The Comparison of the Profile of Phenolic Compounds in Noni (Morinda citrifolia L.) Fruit by Different Drying Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Noni Materials
2.3. Extraction of Polyphenols
2.3.1. Extraction of Free Polyphenols
2.3.2. Extraction of Bound Polyphenols
2.4. Total Polyphenol Content (TPC)
2.5. UPLC-Q-TOF-MS Analysis
2.6. Antioxidant Activity
2.7. Data Analysis
3. Results and Discussion
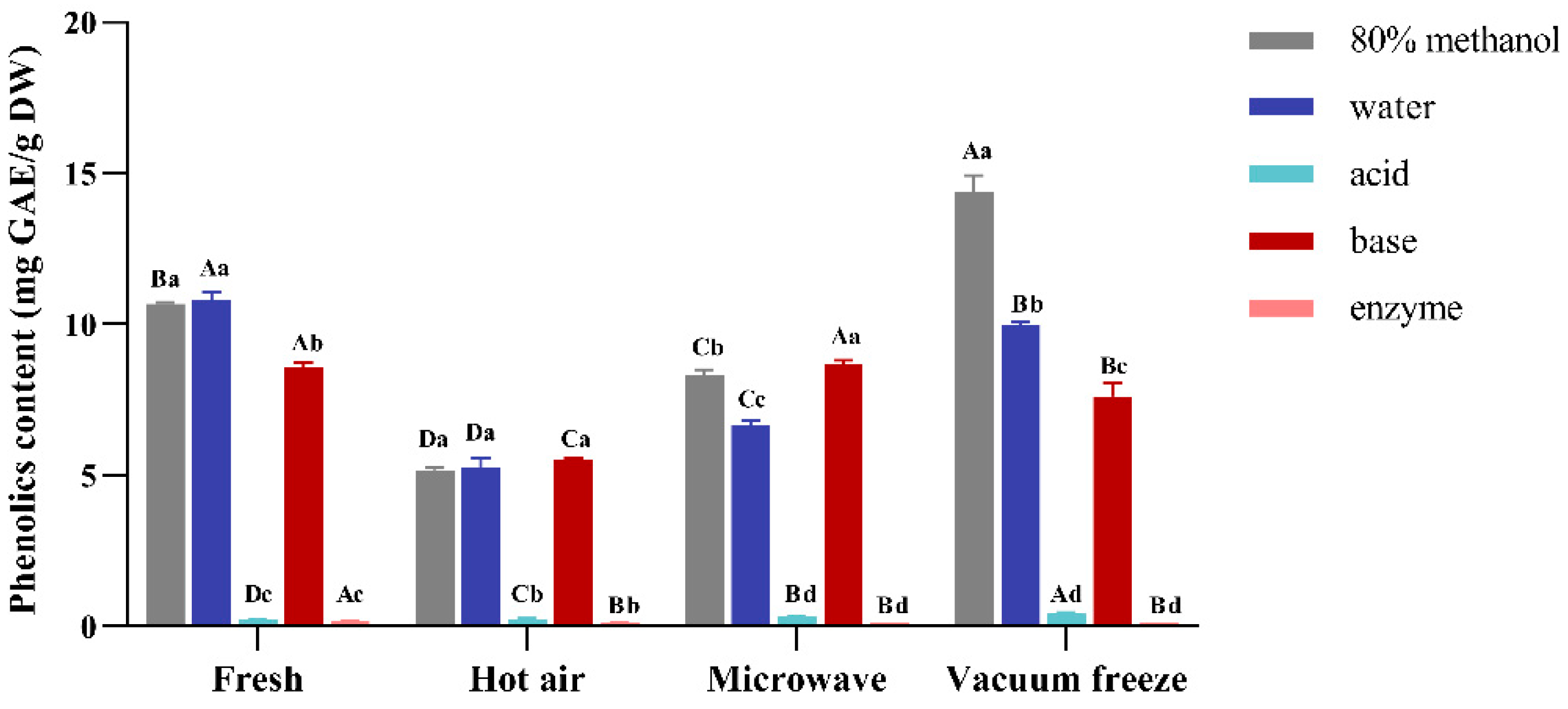
3.1. The TPC of Noni
3.2. Identification of Polyphenols in Noni Fruit
3.3. Quantity of the Polyphenols in Noni Fruit by Different Drying Methods
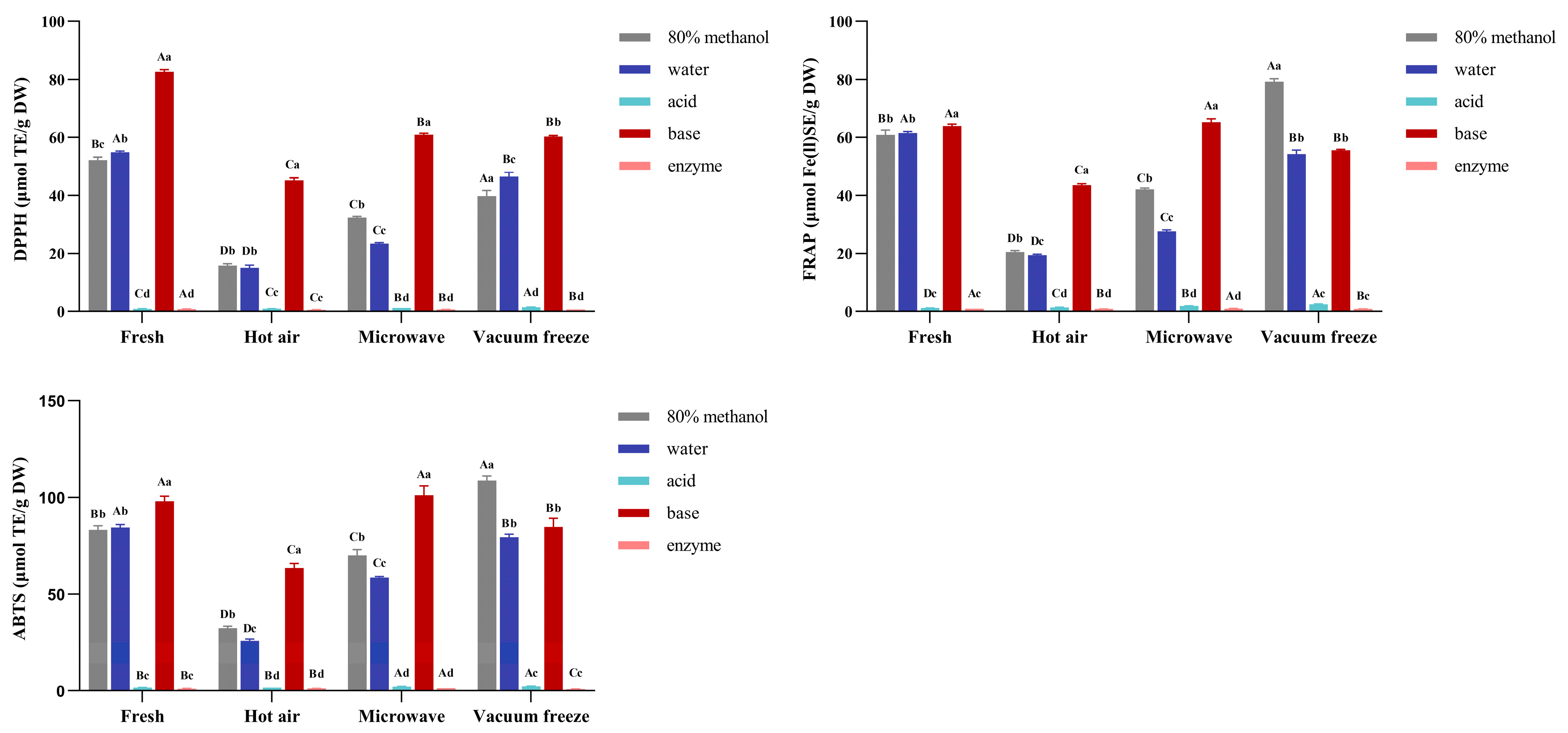
3.4. The Changes in Antioxidant Activity
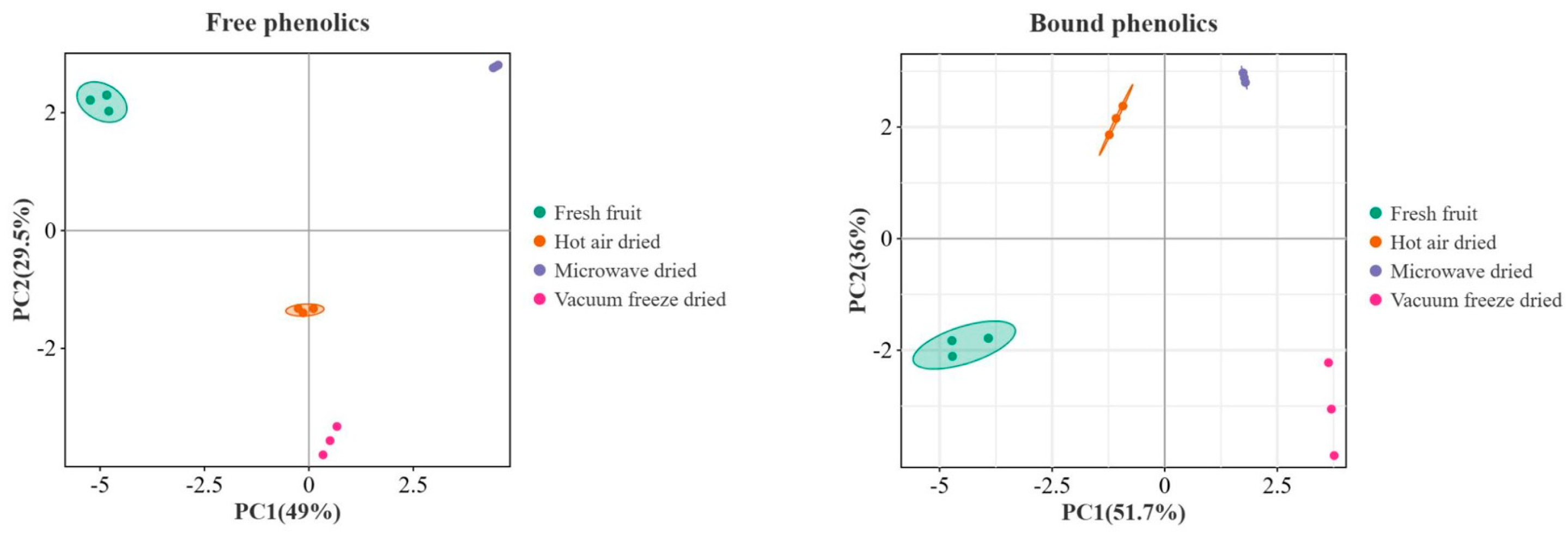
3.5. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From Classification to Therapeutic Potential and Bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary Polyphenols: Review on Chemistry/Sources, Bioavailability/Metabolism, Antioxidant Effects, and Their Role in Disease Management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Y.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Narra, F.; Piragine, G.; Benedetti, G.; Ceccanti, C.; Florio, M.; Spezzini, J.; Troisi, F.; Giovannoni, R.; Martelli, A.; Guidi, L. Impact of thermal processing on polyphenols, carotenoids, glucosinolates, and ascorbic acid in fruit and vegetables and their cardiovascular benefits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13426. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products. Foods 2021, 10, 1538. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Karav, S.; Witkowska, A.M. Dietary Polyphenols, Food Processing and Gut Microbiome: Recent Findings on Bioavailability, Bioactivity, and Gut Microbiome Interplay. Antioxidants 2024, 13, 1220. [Google Scholar] [CrossRef]
- Liu, X.Z.; Lin, X.; Hu, X.B.; Li, C.F.; Wang, L.; Fei, T. Noni (Morinda citrifolia) fruit and by-products: A comprehensive review of its chemical compositions, health-promoting effects. Trends Food Sci. Technol. 2024, 153, 104690. [Google Scholar] [CrossRef]
