Immune Reactivity to Raw and Processed Foods and Their Possible Contributions to Autoimmunity
Abstract
:1. Introduction
- Selection and identification of source materials;
- Grinding and defatting;
- Extraction under specific conditions;
- Antigenic purification;
- Sterilization;
- Product testing.
2. Why Raw Versus Processed Foods Matters
2.1. Detection of IgE, IgG, and IgA Antibodies Against Raw and Modified Food Antigens
2.2. Why Traditional Food Sensitivity Testing Falls Short
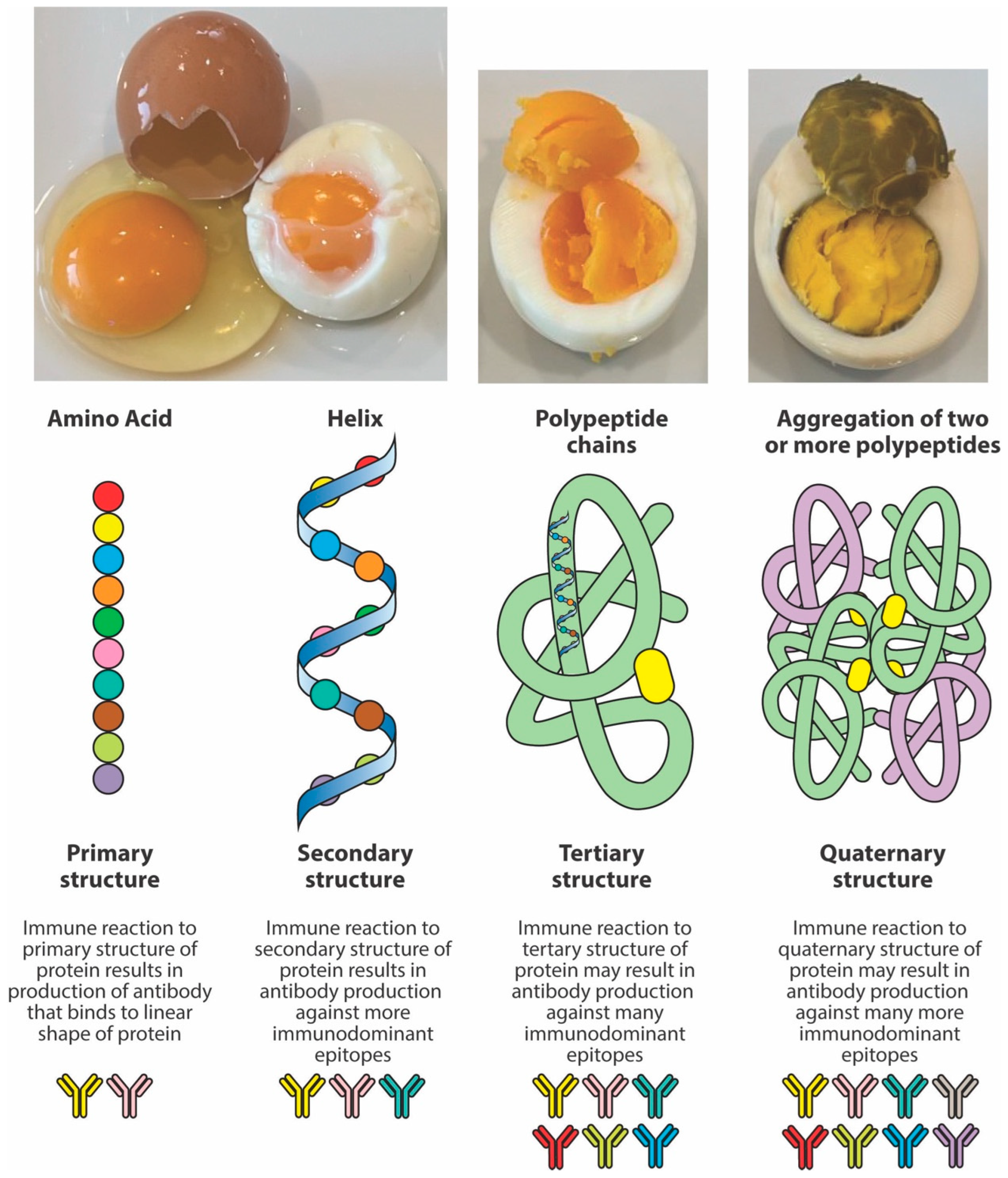
3. Why Immunodominant and Neo-Peptides Matter
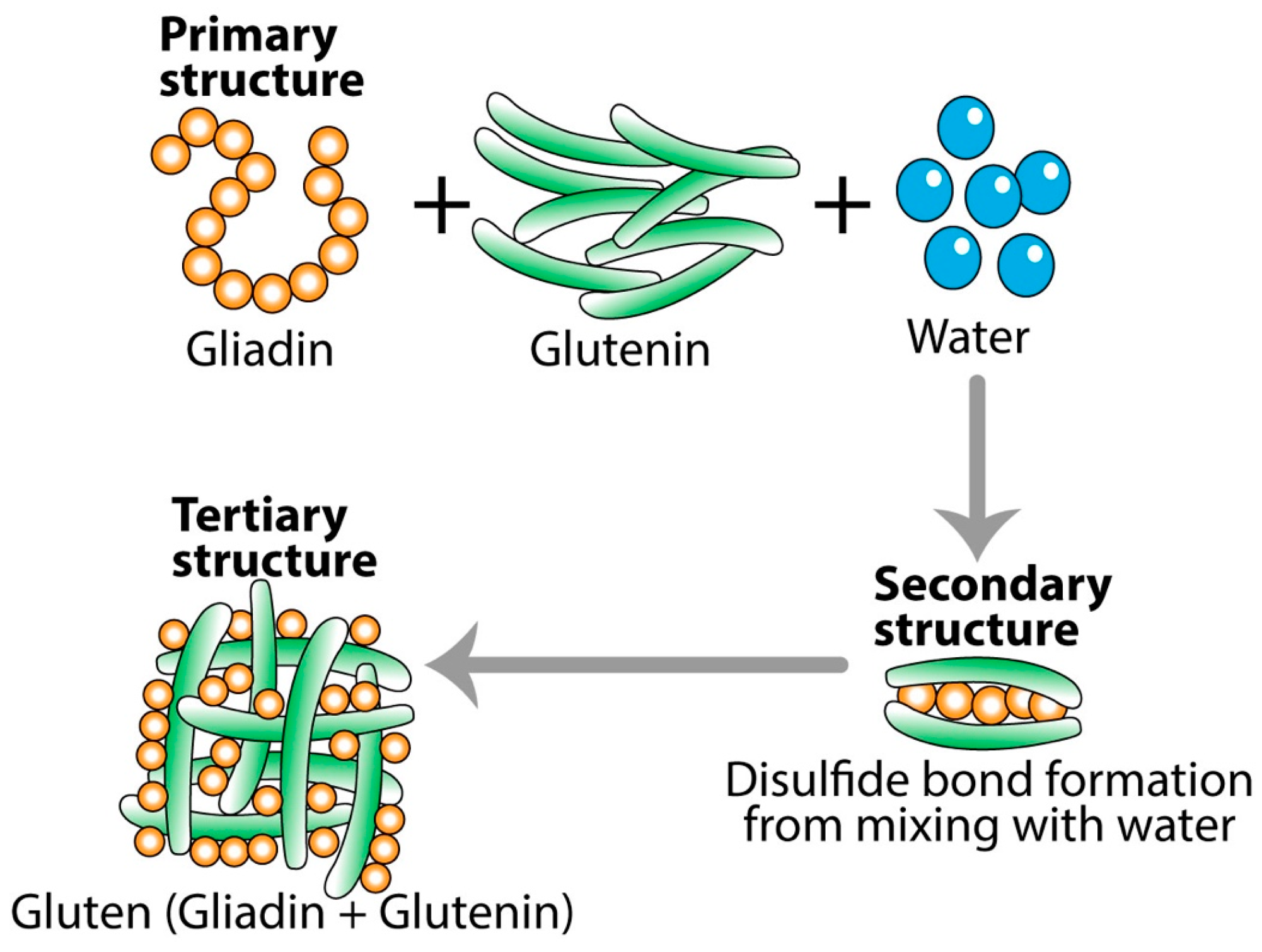
4. The Formation of Neo-Antigens and Immunodominant Epitopes by Thermal and Other Modifications
5. Implications of Food in Autoimmunity
Evidence for the Induction of Autoimmunity by Food Antigens Through Different Mechanisms
- There is molecular mimicry between food proteins/peptides and human tissues;
- Polyclonal and monoclonal antibodies made against food antigens react with human autoantigens;
- Antibodies made against human autoantigens react with various food antigens;
- Affinity-purified antibodies from patients with a known autoimmune disease react with different food antigens;
- Injection of food cross-reactive epitopes into animal models induces autoimmunity;
- Food-specific antibodies and biomarkers of autoimmunity can be simultaneously measured using reliable blood tests.
