The Phytochemical and Functional Characterization of the Aerial Parts of Artemisa alba Turra (Asteraceae) Grown in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Collection and Extraction Protocol
2.3. Phytochemical Analysis
2.4. In Vitro Antioxidant Activity Analysis
2.5. Antiproliferative Activity
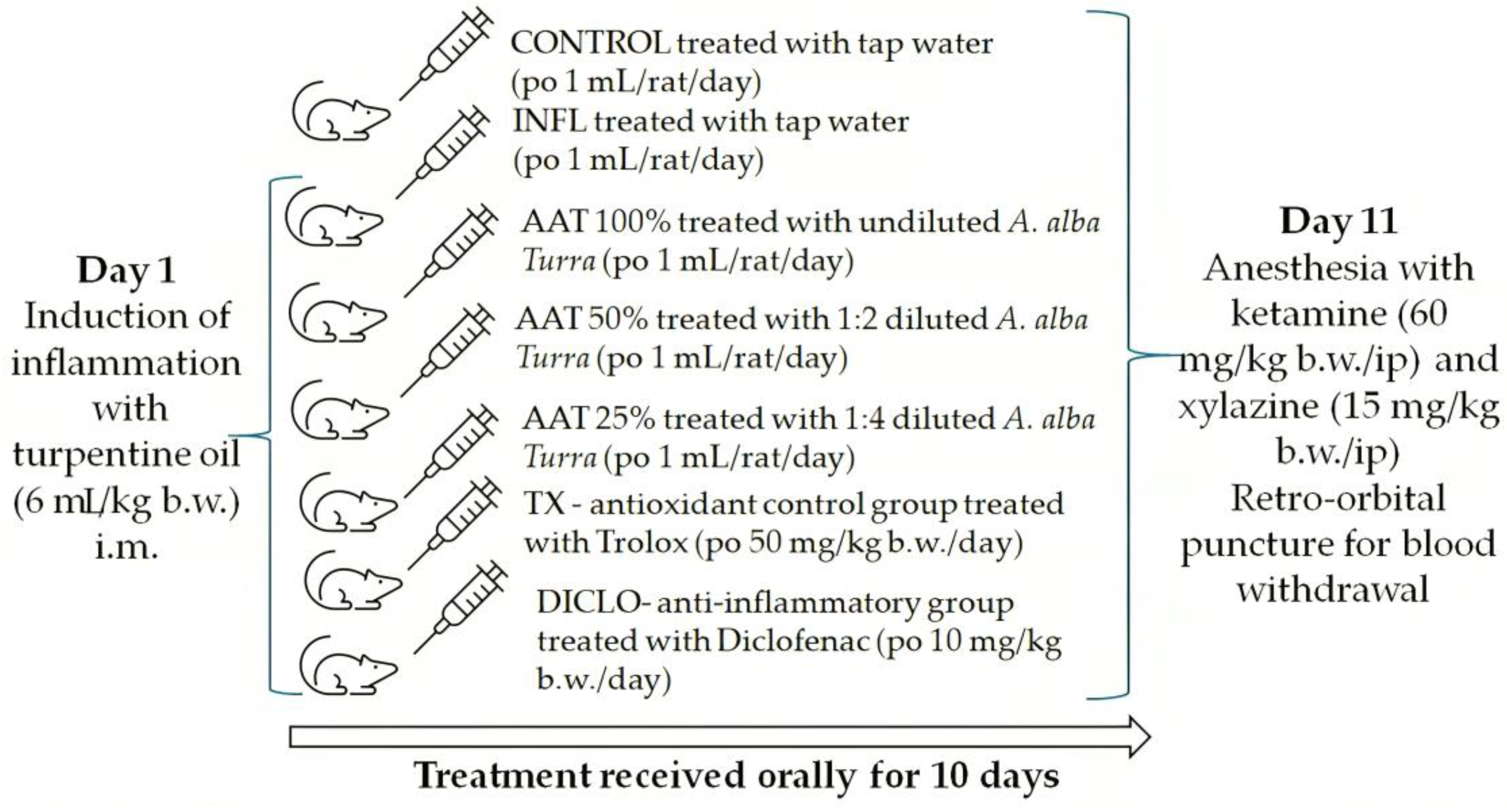
2.6. In Vivo Experimental Design
2.6.1. Animal Subjects
2.6.2. Experimental Protocol
2.6.3. Oxidative Stress Analysis
2.6.4. Inflammatory Markers
2.6.5. Toxicity Assessment
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. In Vitro Antioxidant Activity
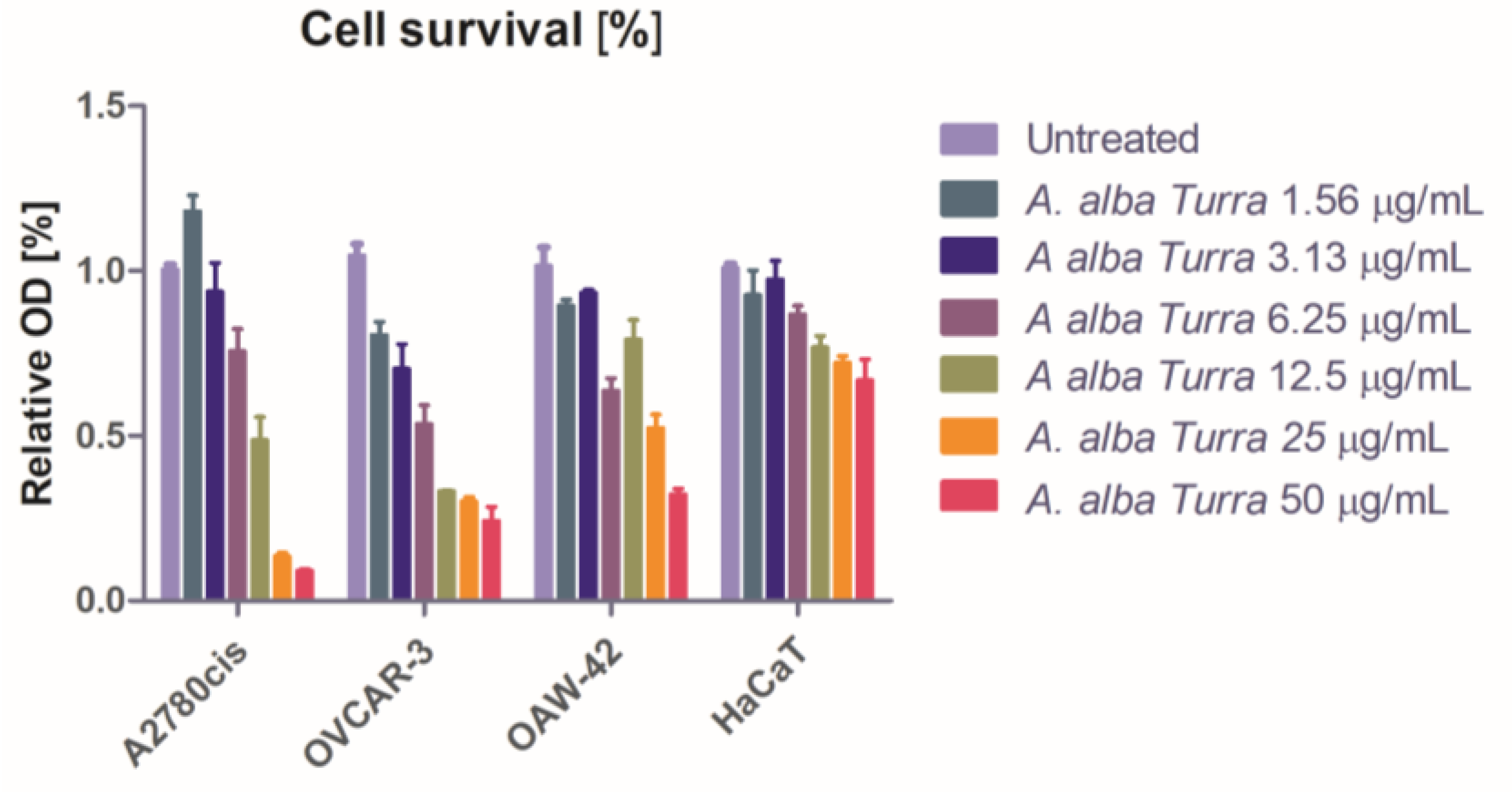
3.3. Antiproliferative Activity
3.4. In Vivo Antioxidant Activity
3.5. In Vivo Anti-Inflammatory Activity
3.6. Liver and Renal Toxicity Assessment
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, Y.; Wang, J.; Yu, C.; Shen, W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front. Immunol. 2021, 11, 609705. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Angiogenesis: Update 2005. J. Thromb. Haemost. 2005, 3, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Chera, E.I.; Pop, R.M.; Pârvu, M.; Sorițău, O.; Uifălean, A.; Cătoi, F.A.; Cecan, A.; Negoescu, A.G.; Achimaș-Cadariu, P.; Pârvu, A.E. Flaxseed Ethanol Extracts’ Antitumor, Antioxidant, and Anti-Inflammatory Potential. Antioxidants 2022, 11, 892. [Google Scholar] [CrossRef]
- Ben Abid, Z.; Feki, M.; Hédhili, A.; Hamdaoui, M.H. Artemisia Herba-Alba Asso (Asteraceae) Has Equivalent Effects to Green and Black Tea Decoctions on Antioxidant Processes and Some Metabolic Parameters in Rats. Ann. Nutr. Metab. 2007, 51, 216–222. [Google Scholar] [CrossRef]
- Jiménez, M.C.; Prieto, K.; Lasso, P.; Gutiérrez, M.; Rodriguez-Pardo, V.; Fiorentino, S.; Barreto, A. Plant Extract from Caesalpinia Spinosa Inhibits Cancer-Associated Fibroblast-like Cells Generation and Function in a Tumor Microenvironment Model. Heliyon 2023, 9, e14148. [Google Scholar] [CrossRef]
- Bou Malhab, L.J.; Harb, A.A.; Eldohaji, L.; Taneera, J.; Al-Hroub, H.M.; Abuhelwa, A.; Alzoubi, K.H.; Abu-Irmaileh, B.; Hudaib, M.; Almaliti, J.; et al. Exploring the Anticancer Effect of Artemisia Herba-Alba on Colorectal Cancer: Insights from Eight Colorectal Cancer Cell Lines. Food Sci. Nutr. 2025, 13, e4715. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Huang, L. Anticancer Activities of Phytoconstituents and Their Liposomal Targeting Strategies against Tumor Cells and the Microenvironment. Adv. Drug Deliv. Rev. 2020, 154–155, 245–273. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Abd El-Razek, M.H.; Saleh, I.A.; Ali, S.K.; Abd El Aty, A.A.; Paré, P.W.; Hegazy, M.E.F. Artemisia Herba-Alba Sesquiterpenes: In Silico Inhibition in the ATP-Binding Pocket. RSC Adv. 2023, 13, 19530–19539. [Google Scholar] [CrossRef]
