–72. [Google Scholar] [CrossRef]
- Lima, É.; Oliveira, M.B.; Freitas, A. Antibiotics in intensive egg production: Food Safety tools to ensure regulatory compliance. Food Chem. Adv. 2023, 3, 100548. [Google Scholar] [CrossRef]
- Zhao, B.C.; Pan, Y.Y.; Liao, S.J.; He, Z.K.; Xie, W.; Shen, A.G. Gold nanoparticles decorated hydrogen-bonded organic frameworks (HOFs) as self-cleaning SERS substrates. Chem. Eng. J. 2024, 497, 154454. [Google Scholar] [CrossRef]
- Zhang, A.; Feng, J.J.; Yan, J.C.; Hu, M.Y.; Zhang, L.; Zeng, H.P. Laser reshaping of gold nanoparticles for highly sensitive SERS detection of ciprofloxacin. Appl. Surf. Sci. 2022, 583, 152543. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kondo, T.; Osasa, T.; Kotsugai, A.; Shitanda, I.; Hoshi, Y.; Itagaki, M.; Aikawa, T.; Tojo, T.; Yuasa, M. Sensitive electrochemical detection of ciprofloxacin at screen-printed diamond electrodes. Carbon 2020, 159, 247–254. [Google Scholar] [CrossRef]
- Xu, L.; Li, H.N.; Yan, P.C.; Xia, J.X.; Qiu, J.X.; Xu, Q.; Zhang, S.Q.; Li, H.M.; Yuan, S.Q. Graphitic carbon nitride/BiOCl composites for sensitive photoelectrochemical detection of ciprofloxacin. J. Colloid Interface Sci. 2016, 483, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.S.; He, L.L. Recent advance in SERS techniques for food safety and quality analysis: A brief review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Xie, H.H.; Li, Y.Y.; Wang, J.; Lei, Y.; Koidis, A.; Li, X.M.; Shen, X.; Xu, Z.L.; Lei, H.T. Broad-specific immunochromatography for simultaneous detection of various sulfonylureas in adulterated multi-herbal tea. Food Chem. 2022, 370, 131055. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Huang, H.C.; Jian, D.; Lu, Y.A.; Shan, Y.K.; Wang, S.Y.; Liu, F. Quantitative ciprofloxacin on-site rapid detections using quantum dot microsphere based immunochromatographic test strips. Food Chem. 2021, 335, 127596. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, S.; Xu, C.Y.; Yu, Q.; Bai, W.L.; Zhang, L.; Li, G.H.; Wang, C.W.; Gu, B. 3D multilayered sheet-like nanozyme enables the multiplex, flexible, and ultrasensitive detection of small-molecule drugs by immunochromatographic assay. Chem. Eng. J. 2024, 502, 158162. [Google Scholar] [CrossRef]
- Lu, Z.W.; Gong, Y.H.; Shen, C.A.; Chen, H.R.; Zhu, W.L.; Liu, T.; Wu, C.; Sun, M.M.; Su, G.H.; Wang, X.X.; et al. Portable, intelligent MIECL sensing platform for ciprofloxacin detection using a fast convolutional neural networks-assisted Tb@Lu2O3 nanoemitter. Food Chem. 2024, 444, 138656. [Google Scholar] [CrossRef]
- Wang, X.C.; Yang, T.T.; Fang, L.X.; Yang, Y.; Zhang, H.T.; Yang, J.H.; Wang, C.B.; Fan, L.M.; Zang, X.L.; Meng, S.L.; et al. Citizen science in action: Time-resolved immunofluorescence-based field detection of antibiotics with portable analytical kit. Sci. Total Environ. 2024, 934, 173251. [Google Scholar] [CrossRef] [PubMed]
- Çevik, K.; Yildiz, I.; Yildiz, A.; Nas, M.S.; Alma, M.H.; Calimli, M.H. PdRuO2/PVP nanomaterial as a highly selective, stable, and applicable potentiometric sensor for the detection of Cr3+. Microchim. Acta 2024, 191, 3046. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lin, C.M.; Li, F.; Wei, X.H.; Chen, Y.S.; Fu, Y.Y.; Yu, X.P.; Zhang, B.; Ye, Z.H. An Immunochromatographic Test Strip for Rapid Quantitative Control of Monoclonal Antibodies against Programmed Cell Death Protein 1. Molecules 2024, 29, 3046. [Google Scholar] [CrossRef]
- DB34/T 3637-2020; Anhui Provincial Market Supervision Administration. Determination of Ofloxacin and Norfloxacin Residues in Aquatic Products—Colloidal Gold Immunochromatography. Anhui Provincial Standards: Hefei, China, 2020.
- DB34/T 821-2024; Anhui Provincial Market Supervision Administration. Determination of Ofloxacin and Norfloxacin Residues in Aquatic Products—Liquid Chromatography-Tandem Mass Spectrometry. Anhui Provincial Standards: Hefei, China, 2024.