- Key point 1: There is molecular mimicry between food proteins/peptides and human tissues
- Key point 2: Polyclonal and monoclonal antibodies made against food antigens react with human autoantigens
- Key point 3: Antibodies made against human autoantigens react with various food antigens
- Key point 4: Affinity-purified antibody from patients with a known autoimmune disease react with different food antigens
- Key point 5: Injection of food cross-reactive epitopes into animal models induces autoimmunity
- Key point 6: Food-specific antibodies and biomarkers of autoimmunity can be simultaneously measured using reliable blood tests
6. Correlation Between Food Modification, Formation of Advanced Glycation End Products (AGEs), and Protein Lipid Peroxidation and Their Contribution to Neuro-Autoimmunity
7. Conclusions: It Is Vital to Measure Antibodies Against Both Raw and Processed Food Antigens to Prevent or Treat Food Immune Reactivity and Autoimmunity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahna, S.L. History of food allergy and where we are today. World Allergy Organ. J. 2024, 17, 100912. [Google Scholar] [CrossRef]
- Portier, P.; Richet, C. De l’action anaphylactique de certain venin. C. R. Seances Soc. Biol. 1902, 54, 170. [Google Scholar]
- Huber, B. 100 years of allergy: Clemens von Pirquet—His idea of allergy and its imminent cconcept of disease. Wien. Klin. Wochenschr. 2006, 118, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Uzzaman, A.; Cho, S.H. Chapter 28: Classification of hypersensitivity reactions. Allergy Asthma Proc. 2012, 33 (Suppl. S1), 96–99. [Google Scholar] [CrossRef]
- Tanno, L.K.; Demoly, P. Food allergy in the World Health Organization’s international classification of diseases (ICD)-11. Pediatr. Allergy Immunol. 2022, 33, e13882. [Google Scholar] [CrossRef]
- Ng, A.E.; Boersma, P. Diagnosed allergic conditions in adults: United States, 2021. NCHS Data Brief 2023, 460, 1–8. [Google Scholar] [CrossRef]
- Zablotsky, B.; Black, L.I.; Akinbami, L.J. Diagnosed allergic conditions in children aged 0–17 years: United States, 2021. NCHS Data Brief 2023, 459, 1–8. [Google Scholar] [CrossRef]
- Mustilo, P.; Martin, B.L.; Oppenheimer, J.; Nelson, M.R. Allergen immunotherapy extraction preparation instructional guide. Am. Coll. Allergy Asthma Immunol. 2019, 6, 1–4. [Google Scholar]
- Malsagova, K.; Stepanov, A.; Sinitsyna, A.A.; Izotov, A.; Klyuchnikov, M.S.; Kopylov, A.T.; Kaysheva, A.L. Determination of specific IgG to identify possible food intolerance in athletes using ELISA. Data 2021, 6, 122. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States; summary of the NIAID-sponsored expert panel report. Nutr. Res. 2011, 31, 61–75. [Google Scholar] [CrossRef]
- Schoos, A.M.; Bullens, D.; Chawes, B.L.; Costa, J.; De Vlieger, L.; DunnGalvin, A.; Epstein, M.M.; Garssen, J.; Hilger, C.; Knipping, K.; et al. Immunological outcomes of allergen-specific immunotherapy in food allergy. Front. Immunol. 2020, 11, 568598. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Enin, M.A.; El-Din, E.M.R.S.; Abdelwahab, H.W.; El-Maksoud, A.A.; El-Aziz, A.M.A.; Shaaban, M.I.; Attia, A.N.; Aboukamar, W.A.; Mohei-Aldin, S.; Belal, F. Preparation of chemically stable allergen-specific sublingual immunotherapy from Egyptian allergens. J. Clin. Lab. Anal. 2022, 36, e224261. [Google Scholar] [CrossRef]
- Terlouw, S.; van Boven, F.E.; Zonneveld, M.B.-V.; de Graaf-in ‘t Veld, C.; van Splunter, M.E.; van Daele, P.L.A.; van Maaren, M.S.; Schreurs, M.W.J.; de Jong, N.W. Homemade food allergen extracts for use in skin prick tests in the diagnosis of IgE-mediated food allergy: A good alternative in the absence of commercially available extracts? Nutrients 2022, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- David, N.A.; Penumarti, A.; Slater, J.E. Manufacturing food extracts. In Allergens and Allergen Immunotherapy, 6th ed.; Lockey, R.F., Ledford, D.K., Eds.; Taylor Francis: Boca Raton, FL, USA, 2020. [Google Scholar]
- García, E.; Llorente, M.; Hernando, A.; Kieffer, R.; Wieser, H.; Méndez, E. Development of a general procedure for complete extraction of gliadins for heat processed and unheated foods. Eur. J. Gastroenterol. Hepatol. 2005, 17, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Um, H.; Kim, N.H.; Kim, D. Potential Alzheimer’s disease therapeutic nano-platform: Discovery of amyloid-beta plaque disaggregating agent and brain-targeted delivery system using porous silicon nanoparticle. Bioact. Mater. 2023, 24, 497–506. [Google Scholar] [CrossRef]
- Vojdani, A. Detection of IgE, IgG, IgA, and IgM antibodies against raw and processed food antigens. Nutr. Metab. 2009, 6, 22. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-mediated gastrointestinal food allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef]
- Hill, D.A.; Grundmeier, R.W.; Ram, G.; Spergel, J.M. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: A retrospective cohort study. BMC Pediatr. 2016, 16, 133. [Google Scholar] [CrossRef]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.K.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B.-W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 21. [Google Scholar] [CrossRef]
- Prausnitz, C.; Küstner, H. Studies on supersensitivity. Cent. Für Bakteriol. 1921, 86, 160–169. [Google Scholar]
- Spies, J.R. Allergens. J. Agric. Food Chem. 1974, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Malanin, K.; Lundberg, M.; Johansson, S.G. Anaphylactic reaction caused by neoallergens in heated pecan nut. Allergy 1995, 50, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Amirdivani, S.; Khorshidian, N.; Fidelis, M.; Granata, D.; Koushki, M.R.; Mohamadi, M.; Khostinat, K.; Mortazavian, A.M. Effects of transglutaminase on health properties of food products. Curr. Opin. Food Sci. 2018, 22, 74–80. [Google Scholar] [CrossRef]
- Bauer, A.; Rosiek, P.; Bauer, T. Microbial transglutaminase—The food additive, a potential inducing factor in primary biliary cholangitis. Molecules 2025, 30, 762. [Google Scholar] [CrossRef]
- Hong, G.P.; Xiong, Y.L. Microbial transglutaminase-induced structural and rheological changes of cationic and anionic myofibrillar properties. Meat Sci. 2012, 91, 36–42. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C.; Vojdani, A. The frequently used industrial food process additive, microbial transglutaminase: Boon or bane. Nutr Rev. 2024, 83, e1286–e1294. [Google Scholar] [CrossRef]
- Leduc, V.; Moneret-Vautrin, D.A.; Guerin, L.; Morisset, M.; Kanny, G. Anaphylaxis to wheat isolates: Immunochemical study of a case proved by means of double-blind, placebo-controlled food challenge. J. Allergy Clin. Immunol. 2003, 111, 897–899. [Google Scholar] [CrossRef]