- Bekka-Hadji, F.; Bombarda, I.; Djoudi, F.; Bakour, S.; Touati, A. Chemical Composition and Synergistic Potential of Mentha pulegium L. and Artemisia herba alba Asso. Essential Oils and Antibiotic against Multi-Drug Resistant Bacteria. Molecules 2022, 27, 1095. [Google Scholar] [CrossRef] [PubMed]
- Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia Herba-Alba and Origanum Majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Baldan, V.; Sut, S.; Faggian, M.; Roccabruna, L.; Zanini, D.; Manzini, P.; Maggi, F.; Dall’Acqua, S. Phytochemical Investigations on Artemisia Alba Turra Growing in the North-East of Italy. Nat. Prod. Res. 2017, 31, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Biswas, P.; Bondhon, T.A.; Jannat, K.; Paul, T.K.; Paul, A.K.; Jahan, R.; Nissapatorn, V.; Mahboob, T.; Wilairatana, P.; et al. Can Artemisia Herba-Alba Be Useful for Managing COVID-19 and Comorbidities? Molecules 2022, 27, 492. [Google Scholar] [CrossRef]
- Eltaysh, R.; Rizk, M.; Sayed, S.; Abouelnasr, K.; Abdallah, A.; Igarashi, I. Evaluation of the in Vitro and in Vivo Inhibitory Effects of Artemisia Herba-Alba against the Growth of Piroplasm Parasites. J. Adv. Vet. Anim. Res. 2022, 9, 267. [Google Scholar] [CrossRef]
- Pecheva, D.; Danova, K. Light and Auxin Treatments Affect Morphogenesis and Polyphenolics Productivity in Artemisia Alba Turra Cell Aggregates in Vitro. BioRisk 2022, 17, 213–225. [Google Scholar] [CrossRef]
- Țicolea, M.; Pop, R.M.; Pârvu, M.; Usatiuc, L.-O.; Uifălean, A.; Ranga, F.; Pârvu, A.E. Phytochemical Composition Antioxidant and Anti-Inflammatory Activity of Artemisia Dracunculus and Artemisia Abrotanum. Antioxidants 2024, 13, 1016. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes Versicolor: Medicinal Mushroom with Important Health Benefits. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Erhan, S.E.; Pârvu, A.E.; Ciorîță, A.; Putri, A.A.; Molina, A.J.V.; Pârvu, M.; Moț, A.C. Chemical Composition and Anti-Inflammatory Effect of Phellodendron Amurense Rupr. Stem Bark Extract. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13306. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Miklášová, N.; Fischer-Fodor, E.; Lönnecke, P.; Schrepler, M.P.; Virag, P.; Tatomir, C.; Cernea, V.I.; Hey-Hawkins, E.; Silaghi-Dumitrescu, L. Antiproliferative Effect and Genotoxicity of Novel Synthesized Palladium Complexes with Organoarsenic Ligands. J. Inorg. Biochem. 2009, 103, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Trouillas, P.; Gašparović, A.Č.; Gazzano, E.; Assaraf, Y.G.; Riganti, C. Phospholipids and Cholesterol: Inducers of Cancer Multidrug Resistance and Therapeutic Targets. Drug Resist. Updates 2020, 49, 100670. [Google Scholar] [CrossRef] [PubMed]
