Structural Characteristics, Gelling Properties, In Vitro Antioxidant Activity and Immunomodulatory Effects of Rhamnogalacturonan-I Rich Pectic Polysaccharides Alkaline-Extracted from Wax Apple (Syzygium samarangense)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Alkaline Extraction of Syzygium samarangense Pectic Polysaccharide
2.3. Determination of Total Polysaccharide and Total Protein Content of SSPs
2.4. Carbohydrate Analysis
2.5. Gelling Properties
2.5.1. Preparation of Ca2+-Induced Gel
2.5.2. Effect of Pectin Concentration and Ca2+ Concentration on Gelling Properties of SSP-AK Sample
2.5.3. Rheological Behaviors
2.5.4. Water-Holding Capacity
2.5.5. Gel Hardness
2.5.6. Scanning Electron Microscopy (SEM) Analysis
2.6. Antioxidant Activity of SSP-AK
2.7. Immunomodulatory Activity of SSP-AK
2.8. Statistics Analysis
3. Results and Discussions
3.1. Chemical Components and Monosaccharide Composition of SSPs
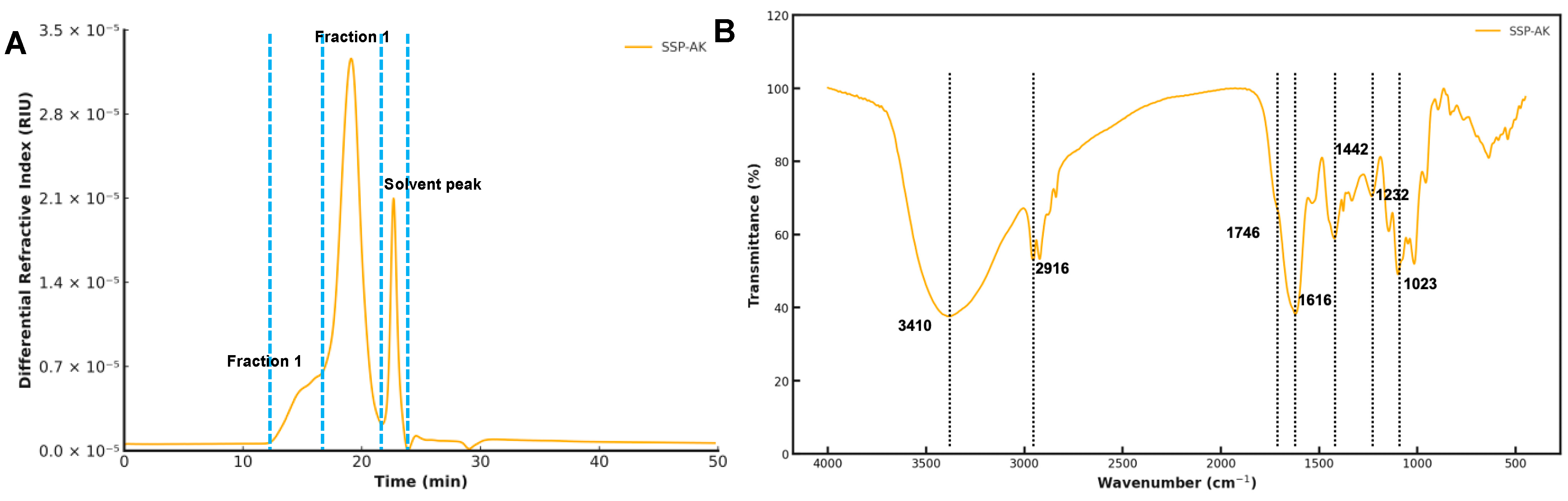
3.2. Molecular Weight Distribution of SSPs
3.3. FT-IR Analysis of SSP-AK
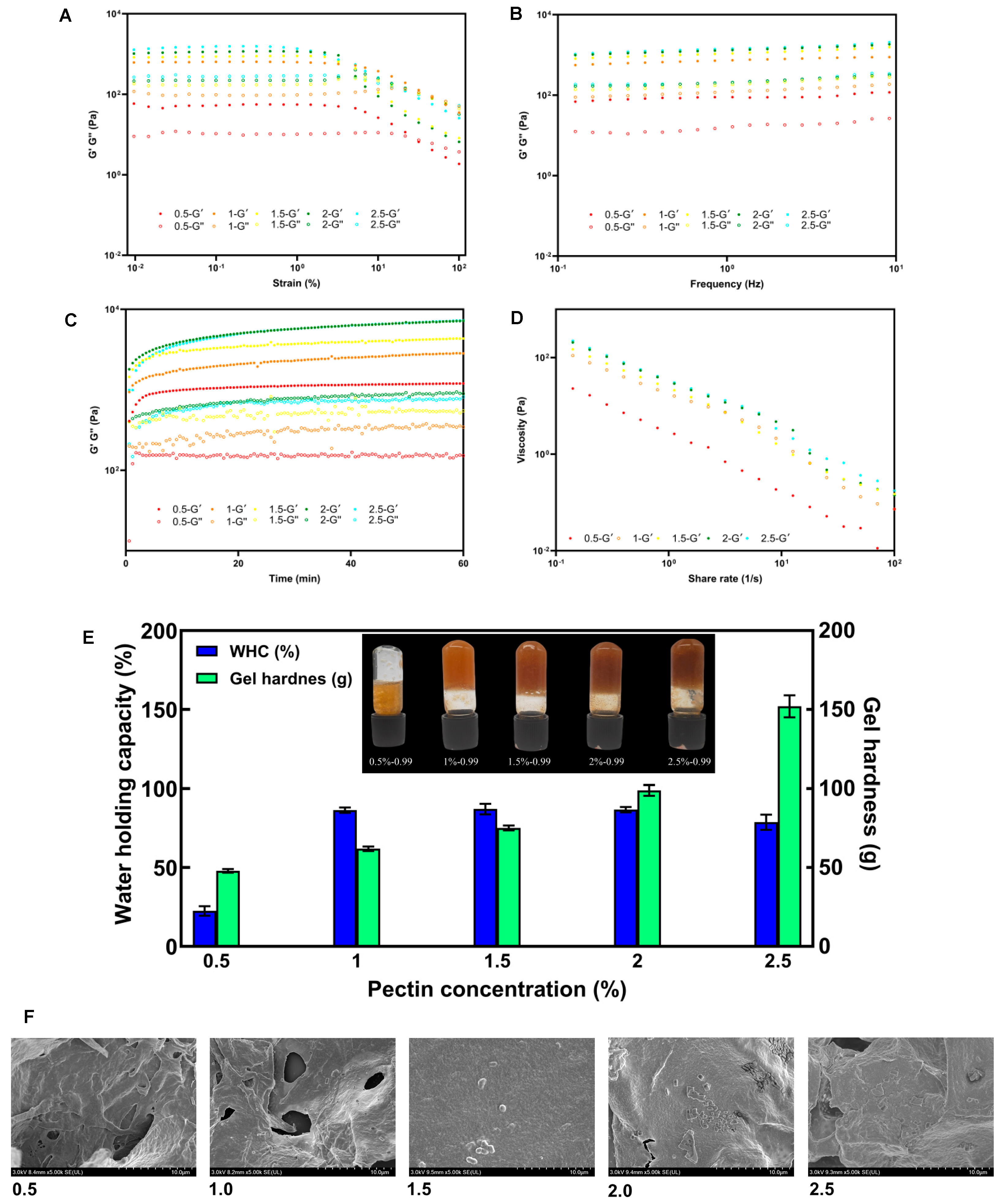
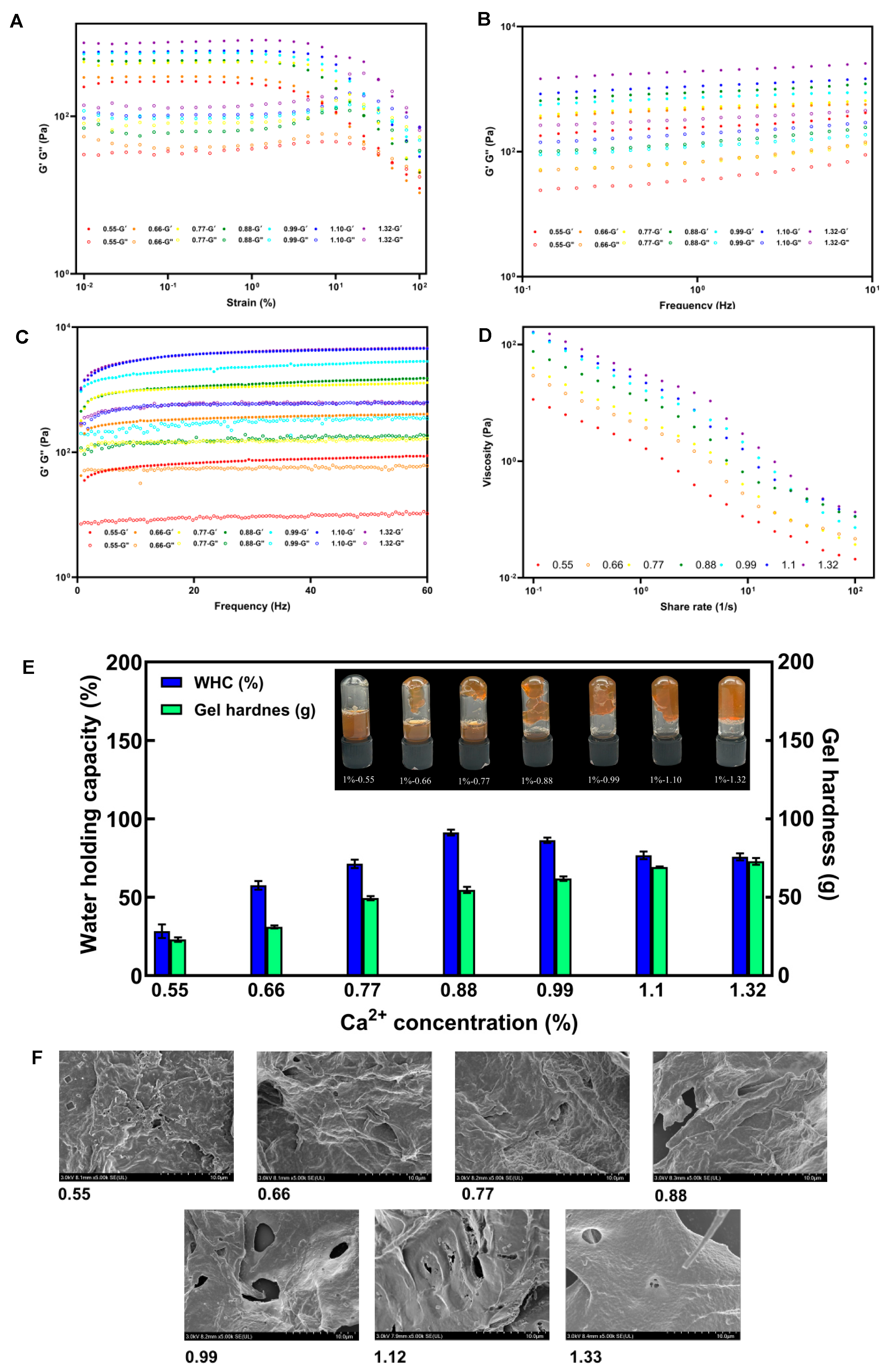
3.4. Rheological Properties and Gelling Properties of SSP-AK
3.5. Effect of Pectin Concentrations on Gelling Properties of SSP-AK
3.6. Effect of Ca2+ Concentrations on Gelling Properties of SSP-AK
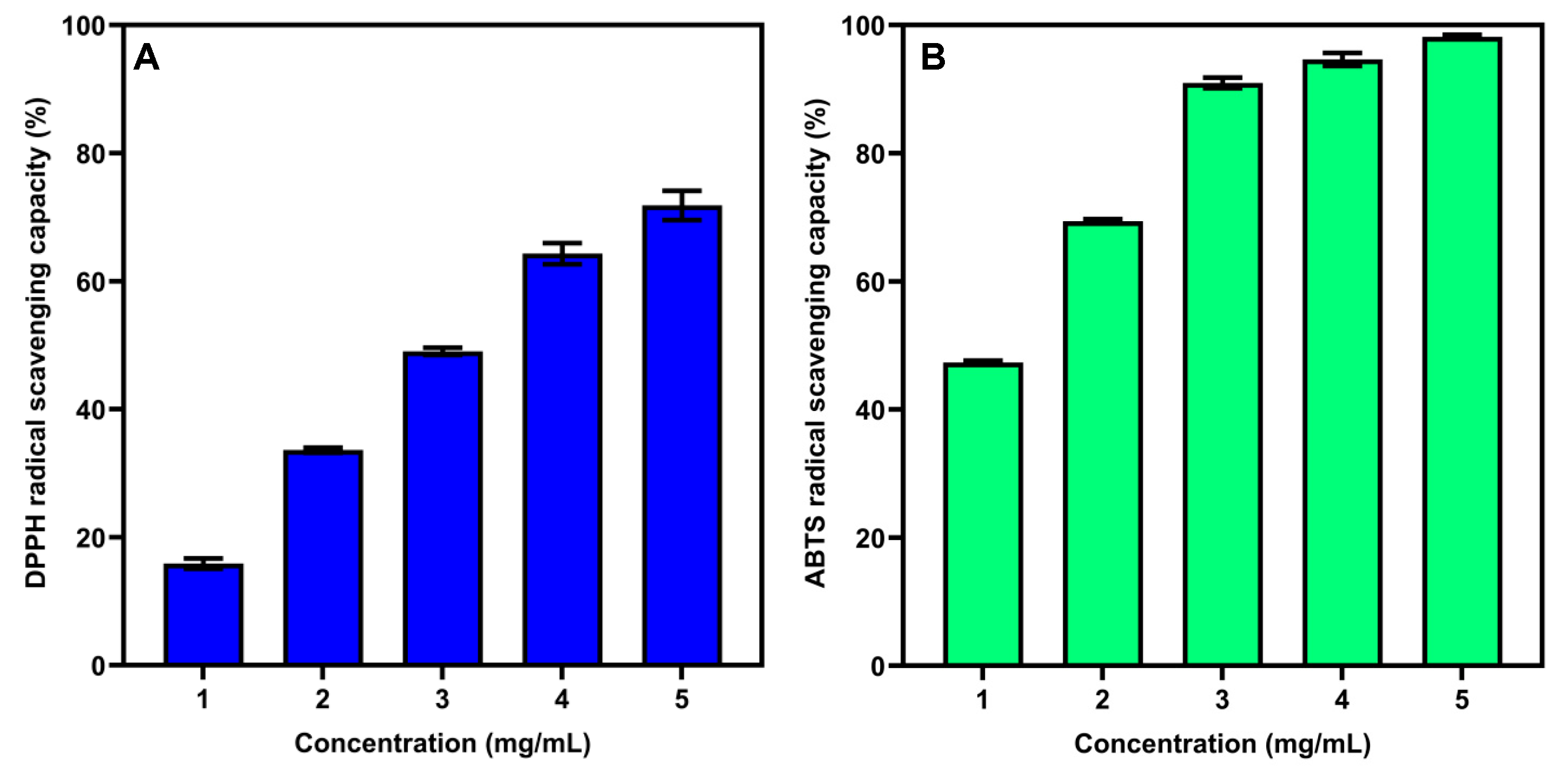
3.7. Antioxidant Activity of SSP-AK
3.8. Immunomodulatory Activity of SSP-AK
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banadka, A.; Wudali, N.S.; Al-Khayri, J.M.; Nagella, P. The role of Syzygium samarangense in nutrition and economy: An overview. S. Afr. J. Bot. 2022, 145, 481–492. [Google Scholar] [CrossRef]