- Maleki, S.J.; Chung, S.; Champagne, E.; Raufman, J.P. The effects of roasting on the allergenic properties of peanuts. J. Allergy Clin. Immunol. 2000, 106, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Sandin, D.I.; San Ireneo, M.M.; Fernandez-Caldas, E.F.; Lebreo, E.A.; Borrego, T.L. Specific IgE determinations to crude and boiled lentil (Lens culinaris) extracts in lentil-sensitive children and controls. Allergy 1999, 54, 1209–1214. [Google Scholar] [CrossRef]
- Su, M.; Venkatachalam, M.V.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Impact of irradiation and thermal processing on the antigenicity of almond, cashew nut, and walnut proteins. J. Sci. Food Agric. 2004, 84, 1119–1125. [Google Scholar] [CrossRef]
- Codina, R.; Oehling, A.G., Jr.; Lockey, R.F. Neoallergens in heated soybean hull. Int. Arch. Allergy Immunol. 1998, 117, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Hefle, S.L.; Lambrecht, O.M.; Nordlee, J.A. Soy sauce retains allergenicity through the fermentation production process. J. Allergy Clin. Immunol. 2005, 115, S32. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Ortolani, C.; Farioli, L.; Pravettoni, V.; Ispano, M.; Borga, A.; Bengtsson, A.; Incorvaia, C.; Berti, C.; Zanussi, C. Allergenic cross-reactivity among peach, apricot, plum, and cherry in patients with oral allergy syndrome: An in vivo and in vitro study. J. Allergy Clin. Immunol. 1994, 94, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Fremont, S. Consequences of heat treatment and processing of food on the structure and allergenicity of component proteins. Rev. Fr. Allergol. Immunol. Clin. 2003, 43, 13–20. [Google Scholar]
- Sathe, S.K.; Teuber, S.S.; Roux, K.H. Effects of food processing on the stability of food allergens. Biotechnol. Adv. 2005, 23, 423–429. [Google Scholar] [CrossRef]
- Bock, A.U.; Atkins, F.M. Patterns of food hypersensity during sixteen years of double-blind placebo-controlled food challenges. J. Pediatr. 1990, 117, 561–567. [Google Scholar] [CrossRef]
- Rosen, J.P.; Selcow, E.; Mendelcon, L.M.; Grodofsky, M.P.; Factor, J.M.; Sampson, H.A. Skin testing with natural foods in patients suspected of having food allergies: Is it a necessity? J. Allergy Clin. Immunol. 1994, 93, 1068–1070. [Google Scholar] [CrossRef]
- Davis, P.J.; Williams, S.C. Protein modification by thermal processing. Allergy 1998, 53, 102–105. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-processed foods and food additives in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 406–427. [Google Scholar] [CrossRef]
- Chang, K.; Gunter, M.J.; Rauber, F.; Levy, R.B.; Huybrechts, I.; Kliemann, N.; Millett, C.; Vamos, E.P. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. eClinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef]
- Snelson, M.; Tan, S.M.; Clarke, R.E.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.-V.; Penfold, S.A.; Harcourt, B.E.; Sourris, K.C.; Lindblom, R.S.; et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci. Adv. 2021, 7, eabe4841. [Google Scholar] [CrossRef] [PubMed]
- Yazici, D.; Ogulur, I.; Pat, Y.; Babayev, H.; Barletta, E.; Ardicli, S.; Imam, M.B.; Huang, M.; Koch, J.; Li, M.; et al. The epithelial barrier: The gateway to allergic, autoimmune, and metabolic diseases and chronic neuropsychiatric conditions. Semin. Immunol. 2023, 70, 101846. [Google Scholar] [CrossRef]
- Sen, M.; Kopper, R.; Pons, L.; Abraham, E.C.; Burks, A.W.; Bannon, G.A. Protein structure plays a critical role in peanut allergen stability. J. Immunol. 2002, 169, 882–890. [Google Scholar] [CrossRef]
- Davis, P.J.; Smales, C.M.; James, D.C. How can thermal processing modify the antigenicity of proteins? Allergy 2001, 56, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Aminov, R.; Matthias, T. Dysbiosis may trigger autoimmune diseases via inappropriate posttranslational modification of host proteins. Front. Microbiol. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Tian, Y.; Fu, W.; Xue, W. In vitro digestibility and immunoreactivity of thermally processed peanut. Food Agri. Immunol. 2018, 29, 989–1001. [Google Scholar] [CrossRef]
- Cuadrado, C.; Sanchiz, A.; Linacero, R. Nut allergenicity: Effect of food processing. Allergies 2021, 1, 150–162. [Google Scholar] [CrossRef]
- Gonzalez, P.M.; Cassin, A.M.; Durban, R.; Upton, J.E.M. Effects of food processing on allergenicity. Curr. Allewrgy Asthma Rep. 2025, 25, 9. [Google Scholar] [CrossRef]
- Vickery, B.P.; Scurlock, A.M.; Jones, S.M.; Burks, A.W. Mechanisms of immune tolerance relevant to food allergy. J. Allergy Clin. Immunol. 2011, 127, 576–586. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Processed food additive microbial transglutaminase and its cross-linked gliadin complexes are potential public health concerns in celiac disease. Internat. J. Mol. Sci. 2020, 21, 1127. [Google Scholar] [CrossRef]
- Böhme, B.; Moritz, B.; Wendler, J.; Hertel, T.C.; Ihling, C.; Brandt, W.; Pietzsch, M. Enzymatic activity and thermoresistance of improved microbial transglutaminase variants. Amino Acids. 2020, 52, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Ramesh, A.; Matthias, T. The temperature and pH repertoire of the transglutaminase family is expanding. FEBS Open Bio. 2020, 10, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Jensen-Jarolim, E. The role of protein digestibility and antiacid on food allergy outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C.; Vojdani, A. The potential harmful effects of genetically engineered microorganisms (GEMs) on the intestinal microbiome and public health. Microorganisms 2024, 12, 238. [Google Scholar] [CrossRef]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Gerrard, J.; Larsen, N.; Domigan, L. The effect of baking time and temperature on gluten protein structure and celiac peptide digestibility. Food Res Int. 2021, 140, 109988. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zheng, J.; Chen, F. Effect of domestic cooking on rice protein digestibility. Food Sci. Nutr. 2019, 7, 608–616. [Google Scholar] [CrossRef]
- Lan, X.; Liu, P.; Xia, S.; Jia, C. Temperature effect on the non-volatile compounds of Maillard reaction products derived from xylose-soybean peptide system: Further insights into thermal degradation and cross-linking. Food Chem. 2010, 120, 967–972. [Google Scholar] [CrossRef]
- Cerpa, R.; Cohen, F.E.; Kuntz, I. Conformational switching in designed peptides: The helix/sheet transition. Fold. Des. 1996, 1, 91–101. [Google Scholar] [CrossRef]
- Gershteyn, I.; Ferreira, L. Immunodietica: A data-driven approach to investigate interactions between diet and autoimmune disorders. J. Transl. Autoimmun. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Mackay, I.R.; Rowley, M.J. Autoimmune epitopes: Autoepitopes. Autoimmun. Rev. 2004, 3, 487–492. [Google Scholar] [CrossRef]