- Tudor, D.V.; Bâldea, I.; Olteanu, D.E.; Fischer-Fodor, E.; Piroska, V.; Lupu, M.; Călinici, T.; Decea, R.M.; Filip, G.A. Celecoxib as a Valuable Adjuvant in Cutaneous Melanoma Treated with Trametinib. Int. J. Mol. Sci. 2021, 22, 4387. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Method to Measure Total Antioxidant Response against Potent Free Radical Reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A Novel and Automated Assay for Thiol/Disulphide Homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Nandakumar, A.; Nataraj, P.; James, A.; Krishnan, R.; Mahesh, K.M. Estimation of Salivary 8-Hydroxydeoxyguanosine (8-OHdG) as a Potential Biomarker in Assessing Progression towards Malignancy: A Case-Control Studyoxidative. Asian Pac. J. Cancer Prev. 2020, 21, 2325–2329. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Mitev, D.; Gradeva, H.; Stoyanova, Z.; Petrova, N.; Karova, N.; Dimov, D.; Iliev, V.; Koychev, A.; Prakova, G.; Vlaykova, T. Evaluation of thiol compounds and lipid peroxidative products in plasma of patients with COPD. Trakia J. Sci. 2010, 8, 306–314. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Ahsan, H. 3-Nitrotyrosine: A Biomarker of Nitrogen Free Radical Species Modified Proteins in Systemic Autoimmunogenic Conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Benchohra, M.; Ahmed, A.; Merah, O. Relationship between Variations in Ecological Conditions and the Dynamics of Intra-Specific Morphological Diversity of Artemisia Herba-Alba Asso in Algeria. Ekol. Bratisl. 2023, 42, 209–217. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hedayati, M.; Biabani, H. Protein Precipitation Methods Evaluated for Determination of Serum Nitric Oxide End Products by the Griess Assay. J. Med. Sci. Res. 2007, 15, 29–32. [Google Scholar]
- Abdel-Ghany, H.S.M.; Abdel-Shafy, S.; Abuowarda, M.; El-Khateeb, R.M.; Hoballah, E.M.; Fahmy, M.M. Acaricidal Activity of Artemisia Herba-Alba and Melia Azedarach Oil Nanoemulsion against Hyalomma Dromedarii and Their Toxicity on Swiss Albino Mice. Exp. Appl. Acarol. 2021, 84, 241–262. [Google Scholar] [CrossRef]
- Sekiou, O.; Boumendjel, M.; Taibi, F.; Tichati, L.; Boumendjel, A.; Messarah, M. Nephroprotective Effect of Artemisia Herba Alba Aqueous Extract in Alloxan-Induced Diabetic Rats. J. Tradit. Complement. Med. 2021, 11, 53–61. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Genova, V.; Peter, S.; Wolfram, E.; Danova, K.; Evstatieva, L. Phenolic Profile of Artemisia Alba Turra. Chem. Biodivers. 2018, 15, e1800109. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Anand, U.; Altemimi, A.B.; Tripathi, V.; Guo, Y.; Pratap-Singh, A. Phenolic Composition, Antioxidant Capacity and Antibacterial Activity of White Wormwood (Artemisia Herba-Alba). Plants 2021, 10, 164. [Google Scholar] [CrossRef]
- Teterovska, R.; Sile, I.; Paulausks, A.; Kovalcuka, L.; Koka, R.; Maurina, B.; Bandere, D. The Antioxidant Activity of Wild-Growing Plants Containing Phenolic Compounds in Latvia. Plants 2023, 12, 4108. [Google Scholar] [CrossRef]