- Zhi, Z.; Chen, J.; Li, S.; Wang, W.; Huang, R.; Liu, D.; Ding, T.; Linhardt, R.J.; Chen, S.; Ye, X. Fast preparation of RG-I enriched ultra-low molecular weight pectin by an ultrasound accelerated Fenton process. Sci. Rep. 2017, 7, 541. [Google Scholar] [CrossRef]
- Yue, F.; Xu, J.; Zhang, S.; Hu, X.; Wang, X.; Lü, X. Structural features and anticancer mechanisms of pectic polysaccharides: A review. Int. J. Biol. Macromol. 2022, 209, 825–839. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Makarova, E.N.; Belyy, V.A. Structural studies of biologically active pectin-containing polysaccharides of pomegranate Punica granatum. Int. J. Biol. Macromol. 2019, 122, 29–36. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Li, H.; Rao, J.; Chen, B. Tyramine modification of high and low methoxyl pectin: Physicochemical properties, antioxidant activity, and gelation behavior. Food Hydrocoll. 2023, 144, 108949. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Yu, S.; Guo, X.; Ai, C.; Tang, X.; Chen, H.; Lin, J.; Zhang, X.; Meng, H. Effects of pH and temperature on the structure, rheological and gel-forming properties of sugar beet pectins. Food Hydrocoll. 2021, 116, 106646. [Google Scholar] [CrossRef]
- Liu, D.; Xia, W.; Liu, J.; Wang, X.; Xue, J. Ultrasound-assisted alkali extraction of RG-I enriched pectin from thinned young apples: Structural characterization and gelling properties. Food Hydrocoll. 2024, 151, 109879. [Google Scholar] [CrossRef]