- Kamath, S.D.; Bublin, M.; Kitamura, K.; Matsui, T.; Ito, K.; Lopata, A.L. Cross-reactive epitopes and their role in food allergy. J. Allergy Clin. Immunol. 2023, 151, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Paul, F.; Franciotta, D.; Waters, P.; Zipp, F.; Hohlfeld, R.; Vincent, A.; Wildemann, B. Mechanisms of disease: Aquaporin-4 antibodies in neuromyelitis optica. Nat. Clin. Pract. Neurol. 2008, 4, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Gershteyn, I.M.; Burov, A.A.; Miao, B.Y.; Morais, V.H.; Ferreira, L.M.R. Immunodietica: Interrogating the role of diet in autoimmune disease. Intern. Immunol. 2020, 32, 771–783. [Google Scholar] [CrossRef]
- Andrade, L.J.d.O.; de Oliveira, G.C.M.; de Oliveira, L.C.M.; Bittencourt, A.M.V.; Baumgarth, Y. Decoding the relationship between cow’s milk proteins and development of type 1 diabetes mellitus. Arch. Endocrinol. Metab. 2024, 68, e230248. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Mukherjee, P.S.; Berookhim, J.; Kharrazian, D. Detection of antibodies against human and plant aquaporins in patients with multiple sclerosis. Autoimmune Dis. 2015, 2015, 905208. [Google Scholar] [CrossRef]
- Brandtzaeg, P.E. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann. N. Y. Acad. Sci. 2002, 964, 13–45. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Development and basic mechanisms of human gut immune function. Nutr. Rev. 1998, 56 Pt 2, S5–S18. [Google Scholar] [CrossRef]
- Vojdani, A. Oral tolerance and its relationship to food immune reactivities. Alt. Ther. Health Med. 2015, 21, 31–45. [Google Scholar]
- Resigno, M. Dendritic cells in oral tolerance in the gut. Cell Microbiol. 2011, 13, 1312–1318. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Xiao, L.; Lu, A.; Zhang, G. Alterations of the gut microbiome in rheumatoid arthritis. Osteoarthr. Cartil. 2017, 25, S287–S288. [Google Scholar] [CrossRef]
- Macdougall, J.D.; Kim, E.H. A clinical focus on oral tolerance in the development, prevention, and management of food allergy. Cell. Immunol. 2023, 386, 104693. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lyu, J.; Wang, S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food-based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Bonds, R.S.; Midoro-Horiuti, T.; Goldblum, R. A structural basis for food allergy: The role of cross-reactivity. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 82–86. [Google Scholar] [CrossRef]
- Vojdani, A.; Gushgari, L.R.; Vojdani, E. Interaction between food antigens and the immune system: Association with autoimmune disorders. Autoimmun. Rev. 2020, 19, 102459. [Google Scholar] [CrossRef]
- Vojdani, A. Molecular mimicry as a mechanism for food immune reactivities and autoimmunity. Altern. Ther. Health Med. 2015, 21 (Suppl. S1), 34–45. [Google Scholar]
- Stefferl, A.; Schubart, A.; Storch, M.; Amini, A.; Mather, I.; Lassman, H.; Linington, C. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J. Immunol. 2000, 165, 2859–2865. [Google Scholar] [CrossRef]
- Cavallo, M.G.; Fava, D.; Monetini, L.; Barone, F.; Pozzilli, P. Cell-mediated immune response to beta casein in recent-onset insulin-dependent diabetes: Implications for disease pathogenesis. Lancet 1996, 348, 926–928. [Google Scholar] [CrossRef]
- Jarius, S.; Wildemann, B. AQP4 antibodies in neuromyelitis optica: Diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 2010, 6, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, W.; Li, X.; Jung, I.J.; Kim, H. Clinical spectrum of CNS aquaporin-4 autoimmunity. Neurology 2012, 78, 1179–1185. [Google Scholar] [CrossRef]
- Bradl, M.; Lassmann, D.H. Anti-aquaporin-4 antibodies in neuro-myelitis optica: How to prove their pathogenetic relevance? Int. MS J. 2008, 15, 75–78. [Google Scholar]
- Vaishnav, R.A.; Liu, R.; Chapman, J.; Roberts, A.M.; Ye, H.; Rebolledo-Mendez, J.D.; Tabira, T.; Fitzpatrick, A.H.; Achiron, A.; Running, M.P.; et al. Aquaporin-4 molecular mimicry and implications for neuromyelitis optica. J. Neuroimmunol. 2013, 260, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Das, K.M.; Bajpai, M. Tropomyosins in human diseases: Ulcerative colitis. Adv. Exp. Med. Biol. 2008, 644, 158–167. [Google Scholar] [CrossRef]
- Das, K.M.; das Gupta, A.; Mandal, A.; Geng, X. Autoimmunity to cytoskeletal protein tropomyosin: A clue to pathogenic mechanisms for ulcerative colitis. J. Immunol. 1993, 150, 2487–2493. [Google Scholar] [CrossRef]
- Mirza, Z.K. Autoimmunity against human tropomyosin isoforms in ulcerative colitis: Localization of specific human tropomyosin isoforms in the intestine and extraintestinal organs. Inflamm. Bowel Dis. 2006, 12, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C.; Geng, X.; Bajpai, M.; Pan, Z.; Tatar, E.; Das, K.M. Antibody to tropomyosin isoform 5 and complement induce the lysis of colonocytes in ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 2996–3003. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, F.; Ren, Y.; Wu, S.; Wu, Y.; Tang, Y. The clinical significance and biological function of tropomyosin 3 in ulcerative colitis. Tissue Cell 2025, 93, 102770. [Google Scholar] [CrossRef]
- Vojdani, A.; O’Bryan, T.; Green, J.; McCandless, J.; Woeller, K.; Vojdani, E.; Nourian, A.; Cooper, E. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr. Neurosci. 2004, 7, 151–161. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jimenez, P.; Monsalve, D.M.; Pacheco Nieva, Y.; Acosta Ampudia, Y.Y.; Ramírez Santana, H.C.; Leung, P.S.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Tarash, I. Cross-reaction between gliadin and different food and tissue antigens. Food Nutr. Sci. 2013, 4, 20–32. [Google Scholar] [CrossRef]
- Vojdani, A. Reaction of food-specific antibodies with different tissue antigens. Int. J. Food Sci. Technol. 2020, 55, 1800–1815. [Google Scholar] [CrossRef]
- Natter, S.; Granditsch, G.; Reichel, G.L.; Baghestanian, M.; Valent, P.; Elfman, L.; Grönlund, H.; Kraft, D.; Valenta, R. IgA cross-reactivity between a nuclear autoantigen and wheat proteins suggests molecular mimicry as a potential mechanism in celiac disease. Eur. J. Immunol. 2001, 31, 918–928. [Google Scholar] [CrossRef]
- Lerner, B.; BenZvi, C.; Vojdani, A. Gluten is a proinflammatory inducer of autoimmunity. J. Transl. Gastro. 2024, 2, 109–124. [Google Scholar] [CrossRef]
- Alaedini, A.; Okamoto, H.; Briani, C.; Wollenberg, K.; Shill, H.A.; Bushara, K.O.; Sander, H.W.; Green, P.H.R.; Hallett, M.; Latov, N. Immune cross-reactivity in celiac disease: Anti-gliadin antibodies bind to neuronal synapsin I. J. Immunol. 2007, 178, 5590–6595. [Google Scholar] [CrossRef] [PubMed]
- Guggenmos, J.; Schubart, A.S.; Ogg, S.; Andersson, M.; Olsson, T.; Mather, I.H.; Linington, C. Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. J. Immunol. 2004, 172, 661–668. [Google Scholar] [CrossRef]