- Dordević, S.; Stanisavljević, D.; Ristić, M.; Milenković, M.; Veličković, D.; Stojičević, S.; Zlatković, B. Chemical, Antioxidant and Antimicrobial Analysis of the Essential Oil and Extract of Artemisia Alba Tura. Dig. J. Nanomater. Biostruct. 2013, 8, 1377–1388. [Google Scholar]
- Maggioa, A.; Rossellia, S.; Brancazioa, C.L.; Spadaro, V.; Raimondoa, F.M.; Bruno, M. Metabolites from the Aerial Parts of the Sicilian Population of Artemisia Alba. Nat. Prod. Commun. 2013, 8, 283–286. [Google Scholar] [CrossRef]
- Sohail, J.; Zubair, M.; Hussain, K.; Faisal, M.; Ismail, M.; Haider, I.; Mumtaz, R.; Khan, A.A.; Khan, M.A. Pharmacological Activities of Artemisia Absinthium and Control of Hepatic Cancer by Expression Regulation of TGFβ1 and MYC Genes. PLoS ONE 2023, 18, e0284244. [Google Scholar] [CrossRef] [PubMed]
- Réggami, Y.; Benkhaled, A.; Boudjelal, A.; Berredjem, H.; Amamra, A.; Benyettou, H.; Larabi, N.; Senator, A.; Siracusa, L.; Ruberto, G. Artemisia Herba-Alba Aqueous Extract Improves Insulin Sensitivity and Hepatic Steatosis in Rodent Model of Fructose-Induced Metabolic Syndrome. Arch. Physiol. Biochem. 2021, 127, 541–550. [Google Scholar] [CrossRef]
- Sendi, N.; Mkadmini-Hammi, K.; Ben Mansour, R.; Selmi, S.; Trabelsi, N.; Isoda, H.; Ksouri, R.; Megdiche-Ksouri, W. Simultaneous Optimization of Ultrasound-Assisted Extraction of Flavonoid Compounds and Antiradical Activity from Artemisia Herba-Alba Using Response Surface Methodology. Prep. Biochem. Biotechnol. 2020, 50, 943–953. [Google Scholar] [CrossRef]
- El Ouardi, M.; Drioiche, A.; El Makhoukhi, F.; Mabrouki, J.; Hakmi, M.; Al Kamaly, O.; Alsfouk, B.A.; Eddamsyry, B.; Khamar, H.; Zair, T.; et al. Chemical Composition, Antimicrobial, and Antioxidant Properties of Essential Oils from Artemisia Herba-Alba Asso. and Artemisia Huguetii Caball. from Morocco: In Vitro and in Silico Evaluation. Front. Chem. 2024, 12, 1456684. [Google Scholar] [CrossRef]
- Abushwereb, H.; Tolba, M. Gastroprotective Activity of Artemisia Herba Alba Aqueous Extract on Aspirin-Induced Gastric Lesions in Albino Rats. J. Pharm. Appl. Chem. 2016, 2, 141–145. [Google Scholar] [CrossRef]
- Alghonmeen, R.; Dmour, S.; Saghir, S.; Abushattal, S.; Alnaimat, S.; Zharani, M.; Nasr, F.; Althunibat, O. Anti-MRSA and Cytotoxic Activities of Different Solvent Extracts from Artemisia Herba-Alba Grown in Shubak, Jordan. Open Vet. J. 2024, 14, 990–1001. [Google Scholar] [CrossRef]
- Moufid, A.; Eddouks, M. Artemisia Herba Alba: A Popular Plant with Potential Medicinal Properties. Pak. J. Biol. Sci. 2012, 15, 1152–1159. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and Pharmacological Activity of the Genus Artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef]