- Mikshina, P.V.; Makshakova, O.N.; Petrova, A.A.; Gaifullina, I.Z.; Idiyatullin, B.Z.; Gorshkova, T.A.; Zuev, Y.F. Gelation of rhamnogalacturonan I is based on galactan side chain interaction and does not involve chemical modifications. Carbohydr. Polym. 2017, 171, 143–151. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Zhang, H.; Wu, D.; Ye, X.; Linardt, R.J.; Chen, S. Gelling mechanism of RG-I enriched citrus pectin: Role of arabinose side-chains in cation- and acid-induced gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- Cui, J.; Ren, W.; Zhao, C.; Gao, W.; Tian, G.; Bao, Y.; Lian, Y.; Zheng, J. The structure–property relationships of acid- and alkali-extracted grapefruit peel pectins. Carbohydr. Polym. 2020, 229, 115524. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.-Y.; Yuan, Q.; Nie, X.-R.; Zhou, J.; Wei, S.-Y.; Du, G.; Zhao, L.; Wang, S.-P.; Zhang, Q.; et al. Structural characterization, antioxidant activity, and immunomodulatory activity of non-starch polysaccharides from Chuanminshen violaceum collected from different regions. Int. J. Biol. Macromol. 2020, 143, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of microwave-assisted extraction and structural characterization of pectin from sweet lemon peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Li, J.; Wang, J.; Lei, J.; Gan, R.-Y.; Qin, P.; Hu, Y.-C.; Wu, X.-Y.; Zou, L. Comparison of soluble dietary fibers from various quinoa microgreens: Structural characteristics and bioactive properties. Food Res. Int. 2024, 181, 114108. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J.-Y. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Tang, X.; Chen, Y.; You, Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015, 188, 111–118. [Google Scholar] [CrossRef]