- Riemekasten, G.; Marell, J.; Hentschel, C.; Klein, R.; Burmester, G.R.; Schoessler, W.; Hiepe, F. Casein is an essential cofactor in autoantibody reactivity directed against the C-terminal SmD1 peptide AA 83-119 in systemic lupus erythematosus. Immunobiology 2002, 206, 537–545. [Google Scholar] [CrossRef]
- Monetini, L.; Barone, F.; Stefanini, L.; Petrone, A.; Walk, T.; Jung, G.; Thorpe, R.; Pozzilli, P.; Cavallo, M. Establishment of T cell lines to bovine beta-casein and beta-casein-derived epitopes in patients with type 1 diabetes. J. Endocrinol. 2003, 176, 143–150. [Google Scholar] [CrossRef]
- Chunder, R.; Weier, A.; Mäurer, H.; Luber, N.; Enders, M.; Luber, G.; Heider, T.; Spitzer, A.; Tacke, S.; Becker-Gotot, J.; et al. Antibody cross-reactivity between casein and myelin associated glycoprotein results in central nervous system demyelination. Proc. Natl. Acad. Sci. USA 2022, 119, e2117034119. [Google Scholar] [CrossRef] [PubMed]
- Wildner, G.; Diedrichs-Möhring, M. Autoimmune uveitis induced by molecular mimicry of peptides from rotavirus, bovine casein, and retinal S-antigen. Eur. J. Immunol. 2003, 33, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Borba, V.V.; Lerner, A.; Matthias, T.; Shoenfeld, Y. Bovine milk proteins as a trigger for autoimmune disease: Myth or reality? Int. J. Celiac Dis. 2020, 8, 10–21. [Google Scholar]
- Kinoshita, M.; Nakatsuji, Y.; Kimura, T.; Moriya, M.; Takata, K.; Okuno, T.; Kumanogoh, A.; Kajiyama, K.; Yoshikawa, H.; Sakoda, S. Anti-aquaporin-4 antibody induces astrocytic cytotoxicity in the absence of CNS antigen-specific T cells. Biochem. Biophys. Res. Commun. 2010, 394, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Mejia, S.; Vojdani, A. Plant and human aquaporins: Pathogenesis from gut to brain. Immunol. Res. 2019, 67, 12–20. [Google Scholar] [CrossRef]
- Shangzu, Z.; Dingxiong, X.; ChengJun, M.; Yan, C.; Yangyang, L.; Zhiwei, L.; Ting, Z.; Zhiming, M.; Yiming, Z.; Liying, Z.; et al. Aquaporins: Important players in cardiovascular pathophysiology. Pharmacol. Res. 2022, 183, 106363. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E. Chapter 7: Molecular mechanism for food immune reaction and autoimmunity. In Food-Associated Autoimmunities; Vojdani, A., Bautista, J., Eds.; A&G Press: Los Angeles, CA, USA, 2019; pp. 207–208. [Google Scholar]
- Dai, H.; Dong, H.-L.; Gong, F.-Y.; Sun, S.-L.; Liu, X.-Y.; Li, Z.-G.; Xiong, S.-D.; Gao, X.-M. Disease association and arthritogenic potential of circulating antibodies against the α-1,4-polygalacturonic acid moiety. J. Immunol. 2014, 192, 4533–4540. [Google Scholar] [CrossRef]
- Dai, H.; Gao, X.M. Elevated levels of serum antibodies against alpha-1,6-glucan in patients with systemic lupus erythematosus or rheumatoid arthritis. Protein Cell. 2011, 2, 739–744. [Google Scholar] [CrossRef]
- Lunardi, C.; Nanni, L.; Tiso, M.; Mingari, M.C.; Bason, C.; Oliveri, M.; Keller, B.; Millo, R.; De Sandre, G.; Corrocher, R.; et al. Glycine-rich cell wall proteins act as specific antigen targets in autoimmune and food allergy disorders. Int. Immunol. 2000, 12, 647–657. [Google Scholar] [CrossRef]
- Newman, H.A.; Romeo, M.J.; Lewis, S.E.; Yan, B.C.; Orlean, P.; Levin, D.E. Gpi19 he Saccharomyces cerevisiae homologue of mammalian PIG-P, is a subunit of the initial enzyme for glycosylphosphatidylinositol anchor biosynthesis. Eukaryot. Cell 2005, 4, 1801–1807. [Google Scholar] [CrossRef]
- Vojdani, A.; Lerner, A.; Vojdani, E. Cross-reactivity and sequence homology between alpha-synuclein and food products: A step further for Parkinson’s disease synucleinopathy. Cells 2021, 10, 1111. [Google Scholar] [CrossRef]
- Vojdani, A.; Afar, D.; Vojdani, A. Reaction of lectin-specific antibody with human tissue: Possible contribution to autoimmunity. J. Immunol. Res. 2020, 2020, 1438957. [Google Scholar] [CrossRef] [PubMed]
- Kitano, N.; Taminato, T.; Ida, T.; Seno, M.; Seino, Y.; Matsukura, S.; Kuno, S.; Imura, H. Detection of antibodies against wheat germ agglutinin bound glycoproteins on the islet cell membrane. Diabet. Med. 1988, 5, 139–144. [Google Scholar] [CrossRef]
- Debbage, P.L.; Hanisch, U.-K.; Reisinger, P.W.M.; Lange, W. Visualization of lectin-like proteins in human placenta by means of anti-plant lectin antibodies. Anat. Embryol. 1993, 187, 465–473. [Google Scholar] [CrossRef]
- Jankovic, M. Identification of human placental wheat germ agglutinin immunoreactive protein by mass spectrometry. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 369–374. [Google Scholar] [CrossRef]
- Lerner, A.; BenZvi, C.; Vojdani, A. Cross-reactivity and sequence similarity between microbial transglutaminase and human tissue antigens. Sci. Rep. 2023, 13, 17526. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Turnpaugh, C.C. Antibodies against Group A Streptococcus, dopamine receptors, and ganglioside GM1 cross-react with a variety of food antigens, potentially interfering with biomarkers for PANS and PANDAS. Biomark. Neuropsychiatry 2020, 3, 100023. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E. Immunoreactivity of anti-amyloid beta-peptide-42 specific antibody with toxic chemicals and food antigens. J. Alz. Dis. Park. 2018, 8, 441. [Google Scholar] [CrossRef]
- Kharrazian, D.; Herbert, M.; Vojdani, A. Immunological reactivity using monoclonal and polyclonal antibodies of autoimmune thyroid target sites with dietary proteins. J. Thyroid Res. 2017, 2017, 4354723. [Google Scholar] [CrossRef]
- Kharrazian, D.; Herbert, M.; Vojdani, A. Detection of islet cell immune reactivity with low glycemic index foods: Is this a concern for type 1 diabetes? J. Diabet. Res. 2017, 2017, 4124967. [Google Scholar] [CrossRef]
- Cuan-Baltazar, Y.; Soto-Vega, E. Microorganisms associated to thyroid autoimmunity. Autoimmun. Rev. 2020, 19, 102614. [Google Scholar] [CrossRef]
- Bullard-Dillard, R.; Chen, J.; Pelsue, S.; Dao, V.; Agris, P.F. Anti-Sm autoantibodies of systemic lupus erythematosus cross-react with dietary plant proteins. Immunol. Investig. 1992, 21, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Parks, R.; Bowman, L.; Guenther, R.H.; Kovacs, S.A.; Pelsue, S. Plant DNA topoisomerase I is recognized and inhibited by human Scl-70 sera autoantibodies. Exp. Cell Res. 1990, 189, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Petersen, F. A methodological review of induced animal models of autoimmune diseases. Autoimmun. Rev. 2018, 17, 473–479. [Google Scholar] [CrossRef]
- Kabat, E.A.; Wolf, A.; Bezer, A.E. The rapid production of acute disseminated encephalomyelitis in Rhesus monkeys by injection of heterologous and homologous brain tissue with adjuvant. J. Exp. Med. 1947, 85, 117–130. [Google Scholar] [CrossRef]
- Morel, L. Animal models of autoimmunity: A relentless pursuit of accurate pre-clinical models. Autoimmunity 2025, 58, 2461072. [Google Scholar] [CrossRef]