- Alshehri, M.A. Cardioprotective Properties of Artemisia Herba Alba Nanoparticles against Heart Attack in Rats: A Study of the Antioxidant and Hypolipidemic Activities. Saudi J. Biol. Sci. 2022, 29, 2336–2347. [Google Scholar] [CrossRef]
- Rasool, R.; Ullah, I.; Shahid, S.; Mubeen, B.; Imam, S.S.; Alshehri, S.; Kazmi, I. In Vivo Assessment of the Ameliorative Impact of Some Medicinal Plant Extracts on Lipopolysaccharide-Induced Multiple Sclerosis in Wistar Rats. Molecules 2022, 27, 1608. [Google Scholar] [CrossRef]
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and Anti-Oxidant, Anti-Cancer and Anti-Inflammatory Activities of Artemisia Herba-Alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013, 55, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Grzelak, A.; Janaszewska, A.; Jakubowski, W.; Koziol, S.; Marszalek, M.; Rychlik, B.; Soszynski, M.; Bilinski, T.; Bartosz, G. Thiols as Major Determinants of the Total Antioxidant Capacity. BioFactors 2003, 17, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Deneke, S.M. Thiol-Based Antioxidants. Curr. Top. Cell Regul. 2001, 36, 151–180. [Google Scholar] [CrossRef]
- Li, X.; Gluth, A.; Zhang, T.; Qian, W.J. Thiol Redox Proteomics: Characterization of Thiol-Based Post-Translational Modifications. Proteomics 2023, 23, e2200194. [Google Scholar] [CrossRef]
- Rahimi, M.; Marefati, N.; Beheshti, F.; Ahmadabady, S.; Rakhshandeh, H.; Hosseini, M. The Effects of Artemisia Absinthium L. on Scopolamine-Induced Learning and Memory Impairment and Brain Tissue Oxidative Damage in Adult Rats. Avicenna J. Phytomed. 2023, 13, 70–84. [Google Scholar] [CrossRef]
- He, M.; Yasin, K.; Yu, S.; Li, J.; Xia, L. Total Flavonoids in Artemisia Absinthium L. and Evaluation of Its Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 16348. [Google Scholar] [CrossRef]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef]
- El Ouahdani, K.; Es-sa, I.; Mechchate, H.; Al-zahrani, M.; Qurtam, A.A.; Aleissa, M.; Bari, A.; Bousta, D. Thymus Algeriensis and Artemisia Herba-Alba Essential Oils: Chemical Analysis, Antioxidant Potential and In Vivo Anti-Inflammatory, Analgesic Activities, and Acute Toxicity. Molecules 2021, 26, 6780. [Google Scholar] [CrossRef]
- Wibisana, J.N.; Okada, M. Encoding and Decoding NF-ΚB Nuclear Dynamics. Curr. Opin. Cell Biol. 2022, 77, 102103. [Google Scholar] [CrossRef]
- Bacher, S.; Schmitz, M.L. Open Questions in the NF-ΚB Field. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119469. [Google Scholar] [CrossRef]
- Mohamed, A.E.-H.H.; Esmail, A.M.; El-Saade, A.M. Terpenes from Artemisia Herba-Alba. Z. Naturforsch C J. Biosci. 2013, 68, 343. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef]