- Yang, M.; Shen, Q.; Li, L.-Q.; Huang, Y.-Q.; Cheung, H.-Y. Phytochemical profiles, antioxidant activities of functional herb Abrus cantoniensis and Abrus mollis. Food Chem. 2015, 177, 304–312. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Wang, C.; Bai, H.; Lu, J.; Tian, L.; Gao, M.; Wu, J.; Bai, C.; Sun, H. Effects of ultra-high pressure enzyme extraction on characteristics and functional properties of red pitaya (Hylocereus polyrhizus) peel pectic polysaccharides. Food Hydrocoll. 2021, 121, 107016. [Google Scholar] [CrossRef]
- Wang, B.-H.; Cao, J.-J.; Zhang, B.; Chen, H.-Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef]
- Song, H.; Han, L.; Zhang, Z.; Li, Y.; Yang, L.; Zhu, D.; Wang, S.; He, Y.; Liu, H. Structural properties and bioactivities of pectic polysaccharides isolated from soybean hulls. LWT 2022, 170, 114079. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Lv, G.-Y.; Song, T.-T.; Xu, Z.-W.; Wang, M.-Y. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. Food Chem. 2024, 445, 138752. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Tang, W.; Liu, D.; Wang, J.-Q.; Huang, X.-J.; Yin, J.-Y.; Geng, F.; Nie, S.-P. Isolation and structure characterization of a low methyl-esterified pectin from the tuber of Dioscorea opposita Thunb. Food Chem. 2021, 359, 129899. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, W.; Feng, Y.; Ma, H. Fine physicochemical, structural, rheological and gelling properties of tomato pectin under infrared peeling technique. Innov. Food Sci. Emerg. Technol. 2023, 85, 103343. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Wang, R.; Dai, J.; Wang, L.; Zhang, J. Extraction of pectins from renewable grapefruit (Citrus paradisi) peels using deep eutectic solvents and analysis of their structural and physicochemical properties. Int. J. Biol. Macromol. 