- Sjölander, A.; Magnusson, K.E.; Latkovic, S. The effect of concanavalin-A and wheat germ agglutinin on the ultrastructure and permeability of rat intestine. A possible model for an intestinal allergic reaction. Int. Arch. Allergy 1984, 75, 230–236. [Google Scholar] [CrossRef]
- Wilson, A.B.; King, T.P.; Clarke, E.M.W.; Pusztai, A. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in rat small intestine: 2 Microbiological studies. J. Comp. Pathol. 1980, 90, 597–602. [Google Scholar] [CrossRef]
- Vojdani, A. Lectins, agglutinins, and their roles in autoimmune reactivities. Altern. Ther. Health Med. 2015, 21 (Suppl. S1), 46–51. [Google Scholar] [PubMed]
- Brauer, R.; Thoss, K.; Henzgen, S.; Waldman, G. Lectin-induced arthritis of rabbit as a model of rheumatoid arthritis. In Proceedings of the Sixth International Lectin Meeting, Poznan, Poland, 2–6 September 1984; Bog-Hansen, T.C., Driessche, E., Eds.; Walter de Gruyter & Company: Berlin, Germany, 1985; Volume 4, pp. 29–38. [Google Scholar]
- Parkkinen, J. Aberrant lectin-binding activity of immunoglobulin G in serum from rheumatoid arthritis patients. Clin. Chem. 1989, 35, 1638–1643. [Google Scholar] [CrossRef]
- Howson, P.; Shepard, N.; Mitchell, N. The antigen-induced arthritis model: The relevance of the method of induction to its use as a model of human disease. J. Rheumatol. 1986, 13, 379–390. [Google Scholar]
- Costes, L.M.M.; Meresse, B.; Cerf-Bensussan, N. The role of animal models in unraveling therapeutic targets in coeliac disease. Best Prac. Res. Clin. Gastroenterol. 2015, 29, 437–450. [Google Scholar] [CrossRef]
- Abadie, V.; Khosla, C.; Jadri, B. A mouse model of celiac disease. Curr. Protoc. 2022, 2, e515. [Google Scholar] [CrossRef]
- Serizawa, K.; Miyake, S.; Katsura, Y.; Yorozu, K.; Kurasawa, M.; Tomizawa-Shinohara, H.; Yasuno, H.; Matsumoto, Y. Intradermal AQP4 peptide immunization induces clinical features of neuromyelitis optica spectrum disorder in mice. J. Neuroimmunol. 2023, 380, 578109. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.K. Food allergies around the world. Front. Nutr. 2024, 11, 1373110. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E. Food allergy: Symptoms and diagnosis. J. Food Allergy 2022, 4, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Benson, T.E.; Arkins, J.A. Cytotoxic testing for food allergy: Evaluation of reproducibility and correlation. J. Allergy Clin. Immunol. 1976, 58, 471–476. [Google Scholar] [CrossRef]
- American Academy of Allergy. Position statements—Controversial techniques. J. Allergy Clin. Immunol. 1981, 67, 333–338. [Google Scholar] [CrossRef]
- Potter, P.C.; Mullineux, J.; Weinberg, E.G.; Haus, M.; Ireland, P.; Buys, C.; Motala, C. The ALCAT test: Inappropriate in testing for food allergy in clinical practice. S. Afr. Med. J. Apr. 1992, 81, 384. [Google Scholar]
- Mullin, G.E.; Swift, K.M.; Lipski, L.; Turnbull, L.K.; Rampertab, S.D. Testing for food reactions: The good, the bad, and the ugly. Nutr. Clin. Pract. 2010, 25, 192–198. [Google Scholar] [CrossRef]
- Hodsdon, W.; Zwickey, H. Reproducibility and reliability of two food allergy testing methods. Nat. Med. J. 2010, 2, 8–13. [Google Scholar]
- Vojdani, A. The evolution of food immune reactivity testing: Why immunoglobulin G or immunoglobulin A antibody for food may not be reproducible from one lab to another. Altern. Ther. Health Med. 2015, 21 (Suppl. S1), 8–22. [Google Scholar] [PubMed]
- U.S. Food & Drug Administration. All Allergenic Extracts for Diagnosis of Food Allergy: FDA Safety Communication—FDA Requires Warning about Anaphylaxis Following False Negative Food Allergen Skin Test Results in the Prescribing Information. Available online: https://www.fda.gov/safety/medical-product-safety-information/all-allergenic-extracts-diagnosis-food-allergy-fda-safety-communication-fda-requires-warning-about-anaphylaxis-following-false-negative-food-allergen-skin-test-results (accessed on 8 April 2025).
- Vojdani, A.; Kharrazian, D.; Mukherjee, P.S. The prevalence of antibodies against wheat and milk proteins in blood donors and their contribution to neuroimmune reactivities. Nutrients 2013, 6, 15–36. [Google Scholar] [CrossRef]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Advanced glycation and lipoxidation end products—Amplifiers of inflammation: The role of food. JPEN 2007, 31, 430–440. [Google Scholar] [CrossRef]
- Virella, G.; Lopes-Virella, M.F. Lipoprotein autoantibodies: Measurement and significance. Clin. Dev. Immunol. 2003, 10, 499–505. [Google Scholar] [CrossRef]
- Turk, Z.; Ljubic, S.; Turk, N.; Benko, B. Detection of autoantibodies against glycated and oxidized food proteins in diabetic patients. Clin. Chim. Acta 2001, 303, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Darkwan, E.R. Advanced glycation end products in metabolic and neurodegenerative disorders. Microorganisms 2022, 10, e1848. [Google Scholar] [CrossRef]
- Mahayana, N.P.K.; Yadmika, N.P.W.P.; Aryaweda, M.D.W.; Mahardana, M.D.P.; Mamangdean, C.T.; Dewi, N.N.A.; Wirawan, C.; Laksmidewi, A.A.A.P. Decoying the enemy: Soluble receptor for advanced glycation end products and cognitive impairment in neurodegenerative diseases—A systemic review and meta-analysis. Egypt J. Neurol. Psychiatry Neurosurg. 2024, 60, 93. [Google Scholar] [CrossRef]
- de Almeida, J.K.A.; Brech, G.C.; Luna, N.M.S.; Iborra, R.T.; Soares-Junior, J.M.; Baracat, E.C.; Greve, J.M.D.; Alonso, A.C.; Machado-Lima, A. Advanced glycation end products consumption and the decline of functional capacity in patients with Parkinson’s disease: Cross-sectional study. Clinics 2024, 79, 100320. [Google Scholar] [CrossRef]
- D’cunha, N.M.; Sergi, D.; Lane, M.M.; Naumovski, N.; Gamage, E.; Rajendran, A.; Kouvari, M.; Gauci, S.; Dissanayka, T.; Marx, W.; et al. The effects of dietary advanced glycation end products on neurocognitive and mental disorders. Nutrients 2022, 14, 2421. [Google Scholar] [CrossRef]
- Kothandan, D.; Singh, D.S.; Yerrakula, G.; Backkiyashree, D.; Pratibha, N.; Ramya, A.; Vg, S.R.; Keshavini, S.; Jagadheeshwari, M.; Dhivya Sr, K.; et al. Advanced glycation end products-induced Alzheimer’s disease and its novel therapeutic approaches: A comprehensive review. Cureus 2024, 16, e61373. [Google Scholar] [CrossRef] [PubMed]
- Polykretis, P. Advanced glycation end products as potential triggering factors in self-reactivity against myelin antigens in multiple sclerosis. Med. Hypotheses 2021, 157, 110702. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Lu, Q.; Humble, M.C. Heritability versus the role of the environment in autoimmunity. J. Autoimmun. 2012, 39, 249–252. [Google Scholar] [CrossRef] [PubMed]
 , cooked
, cooked  , and tandoori chicken
, and tandoori chicken  at a mean cutoff of 0.9 ELISA units. n = 40.
at a mean cutoff of 0.9 ELISA units. n = 40.
 , cooked
, cooked  , and tandoori chicken
, and tandoori chicken  at a mean cutoff of 0.9 ELISA units. n = 40.