- Cester, C.C.; Toutain, P.L. A comprehensive model for enrofloxacin to ciprofloxacin transformation and disposition in dog. J. Pharm. Sci. 1997, 86, 1148–1155. [Google Scholar] [CrossRef]
- Yang, F.; Yang, F.; Wang, D.; Zhang, C.S.; Wang, H.; Song, Z.W.; Shao, H.T.; Zhang, M.; Yu, M.L.; Zheng, Y. Development and Application of a Water Temperature Related Physiologically Based Pharmacokinetic Model for Enrofloxacin and Its Metabolite Ciprofloxacin in Rainbow Trout. Front. Vet. Sci. 2021, 7, 608348. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, J.X.; Yang, Y.Y.; Mao, S.; Li, Z. Lanthanide MOFs based portable fluorescence sensing platform: Quantitative and visual detection of ciprofloxacin and Al3+. Sci. Total Environ. 2024, 922. [Google Scholar] [CrossRef] [PubMed]
- Umesh, N.M.; Jesila, J.A.A.; Wang, S.F. Amperometric detection of antibiotic drug ciprofloxacin using cobalt-iron Prussian blue analogs capped on carbon nitride. Microchim. Acta 2022, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Yu, L.P. Specific and ultrasensitive ciprofloxacin detection by responsive photonic crystal sensor. J. Hazard. Mater. 2014, 280, 46–54. [Google Scholar] [CrossRef]
- Fan, G.Y.; Yang, R.S.; Jiang, J.Q.; Chang, X.Y.; Chen, J.J.; Qi, Y.H.; Wu, S.X.; Yang, X.F. Development of a class-specific polyclonal antibody-based indirect competitive ELISA for detecting fluoroquinolone residues in milk. J. Zhejiang Univ.-Sci. B 2012, 13, 545–554. [Google Scholar] [CrossRef]

| Sample | Add Concentration/(ng/mL) | Mode 1 Test Results | Mode 2 Test Results | ELISA Test Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (ng/mL) | Recovery (%) | CV (%) | Mean (ng/mL) | Recovery (%) | CV (%) | Mean (ng/mL) | Recovery (%) | CV (%) | ||
| Larimichthys crocea | 0.00 | ND 1 | - | - | ND | - | - | ND | - | - |
| 10.00 | 10.03 | 100.30 | 14.97 | 10.27 | 102.70 | 11.50 | 9.50 | 95.00 | 9.48 | |
| 20.00 | 19.60 | 98.00 | 6.83 | 20.03 | 100.30 | 5.97 | 19.67 | 98.30 | 3.15 | |
| Carassius auratus | 0.00 | ND | - | - | ND | - | - | ND | - | - |
| 10.00 | 10.00 | 100.00 | 7.69 | 9.50 | 95.00 | 15.47 | 10.13 | 101.30 | 7.74 | |
| 20.00 | 19.80 | 99.00 | 1.53 | 19.60 | 98.00 | 4.00 | 19.87 | 99.40 | 7.39 | |
| Micropterus salmoides | 0.00 | ND | - | - | ND | - | - | ND | - | - |
| 10.00 | 9.90 | 99.00 | 18.93 | 9.90 | 99.00 | 13.9 | 9.57 | 95.70 | 10.09 | |
| 20.00 | 19.30 | 96.50 | 3.47 | 19.47 | 97.40 | 4.50 | 19.30 | 96.50 | 3.32 | |
| Channa argus | 0.00 | ND | - | -- | ND | - | - | ND | - | - |
| 10.00 | 9.630 | 96.30 | 14.44 | 9.47 | 94.70 | 10.25 | 10.13 | 101.30 | 6.71 | |
| 20.00 | 19.90 | 99.50 | 6.75 | 19.47 | 97.40 | 1.82 | 19.77 | 98.80 | 2.69 | |
| Parabramis pekinensis | 0.00 | ND | - | - | ND | - | - | ND | - | - |
| 10.00 | 9.030 | 90.30 | 19.79 | 10.13 | 101.30 | 9.74 | 9.43 | 94.30 | 11.93 | |
| 20.00 | 19.30 | 96.50 | 5.27 | 19.83 | 99.20 | 2.57 | 19.63 | 98.20 | 1.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Cai, Z.; Zhang, C.; Feng, X.; Zhang, C.; Zhao, H.; Yin, C.; Wang, B.; Yu, X.; Zhang, B. Dual-Mode Quantitative Immunochromatographic Assay for Highly Sensitive On-Site Detection of Ciprofloxacin in Fish Products. Foods 2025, 14, 1132. https://doi.org/10.3390/foods14071132
Shen J, Cai Z, Zhang C, Feng X, Zhang C, Zhao H, Yin C, Wang B, Yu X, Zhang B. Dual-Mode Quantitative Immunochromatographic Assay for Highly Sensitive On-Site Detection of Ciprofloxacin in Fish Products. Foods. 2025; 14(7):1132. https://doi.org/10.3390/foods14071132
Chicago/Turabian StyleShen, Junqi, Zhengyi Cai, Cheng Zhang, Xinyue Feng, Chenzhi Zhang, Huan Zhao, Chuanlin Yin, Bo Wang, Xiaoping Yu, and Biao Zhang. 2025. "Dual-Mode Quantitative Immunochromatographic Assay for Highly Sensitive On-Site Detection of Ciprofloxacin in Fish Products" Foods 14, no. 7: 1132. https://doi.org/10.3390/foods14071132
APA StyleShen, J., Cai, Z., Zhang, C., Feng, X., Zhang, C., Zhao, H., Yin, C., Wang, B., Yu, X., & Zhang, B. (2025). Dual-Mode Quantitative Immunochromatographic Assay for Highly Sensitive On-Site Detection of Ciprofloxacin in Fish Products. Foods, 14(7), 1132. https://doi.org/10.3390/foods14071132