2024, 254, 127785. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Luo, X.; Mo, M.-M.Q.; Feng, J.; Li, W.-B.; Yan, H.; Hu, Y.-C.; Zou, L.; Wu, D.-T. Structural properties and biological effects of pectic polysaccharides extracted from Tartary buckwheat sprouts by high pressure-assisted deep eutectic solvent extraction. LWT 2024, 203, 116397. [Google Scholar] [CrossRef]
- Gallery, C.; Agoda-Tandjawa, G.; Bekaert, D.; Gitto, L. Understanding structure/rheology relationships of amidated low-methoxyl citrus and apple pectin gels: Implications of sucrose, pectin types and characteristics. Food Hydrocoll. 2024, 152, 109950. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Sun, J.; Lei, Y.; Guo, M.; Wang, L. Extraction methods, multiple biological activities, and related mechanisms of Momordica charantia polysaccharide: A review. Int. J. Biol. Macromol. 2024, 263, 130473. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Wang, X.; Wang, J.; Du, Y.; Cui, J.; Zeng, L.; Zheng, J. Arabinan branches in the RG-I region of citrus pectin aid acid-induced gelation. Carbohydr. Polym. 2024, 346, 122668. [Google Scholar] [CrossRef]
- Shivamathi, C.S.; Moorthy, I.G.; Kumar, R.V.; Soosai, M.R.; Maran, J.P.; Kumar, R.S.; Varalakshmi, P. Optimization of ultrasound assisted extraction of pectin from custard apple peel: Potential and new source. Carbohydr. Polym. 2019, 225, 115240. [Google Scholar] [CrossRef]
- Qi, T.; Ren, J.; Li, X.; An, Q.; Zhang, N.; Jia, X.; Pan, S.; Fan, G.; Zhang, Z.; Wu, K. Structural characteristics and gel properties of pectin from citrus physiological premature fruit drop. Carbohydr. Polym. 2023, 309, 120682. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Meng, Y.; Hu, C.; Dong, G.; Qu, Y.; Deng, H.; Guo, Y. Mathematical model of Ca2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 2017, 66, 37–48. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M.; Jia, Z. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Ding, D.; Gao, J.; Hao, L.; Guo, X.; Liu, Y. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J. Food Meas. Charact. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar] [CrossRef]
- Huang, R.; Yu, H. Extraction methods, chemical compositions, molecular structure, health functions, and potential applications of tea polysaccharides as a promising biomaterial: A review. Int. J. Biol. Macromol. 2024, 277, 134150. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Xin, X.; Zhang, M. Effect of the immunoregulation activity of a pectin polysaccharide from Saussurea laniceps petals on macrophage polarization. Int. J. Biol. Macromol. 2024, 278, 134757. [Google Scholar] [CrossRef]
- Steigerwald, H.; Blanco-Pérez, F.; Macías-Camero, A.; Albrecht, M.; Huch, M.; Bender, C.; Schülke, S.; Keller, J.; Krause, M.; Barbas, C.; et al. Effects of pectin methyl-esterification on intestinal microbiota and its immunomodulatory properties in naive mice. Carbohydr. Polym. 2024, 334, 122007. [Google Scholar] [CrossRef]
- Desai, K.; Dobruchowska, J.M.; Elbers, K.; Cybulska, J.; Zdunek, A.; Porbahaie, M.; Jansen, E.; Van Neerven, J.; Albers, R.; Wennekes, T.; et al. Associating structural characteristics to immunomodulating properties of carrot rhamnogalacturonan-I fractions. Carbohydr. Polym. 2025, 347, 122730. [Google Scholar] [CrossRef]