at a mean cutoff of 0.9 ELISA units. n = 40.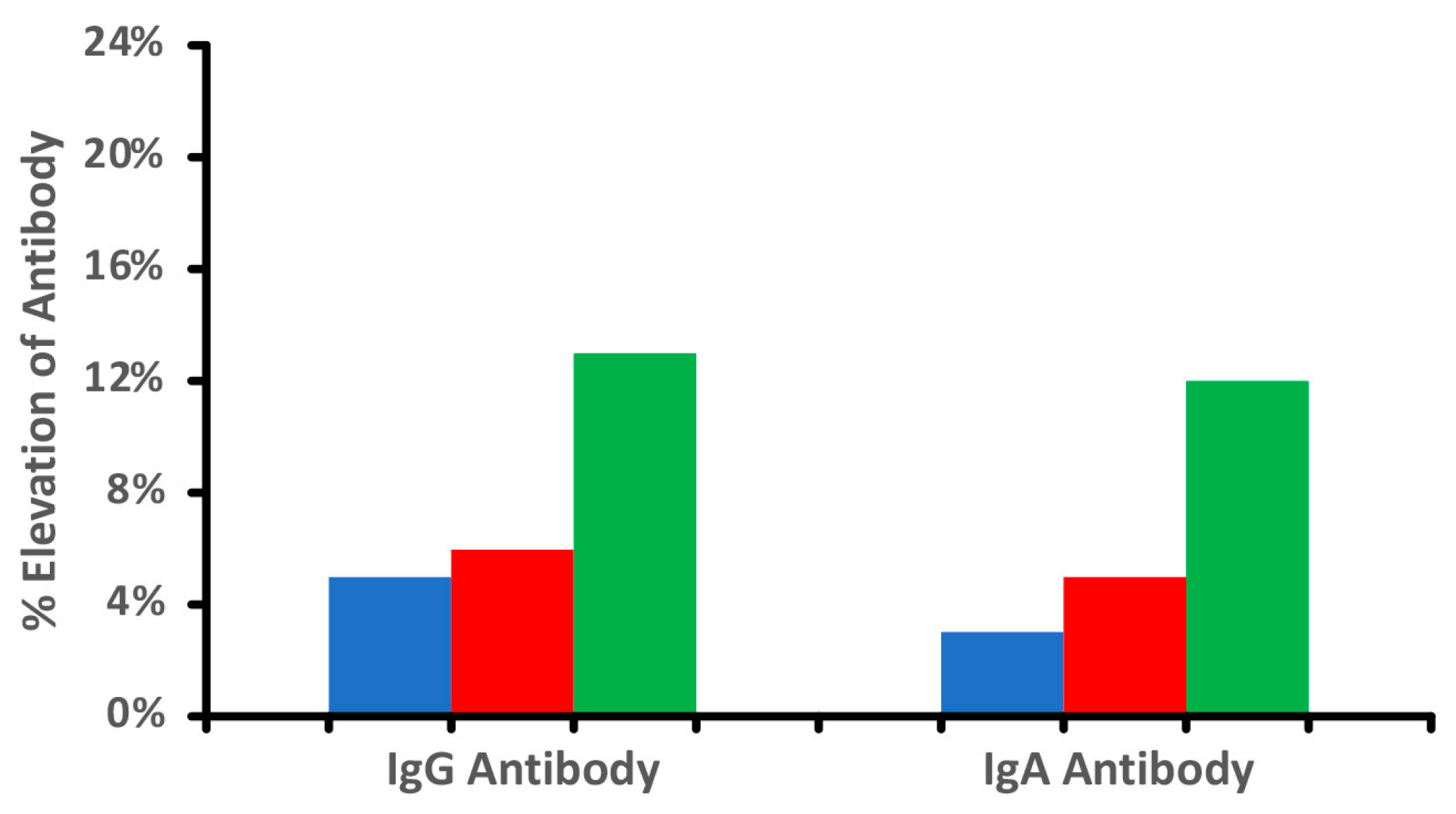
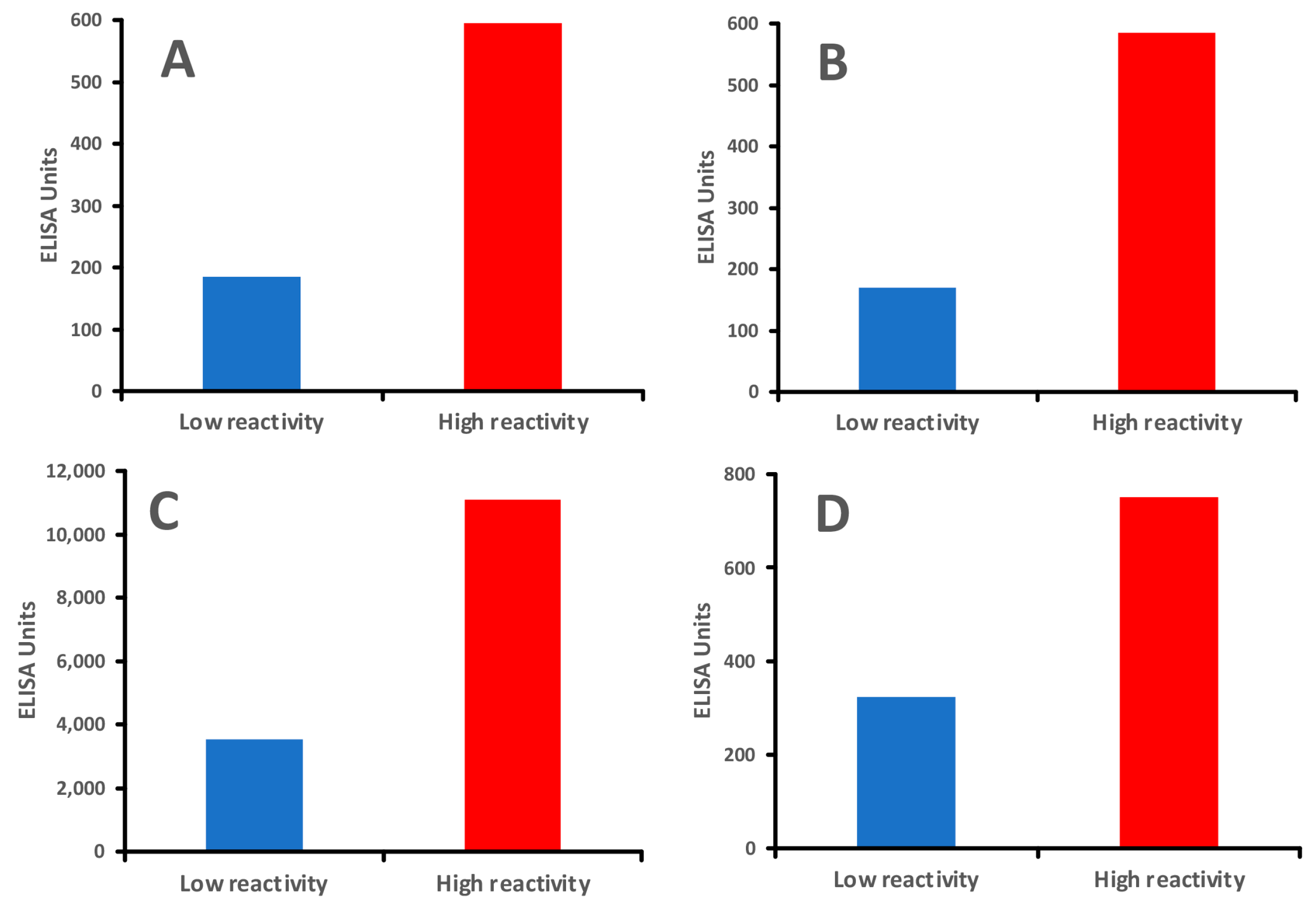
 vs. cooked
vs. cooked  foods. Patient 1 shows no significant reaction to either raw or cooked egg. Patient 2 shows a significant reaction to raw egg but not cooked egg, while Patient 3 shows a significant reaction to cooked egg but not raw egg.
foods. Patient 1 shows no significant reaction to either raw or cooked egg. Patient 2 shows a significant reaction to raw egg but not cooked egg, while Patient 3 shows a significant reaction to cooked egg but not raw egg.
 vs. cooked
vs. cooked  foods. Patient 1 shows no significant reaction to either raw or cooked egg. Patient 2 shows a significant reaction to raw egg but not cooked egg, while Patient 3 shows a significant reaction to cooked egg but not raw egg.
foods. Patient 1 shows no significant reaction to either raw or cooked egg. Patient 2 shows a significant reaction to raw egg but not cooked egg, while Patient 3 shows a significant reaction to cooked egg but not raw egg.