- Su, J.; Yu, M.; Wang, H.; Wei, Y. Natural Anti-inflammatory Products for Osteoarthritis: From Molecular Mechanism to Drug Delivery Systems and Clinical Trials. Phytother. Res. 2023, 37, 4321–4352. [Google Scholar] [CrossRef]
- Kadi, I.; Ouinten, M.; Gourine, N.; Yousfi, M. Synergistic Antinociceptive Activity of Combined Aqueous Extracts of Artemisia Campestris and Artemisia Herba-Alba in Several Acute Pain Models. Nat. Prod. Res. 2019, 33, 875–878. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Ma, Q. Pharmacological Inhibition of the NLRP3 Inflammasome: Structure, Molecular Activation, and Inhibitor-NLRP3 Interaction. Pharmacol. Rev. 2023, 75, 487–520. [Google Scholar] [CrossRef]
- Jahan, S.; Kumar, D.; Chaturvedi, S.; Rashid, M.; Wahajuddin, M.; Khan, Y.A.; Goyal, S.N.; Patil, C.R.; Mohanraj, R.; Subramanya, S.; et al. Therapeutic Targeting of NLRP3 Inflammasomes by Natural Products and Pharmaceuticals: A Novel Mechanistic Approach for Inflammatory Diseases. Curr. Med. Chem. 2017, 24, 1645–1670. [Google Scholar] [CrossRef]
- Tőzsér, J.; Benkő, S. Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1 β Production. Mediat. Inflamm. 2016, 2016, 5460302. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
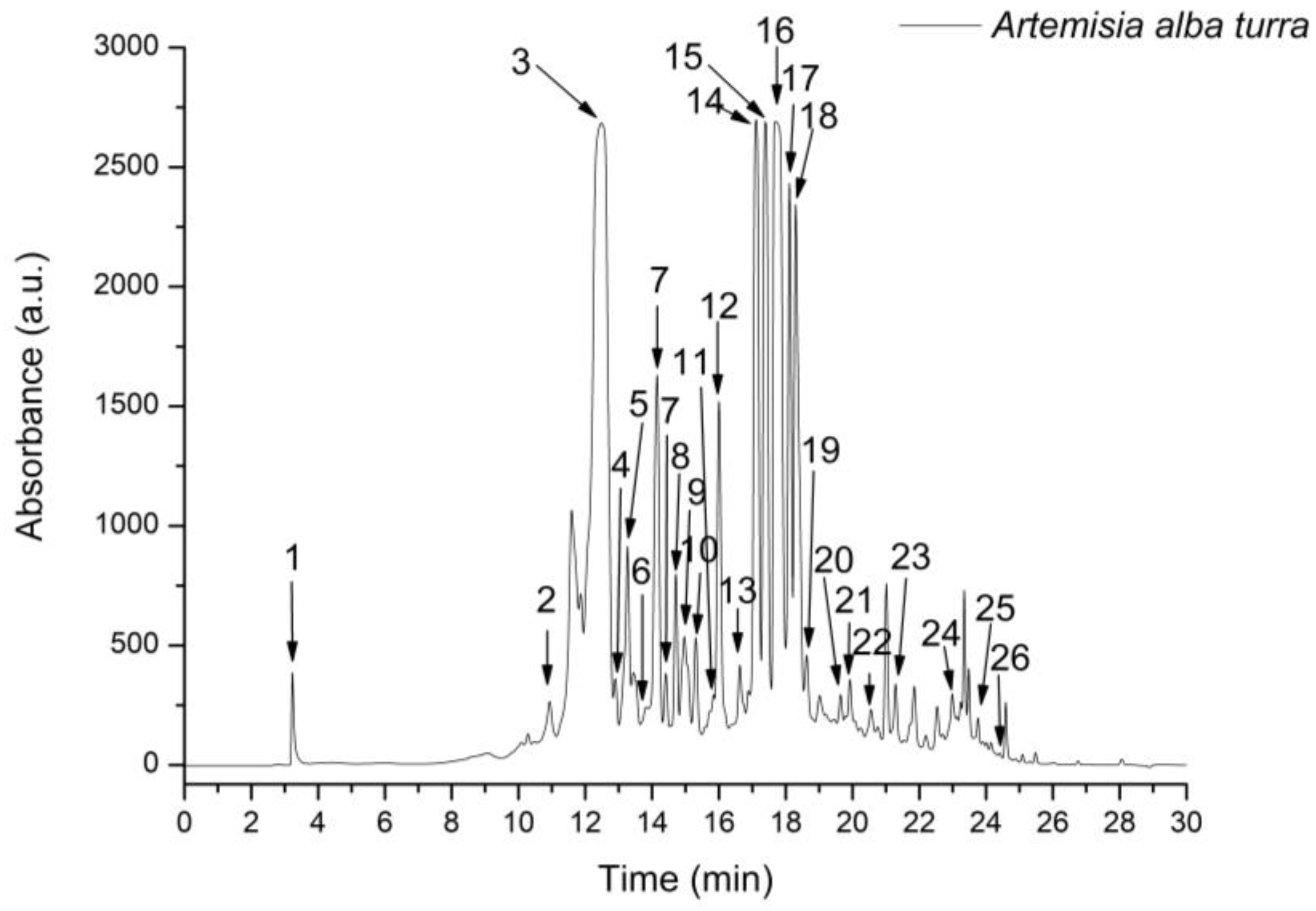

| Peak No. | Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Compound | Subclass | A. alba Turra |
|---|---|---|---|---|---|---|
| 1 | 3.22 | 275 | 155 | 3,5-Dihydroxybenzoic acid | Hydroxybenzoic acid 1 | 74.138 ± 2.12 |
| 2 | 11.04 | 323 | 355, 163 | 3-Caffeoylquinic acid (neochlorogenic acid) | Hydroxycinnamic acid 2 | 290.674 ± 15.66 |
| 3 | 12.57 | 323 | 355, 163 | 5-Caffeoylquinic acid (chlorogenic acid) | Hydroxycinnamic acid 2 | 4843.820 ± 82.94 |
| 4 | 12.95 | 323 | 355, 163 | 4-Caffeoylquinic acid (criptochlorogenic acid) | Hydroxycinnamic acid 2 | 161.934 ± 8.73 |
| 5 | 13.21 | 323 | 343, 163 | Caffeoyl acid-glucoside | Hydroxycinnamic acid 2 | 550.971 ± 22.70 |
| 6 | 13.72 | 324 | 195 | Iso-Ferulic acid | Hydroxycinnamic acid 2 | 160.712 ± 6.78 |
| 7 | 14.24 | 325 | 313 | Caffeoyl tartaric acid | Hydroxycinnamic acid 2 | 1033.149 ± 46.69 |
| 8 | 14.59 | 330, 270 | 565, 271 | Apigenin-arabinosyl-glucoside | Flavone 3 | 106.114 ± 3.04 |
| 9 | 15.02 | 324 | 369, 195 | 3-Feruloylquinic acid | Hydroxycinnamic acid 2 | 442.686 ± 14.24 |
| 10 | 15.47 | 324 | 369, 195 | 4-Feruloylquinic acid | Hydroxycinnamic acid 2 | 285.520 ± 10.31 |
| 11 | 15.88 | 324 | 369, 195 | 5-Feruloylquinic acid | Hydroxycinnamic acid 2 | 187.065 ± 3.03 |
| 12 | 16.03 | 255, 360 | 611, 303 | Quercetin-rutinoside (rutin) | Flavonol 4 | 681.141 ± 14.11 |
| 13 | 16.66 | 330, 270 | 565, 271 | Apigenin-glucosyl-arabinoside | Flavone 3 | 125.737 ± 4.50 |
| 14 | 17.13 | 240, 350 | 625, 317 | Isorhamnetin-rutinoside | Flavonol 4 | 1348.303 ± 80.81 |
| 15 | 17.52 | 323 | 517, 163 | 3,4-Dicaffeoylquinic acid | Hydroxycinnamic acid 2 | 1524.679 ± 98.54 |