| Yield (%) | Polysaccharide (%) | Protein (%) | DE (%) | Monosaccharide Composition | Structure Content (mol%) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rha | GluA | GalA | Glu | Gal | Ara | HG | RG-I | Rha/GalA | (Ara + Gal)/Rha | |||||
| SSP-H | 1.5 ± 0.1 | 82.1 | 1.5 ± 0.3 | 66.3 ± 0.3 | 1.0 | 0.6 | 4.7 | 2.7 | 8.9 | 5.2 | 15.9 | 69.4 | 0.2 | 14.1 |
| SSP-AK | 5.3 ± 0.2 | 88.4 | 5.5 ± 0.8 | 41.3 ± 0.3 | 1.0 | 0.4 | 4.3 | 1.3 | 4.1 | 3.5 | 22.6 | 64.8 | 0.2 | 7.6 |
| SSP-AC | 3.2 ± 0.3 | 80.5 | 3.2 ± 0.3 | 50.5 ± 0.7 | 1.0 | 0.6 | 6.5 | 2.5 | 8.9 | 5.4 | 21.9 | 65.6 | 0.2 | 14.3 |
| SSP-DES | 3.1 ± 0.1 | 76.3 | 3.1 ± 0.1 | 44.8 ± 0.5 | 1.0 | 0.3 | 1.3 | 2.1 | 3.9 | 3.7 | 2.6 | 77.6 | 0.8 | 7.5 |
| SSP-AU | 2.1 ± 0.1 | 83.2 | 2.1 ± 0.2 | 66.0 ± 0.6 | 1.0 | 0.4 | 9.7 | 1.2 | 5.4 | 3.6 | 40.6 | 51.5 | 0.1 | 9.0 |
| SSP-U | 0.8 ± 0.1 | 83.4 | 3.2 ± 0.4 | 51.1 ± 0.7 | 1.0 | 0.7 | 4.9 | 2.8 | 9.6 | 6.0 | 15.6 | 69.7 | 0.4 | 15.5 |
| SSP-M | 0.7 ± 0.1 | 81.3 | 4.1 ± 0.1 | 56.0 ± 0.7 | 1.0 | 0.7 | 2.4 | 2.0 | 9.9 | 5.6 | 6.3 | 80.3 | 0.2 | 15.6 |
| SSP-H | SSP-AK | SSP-AC | SSP-DES | SSP-AU | SSP-U | SSP-M | |
|---|---|---|---|---|---|---|---|
| Molecular weight, Mw (kDa) | |||||||
| Fraction 1 | 741.5 ± (0.8%) | 7658 ± (0.9%) | 1305 ± (0.7%) | 533.1 ± (1.5%) | 68.7 ± (0.9%) | 605.3 ± (0.8%) | 384.6 ± (1.0%) |
| Fraction 2 | - | 345.3 ± (2.8%) | 44.2 ± (2.7%) | 130.9 ± (1.5%) | - | - | - |
| Fraction 3 | - | - | - | 16.9 ± (6.4%) | - | - | - |
| Mass fraction (%) | |||||||
| Fraction 1 | 100 | 15.7 | 8.4 | 6.3 | 100 | 100 | 100 |
| Fraction 2 | - | 84.4 | 91.6 | 19.3 | - | - | - |
| Fraction 3 | - | - | - | 74.4 | - | - | - |
| Mw/Mn (polydispersity) | |||||||
| Fraction 1 | 4.7 | 1.4 | 1.4 | 1.1 | 2.3 | 3.8 | 2.8 |