| Food Proteins/Peptides | Cross-Reactive Tissue | Reference(s) |
|---|---|---|
| Wheat/gliadin Wheat germ agglutinin | Glutamic acid decarboxylase; hepatocytes; cerebellum; myelin basic protein; synapsin; asialoganglioside; amyloid beta; myelin-associated glycoprotein; microtubule-associated protein; thyroid peroxidase; alpha-enolase; tyrosinase; somatotropin; elastin; alpha-myosin | Vojdani et al. [91,93,94] Lerner and BenZvi [56,96] Natter et al. [95] Lerner et al. [96] Alaedini et al. [97] |
| Milk/casein/butyrophilin | Myelin oligodendrocyte glycoprotein; myelin-associated glycoprotein; islet cell antigen; zinc transporter 8; glutamic acid decarboxylase 65; retinal S antigen | Stefferl et al. [80] de Oliveira Andrade et al. [66] Guggenmos et al. [98] Riemekasten et al. [99] Monetini et al. [100] Chunder et al. [101] Wildner and Diedrichs- Möhring [102] Vieira Borba et al. [103] |
| Soy, corn, spinach, and tomato aquaporin 4, and serpin from legumes | Human aquaporin 4 found in the blood–brain barrier, astrocytes, cardiac myocytes, kidneys, lungs, stomach, skeletal muscle, salivary glands, and sensory organs | Vaishnav et al. [85] Kinoshita et al. [104] Lambert et al. [105] Shangzu et al. [106] |
| Corn and legume serpin | Aquaporin 4 | Vaishnav et al. [85] |
| Shrimp and shrimp tropomyosin | Human tropomyosin | Das et al. [86,87] Vojdani and Vojdani [107] |
| Food pectins found in apple, orange, quince, berries, and grapefruit | Joints | Dai et al. [108,109] |
| Glycine-rich food proteins found in gelatin, meat, soy, chicken, eggs, cereals, beans, rice, and some fruits and vegetables | Procollagen; collagen; epidermal keratin | Lunardi et al. [110] |
| Saccharomyces cerevisiae | Complex glycolipids; alpha-synuclein | Newman et al. [111] Vojdani et al. [112] |
| Peanut proteins and lectins | Alpha-synuclein | Vojdani et al. [112] |
| Potato proteins | Alpha-synuclein | Vojdani et al. [112] |
| Food Protein/Peptide Antibodies | Reactive Human Tissue | Reference(s) |
|---|---|---|
| Wheat/Gliadin | Synapsin I; cerebellar; hepatocytes; cytochrome P450; GAD-65; asialoganglioside; myelin basic protein; dopamine receptor; beta nerve growth factor; intrinsic factor; tyrosinase; thyroid peroxidase; alpha-enolase; e-cadherin; transglutaminase 2; elastin; alpha-myosin | Vojdani et al. [91,93,94] Alaedini et al. [97] |
| Wheat germ agglutinin | Myelin basic protein; zonulin; neurotropin; presenilin; tau protein; thyroid peroxidase; alpha-enolase; calmodulin; ovaries; insulin; islet cell antigen; alpha-myosin; acetylcholine receptor; dopamine receptor; neutrophil cytoplasmic antigen; placenta | Vojdani et al. [113] Kitano et al. [114] Debbage et al. [115] Jankovic et al. [116] |
| Bean and phytohemagglutinin | Intrinsic factor; calprotectin; hepatocytes; a+b tubulin; neurotrophin; tyrosinase; thyroid peroxidase; calmodulin; fibulin; platelet glycoprotein; alpha-myosin; dopamine receptor | Vojdani et al. [113] |
| Soy and soy bean agglutinin | Tyrosinase; thyroid peroxidase; alpha-myosin; alpha-enolase; intrinsic factor; asialoganglioside; aquaporin 4 | Vojdani et al. [107,112,113] |
| Peanut and peanut agglutinin | Enteric nerve; cerebellum; tropomyosin; presenilin; ovaries; e-cadherin; insulin; islet cell antigen; dopamine receptor; amyloid beta peptide; glutamate receptor | Vojdani et al. [94,107,113] |
| Microbial transglutaminase | Mitochondrial M2 protein; extractable nuclear antigen; nuclear antigen; somatostatin; S-100B; alpha-myosin; transglutaminase 2; somatotropin; thyroid peroxidase | Lerner et al. [117] |
| Milk | Neutrophil cytoplasmic antigen; hepatocytes; thyroid peroxidase; alpha-enolase; somatotropin, myelin-associated glycoprotein | Vojdani [94] Chunder et al. [101] |
| Corn | Neurotrophin; intrinsic factor; alpha-myosin; thyroid peroxidase; tyrosinase; neutrophil cytoplasmic antigen; DPP-IV; somatotropin; aquaporin 4; myelin basic protein | Vojdani [94] |
| Egg | Zonulin; calprotectin; DPP-IV; alpha-enolase; lysozyme; myelin basic protein; asialoganglioside; aquaporin 4; platelet glycoprotein; fibulin; ovaries; insulin; islet cell antigen; alpha-myosin; dopamine receptor; acetylcholine receptor; amyloid beta peptide; brain-derived neurotrophic factor | Vojdani [94] |
| Tissue Antibodies | Reactive Food | Reference(s) |
|---|---|---|
| Alpha synuclein | Casein; casomorphin; yeast; sorghum; onion; rice protein; bean agglutinin; pea lectin; lentil lectin; wheat germ agglutinin; soybean agglutinin; soy sauce; soy protein; tofu; potato; peanut; peanut butter; peanut agglutinin; macadamia nut; cashew; walnut; bell pepper; broccoli; lettuce; spinach; radish; celery; olive; latex hevein; grapefruit; bromelain; watermelon; shrimp; shrimp tropomyosin | Vojdani et al. [112,113] |
| Dopamine-1 receptor | Mint; eggplant; thyme; basil; cilantro; fish; wine; cherry; latex hevein; seaweed; cabbage; bell pepper; walnut; sesame albumin; chia seed; cashew vicilin; soybean oleosin; lentil lectin; rice protein | Vojdani and Turnpaugh [118] |
| Dopamine-2 receptor | Milk; cheese; wheat; peanut; peanut butter; walnut; seaweed; cherry; tuna; basil; mint; thyme | Vojdani and Turnpaugh [118] |
| Asialoganglioside GM1 | Soy sauce; almond; chia seed; mustard seed; sunflower seed; asparagus; bell pepper; celery; chili pepper; eggplant; radish; spinach; cherry; date; wine; kiwi; orange; peach; basil; cilantro; parsley; thyme; cinnamon; paprika | Vojdani and Turnpaugh [118] |
| Amyloid beta42 peptide | Egg yolk; pea protein; pea lectin; raw and canned tuna; hazelnut; squid; cow’s milk; a+b casein; wheat proteins; alpha gliadin; wheat-alpha amylase; wheat globulin; CXCR3-binding gliadin | Vojdani and Vojdani [119] |
| Triiodothyronine (T3) | Roasted almond; amaranth; buckwheat; cow’s milk; milk chocolate; coffee; hemp; kamut; latex hevein; mustard seed; oat; egg yolk | Vojdani and Vojdani [119] Kharrazian et al. [120] |
| Thyroxine (T4) | Roasted almond; almond; Brussel sprout; Brazil nut; roasted cashew; cashew vicilin; macadamia nut; cooked clam; gelatin; mustard seed; oat; egg yolk | Vojdani and Vojdani [119] Kharrazian et al. [120] |
| Thyroid peroxidase | Pea lectin; lentil lectin; mushroom; okra; seaweed; apricot; avocado (raw and cooked); banana; lemon and lime; orange juice; cod; wheat germ agglutinin; cooked chicken; buckwheat; amaranth; quinoa; soy; potato | Vojdani and Vojdani [119] Kharrazian et al. [120] |
| Glutamic acid decarboxylase (GAD-65) | Buckwheat; amaranth; rice; corn; yeast; potato; quinoa; oat | Vojdani and Vojdani [119] Kharrazian et al. [120] |
| Zinc transporter-8 (ZnT8) | Seaweed; cooked lentil; pea protein; cooked pea; wheat; soybean oleosin; roasted peanut; tilapia; guar gum | Vojdani and Vojdani [119] Kharrazian et al. [120] |
| Islet cell antigen 2 (IA2) | Seaweed; pea lectin; bell pepper; zucchini; spinach; apricot; rice; garlic; guar gum | Vojdani and Vojdani [119] Kharrazian et al. [120] |
| Insulin receptor alpha | Milk butyrophilin; potato; amaranth; quinoa; tapioca | Vojdani and Vojdani [119] Kharrazian et al. [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojdani, A.; Vojdani, E.; Benzvi, C.; Lerner, A. Immune Reactivity to Raw and Processed Foods and Their Possible Contributions to Autoimmunity. Foods 2025, 14, 1357. https://doi.org/10.3390/foods14081357
Vojdani A, Vojdani E, Benzvi C, Lerner A. Immune Reactivity to Raw and Processed Foods and Their Possible Contributions to Autoimmunity. Foods. 2025; 14(8):1357. https://doi.org/10.3390/foods14081357
Chicago/Turabian StyleVojdani, Aristo, Elroy Vojdani, Carina Benzvi, and Aaron Lerner. 2025. "Immune Reactivity to Raw and Processed Foods and Their Possible Contributions to Autoimmunity" Foods 14, no. 8: 1357. https://doi.org/10.3390/foods14081357
APA StyleVojdani, A., Vojdani, E., Benzvi, C., & Lerner, A. (2025). Immune Reactivity to Raw and Processed Foods and Their Possible Contributions to Autoimmunity. Foods, 14(8), 1357. https://doi.org/10.3390/foods14081357