- Wang, R.M.; Wang, L.; Zhang, L.; Wan, S.T.; Li, C.F.; Liu, S.X. Solvents effect on phenolics, iridoids, antioxidant activity, antibacterial activity, and pancreatic lipase inhibition activity of noni (Morinda citrifolia L.) fruit extract. Food Chem. 2022, 377, 131989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Charoensiddhi, S.; Xue, X.; Sun, B.; Liu, Y.; El-Seedi, H.R.; Wang, K. A review on the gastrointestinal protective effects of tropical fruit polyphenols. Crit. Rev. Food Sci. Nutr. 2022, 17, 7197–7223. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, Z.; Xiang, Y.; Deng, T.; Zhao, X.; Shi, S.; Zheng, Q.; Gao, X.; Li, W. The effects of drying methods on chemical profiles and antioxidant activities of two cultivars of Psidium guajava fruits. LWT-Food Sci. Technol. 2020, 118, 108723. [Google Scholar] [CrossRef]
- Minuye, M.; Paulos, G.; Arnaud, L.; Stanley, C.; Kaleab, B. Effects of different drying methods and ascorbic acid pretreatment on carotenoids and polyphenols of papaya fruit in Ethiopia. Food Sci. Nutr. 2021, 9, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Babaei Rad, S.; Mumivand, H.; Mollaei, S.; Khadivi, A. Effect of drying methods on phenolic compounds and antioxidant activity of Capparis spinosa L. fruits. BMC Plant Biol. 2025, 25, 133. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, C.T.; Tran, L.T.; Truong, T. Effects of pretreatment and air drying temperature on Noni fruit powder. Food Sci. Biotechnol. 2021, 30, 1519–1526. [Google Scholar] [CrossRef]
- Oliveira, B.F.; Negreiros, J.K.S.; Sobrinho, M.A.M.; Cavalcante, J.A.; Costa, N.A.; Pereira, T.S. Influence of drying variables on the properties of noni powder obtained by spouted bed. Part. Sci. Technol. 2024, 42, 1324–1332. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Tang, W.; Li, W.; Yang, Y.; Lin, X.; Wang, L.; Li, C.; Yang, R. Phenolic compounds profile and antioxidant capacity of pitahaya fruit peel from two red-skinned species (Hylocereus polyrhizus and Hylocereus undatus). Foods 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Q-TOF LC/MS identification and UHPLC-Online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Van Der Werf, R.; Marcic, C.; Khalil, A.; Sigrist, S.; Marchioni, E. ABTS radical scavenging capacity in green and roasted coffee extracts. LWT-Food Sci. Technol. 2014, 58, 77–85. [Google Scholar] [CrossRef]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Goyal, A.K.; Middha, S.K.; Sen, A. Evaluation of the DPPH radical scavenging activity, total phenols and antioxidant activities in Indian wild Bambusa vulgaris “Vittata” methanolic leaf extract. J. Nat. Pharm. 2010, 1, 40–45. [Google Scholar]
- Süfer, Ö.; Palazoğlu, T.K. A study on hot-air drying of pomegranate. J. Therm. Anal. Calorim. 2019, 137, 1981–1990. [Google Scholar] [CrossRef]
- Yuste, S.; Macià, A.; Motilva, M.J.; Prieto-Diez, N.; Romero, M.P.; Pedret, A.; Solà, R.; Ludwig, I.A.; Rubió, L. Thermal and non-thermal processing of red-fleshed apple: How are (poly)phenol composition and bioavailability affected? Food Funct. 2020, 11, 10436–10447. [Google Scholar] [CrossRef]
- Li, F.; Shao, P.; Han, Y.; Xie, H.; Zhang, L. Effect of drying methods on active component contents and antioxidant activity of jujube pigment. Food Sci. Technol. 2019, 44, 120–124. [Google Scholar]
- Ma, Q.; Bi, J.; Yi, J.; Wu, X.; Li, X.; Zhao, Y. Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments. Food Sci. Hum. Wellness 2021, 10, 174–182. [Google Scholar] [CrossRef]
- Nawawi, N.I.M.; Ijod, G.; Abas, F.; Adzahan, N.M.; Azman, E.M. Influence of Different Drying Methods on Anthocyanins Composition and Antioxidant Activities of Mangosteen (Garcinia mangostana L.) Pericarps and LC-MS Analysis of the Active Extract. Foods 2023, 12, 2351. [Google Scholar] [CrossRef]
- Papoutsis, K.; Vuong, Q.V.; Golding, J.B.; Hasperué, J.H.; Pristijono, P.; Bowyer, M.C.; Scarlett, C.J.; Stathopoulos, C.E. Pretreatment of citrus by-products affects polyphenol recovery: A review. Food Rev. Int. 2018, 34, 770–795. [Google Scholar] [CrossRef]