| Fraction 2 | - | 2.0 | 1.9 | 1.2 | - | - | - |
| Fraction 3 | - | - | - | 1.4 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; He, S.; Zhao, Z.; Liu, C.; Lei, Y.; Liu, M.; Zhang, Q.; Lin, D.; Liu, Y.; Lin, S.; et al. Structural Characteristics, Gelling Properties, In Vitro Antioxidant Activity and Immunomodulatory Effects of Rhamnogalacturonan-I Rich Pectic Polysaccharides Alkaline-Extracted from Wax Apple (Syzygium samarangense). Foods 2025, 14, 1227. https://doi.org/10.3390/foods14071227
Lu Y, He S, Zhao Z, Liu C, Lei Y, Liu M, Zhang Q, Lin D, Liu Y, Lin S, et al. Structural Characteristics, Gelling Properties, In Vitro Antioxidant Activity and Immunomodulatory Effects of Rhamnogalacturonan-I Rich Pectic Polysaccharides Alkaline-Extracted from Wax Apple (Syzygium samarangense). Foods. 2025; 14(7):1227. https://doi.org/10.3390/foods14071227
Chicago/Turabian StyleLu, Yue, Siyu He, Zifan Zhao, Changxin Liu, Ye Lei, Mingyu Liu, Qing Zhang, Derong Lin, Yaowen Liu, Shang Lin, and et al. 2025. "Structural Characteristics, Gelling Properties, In Vitro Antioxidant Activity and Immunomodulatory Effects of Rhamnogalacturonan-I Rich Pectic Polysaccharides Alkaline-Extracted from Wax Apple (Syzygium samarangense)" Foods 14, no. 7: 1227. https://doi.org/10.3390/foods14071227
APA StyleLu, Y., He, S., Zhao, Z., Liu, C., Lei, Y., Liu, M., Zhang, Q., Lin, D., Liu, Y., Lin, S., Lu, X., & Qin, W. (2025). Structural Characteristics, Gelling Properties, In Vitro Antioxidant Activity and Immunomodulatory Effects of Rhamnogalacturonan-I Rich Pectic Polysaccharides Alkaline-Extracted from Wax Apple (Syzygium samarangense). Foods, 14(7), 1227. https://doi.org/10.3390/foods14071227