| 16 | 17.86 | 323 | 517, 163 | 3,5-Dicaffeoylquinic acid | Hydroxycinnamic acid 2 | 2654.119 ± 97.89 |
| 17 | 18.11 | 323 | 517, 163 | Quinic acid derivative | Hydroxycinnamic acid 2 | 972.472 ± 57.03 |
| 18 | 18.35 | 323 | 517, 163 | 4,5-Dicaffeoylquinic acid | Hydroxycinnamic acid 2 | 1369.054 ± 87.50 |
| 19 | 18.73 | 324 | 531, 163 | 3-Feruloyl-4-caffeoylquinic acid | Hydroxycinnamic acid 2 | 312.830 ± 15.64 |
| 20 | 19.42 | 324 | 531, 163 | 4-Feruloyl-5-caffeoylquinic acid | Hydroxycinnamic acid 2 | 153.220 ± 3.98 |
| 21 | 19.84 | 324 | 545, 163 | 3,4-Diferuloylquinic acid | Hydroxycinnamic acid 2 | 308.952 ± 13.67 |
| 22 | 20.44 | 324 | 545, 163 | 3,5-Diferuloylquinic acid | Hydroxycinnamic acid 2 | 171.285 ± 4.62 |
| 23 | 21.32 | 324 | 531, 163 | 5-Caffeoyl-4-feruloyl-quinic acid | Hydroxycinnamic acid 2 | 196.257 ± 5.73 |
| 24 | 22.91 | 323 | 679, 163 | 3,4,5-Tricaffeoylquinic acid | Hydroxycinnamic acid 2 | 193.654 ± 2.34 |
| 25 | 23.68 | 330, 270 | 361 | 3,5-Dihydroxy-6,7,4′-trimethoxyflavone | Flavone 3 | 93.217 ± 5.02 |
| 26 | 24.39 | 330, 270 | 375 | 3,5-Dihydroxy-6,7,3′,4′-tetramethoxyflavone | Flavone 3 | 27.307 ± 2.77 |
| DPPH μg TE/mL | FRAP mg TE/mL | H2O2 Scavenging Activity μg TE/mL | NO Scavenging Activity μg QE/mL | |
|---|---|---|---|---|
| A. alba Turra (1 g/1.2 mL) | 42.66 ± 0.53 | 54.91 ± 0.56 | 38.48 ± 0.40 | 66.55 ± 1.28 |
| TROLOX (mg) | 11.61 ± 0.14 | 15.28 ± 1.15 | 12.04 ± 0.12 | |
| Quercitin (mg) | 20.05 ± 0.18 |
| Groups | TOS (µmol H2O2E/L) | TAC (mmol TE/L) | OSI | AOPP (µmol/L) | MDA (nmol/L) | NO (µmol/L) | 3NT (ng/mL) | 8-OhdG (ng/mL) | SH (µmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 14.72 ± 2.34 | 1.08 ± 0.00 | 15.52 ± 2.16 | 26.93 ± 1.70 | 2.54 ± 0.16 | 25.26 ± 3.41 | 22.14 ± 2.35 | 24.16 ± 1.89 | 340.24 ± 30.14 |
| INFL | 50.00 ± 4.93 a | 1.17 ± 0.08 a | 43.09 ± 4.00 a | 68.28 ± 6.19 a | 4.08 ± 0.35 a | 37.00 ± 6.25 a | 70.24 ± 5.32 a | 87.64 ± 11.72 a | 249.24 ± 18.61 a |
| AAT 100% | 25.75 ± 1.46 b,c | 1.09 ± 0.00 | 23.67 ± 3.70 b,c | 37.51 ± 3.75 b | 2.62 ± 0.20 b | 41.60 ± 7.94 b,d | 45.10 ± 2.58 b | 44.49 ± 3.09 b | 389.50 ± 17.98 b,c,d |
| AAT 50% | 27.68 ± 3.75 b,c | 1.09 ± 0.00 | 25.48 ± 2.14 b,c | 35.81 ± 2.08 b | 2.78 ± 0.19 b | 44.00 ± 9.86 b,d | 59.44 ± 6.96 c,d | 68.34 ± 6.83 b,c,d | 417.80 ± 34.70 b,c,d |
| AAT 25% | 19.09 ± 2.76 b | 1.08 ± 0.00 | 17.61 ± 1.16 b | 29.65 ± 1.76 b | 2.46 ± 0.22 b | 51.62 ± 4.83 d | 53.99 ± 4.10 c,d | 67.68 ± 6.24 b,c,d | 309.40 ± 29.93 b |
| DICLO | 20.24 ± 2.11 b | 1.09 ± 0.00 b | 15.08 ± 1.66 b | 25.85 ± 1.63 b | 2.89 ± 0.12 b | 25.41 ± 3.26 b | 30.22 ± 2.34 b | 48.12 ± 5.04 b | 260.17 ± 27.44 |
| TX | 18.16 ± 1.17 b | 1.09 ± 0.00 b | 15.17 ± 1.92 b | 27.62 ± 2.60 b | 2.72 ± 0.24 b | 38.54 ± 4.23 b | 20.48 ± 2.72 b | 40.06 ± 4.91 b | 280.86 ± 22.45 |