- Zielinska, M.; Sadowski, P.; Błaszczak, W. Freezing/thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.). LWT-Food Sci. Technol. 2015, 62, 555–563. [Google Scholar] [CrossRef]
- Hii, C.L.; Law, C.L.; Cloke, M.; Suzannah, S. Thin layer drying kinetics of cocoa and dried product quality. Biosyst. Eng. 2009, 102, 153–161. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Dolan, K.D. Total phenolics, carotenoids and antioxidant properties of Tommy Atkin mango cubes as affected by drying techniques. LWT-Food Sci. Technol. 2015, 62, 564–568. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Identification and quantification of flavonol aglycons in cactus pear (Opuntia ficus indica) fruit using a commercial pectinase and cellulase preparation. Food Chem. 2011, 124, 1177–1184. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Fazary, A.E.; Ju, Y. Feruloyl esterases as biotechnological tools: Current and future perspectives. Acta Biochim. Biophys. Sin. 2007, 39, 811–828. [Google Scholar] [CrossRef]
- Dussossoy, E.; Brat, P.; Bony, E.; Boudard, F.; Poucheret, P.; Mertz, C.; Giaimis, J.; Michel, A. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.). J. Ethnopharmacol. 2011, 133, 108–115. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Bualuang, O.; Onwude, D.I.; Pracha, K. Microwave drying of germinated corn and its effect on phytochemical properties. J. Sci. Food Agric. 2017, 97, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Dey, S.; Chakraborty, R. Effect of microwave power on drying kinetics, structure, color, and antioxidant activities of corncob. J. Food Process Eng. 2019, 42, e13021. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernandez-Ortega, M.; Hernandez-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragon, H.; Ortiz Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, Hot Air and Freeze-Drying on the phenolic Compounds, Antioxidant Capacity, Enzyme Activity and Microstructure of Cacao PodHusks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Advances in the analytical methods for determining the antioxidant properties of honey: A review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 36–42. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, C.; Luo, J.; Zhou, R.; Sun, X.; Ma, J. Influence of technical processing units on polyphenols and antioxidant capacity of carrot (Daucus carrot L.) juice. Food Chem. 2013, 141, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Deng, J.; Ouyang, D.; Wang, D.; Liang, Y.; Chen, Y.; Sun, Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020, 332, 127429. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Zhang, N.; Qiu, R.; Li, C.; Wang, S.; Ni, Z. Accelerated biodegradation of p-tert-butylphenol in the Phragmites australis rhizosphere by phenolic root exudates. Environ. Exp. Bot. 2020, 169, 103891. [Google Scholar]
| No. | RT (min) | Compounds | Formula | m/z [M-H] | m/z Fragments | Identified in |
|---|---|---|---|---|---|---|
| 1 | 1.67 | quinic acid b | C7H12O6 | 191.0561 | 127.0401 | F, H, M, V |
| 2 | 3.04 | phloroglucinol ab | C6H6O3 | 125.1100 | F, H, M, V | |
| 3 | 3.05 | gallic acid ab | C7H6O5 | 169.0131 | 125.0234 | H, M |
| 4 | 4.87 | helicid ab | C13H16O7 | 329.0878 | 121.0284 | F, H, V |
| 5 | 5.07 | 3,4-dihydroxybenzoic acid ab | C7H6O4 | 153.0181 | 109.0282 | F, H, M, V |
| 6 | 6.54 | p-hydroxybenzoic acid ab | C7H6O3 | 137.0231 | 93.0332 | F, H, V |
| 7 | 7.00 | esculetin b | C9H6O4 | 177.0183 | 133.0284 | F, H, M |
| 8 | 7.15 | caffeic acid ab | C9H8O4 | 179.0337 | 135.0438 | F, H, M, V |
| 9 | 7.17 | vanillic acid ab | C8H8O4 | 167.0340 | 152.0106, 123.0440 | F, H, M, V |
| 10 | 7.27 | syringic acid ab | C9H10O5 | 197.0447 | 182.0214 | F, H, V |
| 11 | 8.10 | benzoic acid ab | C7H6O2 | 121.0283 | 77.0381, 94.0281 | F, H, M, V |
| 12 | 8.89 | rutin ab | C27H30O16 | 609.1467 | 300.0279, 301.0354 | F, H, M, V |