| Groups | NfkB-p65 (ng/mL) | IL-1b (pg/mL) | IL-18 (pg/mL) | Caspase—1 (pg/mL) | GSDMD (ng/mL) |
|---|---|---|---|---|---|
| CONTROL | 138.26 ± 10.09 | 22.13 ± 1.87 | 20.05 ± 1.09 | 12.52 ± 2.00 | 4.77 ± 0.35 |
| INFL | 329.57 ± 20.13 a | 60.16 ± 4.22 a | 60.29 ± 8.41 a | 130.74 ± 10.25 a | 10.13 ± 0.86 a |
| AAT 100% | 138.51 ± 15.33 b | 27.29 ± 1.57 b | 22.60 ± 2.70 b,c | 49.29 ± 6.52 b | 5.44 ± 0.34 b |
| AAT 50% | 187.23 ± 18.76 b | 30.42 ± 2.07 b | 25.20 ± 1.15 b,c | 55.22 ± 4.82 b | 6.03 ± 0.51 b |
| AAT 25% | 754.38 ± 14.18 b | 31.46 ± 4.48 b | 28.54 ± 3.52 b,c | 51.13 ± 2.22 b | 7.09 ± 0.84 b |
| DICLO | 135.22 ± 10.41 b | 25.81 ± 2.44 b | 48.87 ± 2.76 b,c | 42.07 ± 4.83 b | 5.16 ± 0.61 b |
| TX | 150.15 ± 10.28 b | 30.42 ± 4.06 b | 28.46 ± 2.72 b | 50.42 ± 4.21 b | 5.53 ± 0.54 b |
| Groups | ALT (U/L) | AST (U/L) | Creatinine (mg/dL) | Urea (mg/dL) |
|---|---|---|---|---|
| CONTROL | 43.12 ± 2.23 | 58.25 ± 4.18 | 0.72 ± 0.01 | 32.42 ± 3.02 |
| INFL | 49.87 ± 3.71 | 49.58 ± 4.09 | 1.05 ± 0.18 a | 57.32 ± 6.10 a |
| AAT 100% | 49.33 ± 4.95 | 54.95 ± 7.01 | 0.74 ± 0.11 b | 47.06 ± 6.86 b |
| AAT 50% | 38.12 ± 2.62 | 42.68 ± 6.09 | 0.88 ± 0.09 b | 39.56 ± 4.05 b |
| AAT 25% | 43.82 ± 2.33 | 39.56 ± 5.49 | 0.84 ± 0.09 b | 44.98 ± 3.19 b |
| DICLO | 35.24 ± 2.26 | 34.36 ± 3.05 | 0.72 ± 0.01 b | 42.06 ± 3.14 b |
| TX | 35.46 ± 2.09 | 32.53 ± 2.72 | 0.74 ± 0.07 b | 41.29 ± 3.11 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țicolea, M.; Pop, R.M.; Pârvu, M.; Usatiuc, L.-O.; Uifălean, A.; Brito, V.A.; Fischer-Fodor, E.; Ranga, F.; Rusu, C.C.; Crisan, M.; et al. The Phytochemical and Functional Characterization of the Aerial Parts of Artemisa alba Turra (Asteraceae) Grown in Romania. Foods 2025, 14, 1389. https://doi.org/10.3390/foods14081389
Țicolea M, Pop RM, Pârvu M, Usatiuc L-O, Uifălean A, Brito VA, Fischer-Fodor E, Ranga F, Rusu CC, Crisan M, et al. The Phytochemical and Functional Characterization of the Aerial Parts of Artemisa alba Turra (Asteraceae) Grown in Romania. Foods. 2025; 14(8):1389. https://doi.org/10.3390/foods14081389
Chicago/Turabian StyleȚicolea, Mădălina, Raluca Maria Pop, Marcel Pârvu, Lia-Oxana Usatiuc, Ana Uifălean, Valeria Alvarez Brito, Eva Fischer-Fodor, Floricuța Ranga, Crina Claudia Rusu, Maria Crisan, and et al. 2025. "The Phytochemical and Functional Characterization of the Aerial Parts of Artemisa alba Turra (Asteraceae) Grown in Romania" Foods 14, no. 8: 1389. https://doi.org/10.3390/foods14081389
APA StyleȚicolea, M., Pop, R. M., Pârvu, M., Usatiuc, L.-O., Uifălean, A., Brito, V. A., Fischer-Fodor, E., Ranga, F., Rusu, C. C., Crisan, M., Bosca, B., Cătoi, F. A., & Pârvu, A. E. (2025). The Phytochemical and Functional Characterization of the Aerial Parts of Artemisa alba Turra (Asteraceae) Grown in Romania. Foods, 14(8), 1389. https://doi.org/10.3390/foods14081389