| 13 | 8.99 | p-coumaric acid ab | C9H8O3 | 163.0386 | 119.0401 | M, V |
| 14 | 9.00 | vanillin ab | C8H8O3 | 151.0389 | 136.0155 | F, H, M, V |
| 15 | 9.02 | p-hydroxycinnamic acid ab | C9H8O3 | 163.0389 | 119.0491 | F, H, M, V |
| 16 | 9.32 | hyperoside ab | C21H20O12 | 463.0886 | 300.0282, 301.0355 | F, H, M, V |
| 17 | 9.46 | isoquercitrin ab | C21H20O12 | 463.0885 | 300.0276 | F, H, M, V |
| 18 | 9.74 | ferulic acid ab | C10H10O4 | 193.0497 | 178.0265, 134.0363, 149.0599 | F, H, M, V |
| 19 | 9.99 | kaempferol-3-o-rutinoside b | C27H30O15 | 593.1514 | 285.0404 | F, H, V |
| 20 | 10.29 | isoferulic acid ab | C10H10O4 | 193.0498 | 178.0265, 134.0363 | F, H, M, V |
| 21 | 10.60 | kaempferol-3-o-glucoside ab | C21H20O11 | 447.0931 | 284.0328 | V |
| 22 | 11.32 | hesperidin b | C28H34O15 | 609.1829 | 301.0723 | F, H |
| 23 | 13.07 | salicylic acid b | C7H6O3 | 137.0230 | F, H, V | |
| 24 | 13.32 | morin b | C15H14O9 | 301.0359 | 151.0028 | F, V |
| 25 | 14.60 | quercetin ab | C15H10O7 | 301.0352 | 151.0030, 178.9983 | F, H, M, V |
| 26 | 15.65 | cinnamic acid ab | C9H8O2 | 147.0439 | 103.0536 | M, V |
| 27 | 16.43 | kaempferol ab | C15H10O6 | 285.0411 | 151.0014 | F, H, M |
| No. | Compounds | 80% Methanol (mg/kg DW) | Water (mg/kg DW) | Acid (mg/kg DW) | Base (mg/kg DW) | Enzyme (mg/kg DW) |
|---|---|---|---|---|---|---|
| 1 | salicylic acid | 23.15 ± 2.10 | 26.32 ± 2.97 | 3.89 ± 0.22 | 2.57 ± 0.57 | 1.20 ± 0.27 |
| 2 | phloroglucinol | 1.93 ± 0.70 | 1.99 ± 0.37 | 1.30 ± 0.17 | 13.10 ± 2.23 | 0.32 ± 0.09 |
| 3 | 3,4-dihydroxybenzoic acid | 6.07 ± 0.66 | 7.50 ± 1.00 | 1.64 ± 0.34 | 1.36 ± 0.47 | ND |
| 4 | p-hydroxybenzoic acid | 44.12 ± 2.82 | 45.33 ± 2.42 | 3.47 ± 0.20 | 5.50 ± 0.04 | 2.44 ± 0.06 |
| 5 | caffeic acid | 0.13 ± 0.03 | 1.17 ± 0.22 | ND | 0.89 ± 0.06 | ND |
| 6 | syringic acid | 1.72 ± 0.20 | 1.47 ± 0.20 | 0.25 ± 0.05 | 0.68 ± 0.07 | ND |
| 7 | benzoic acid | ND | ND | 69.50 ± 7.95 | 220.67 ± 14.57 | 23.54 ± 2.02 |
| 8 | rutin | 1446.09 ± 21.18 | 1016.21 ± 14.98 | 0.24 ± 0.06 | 77.02 ± 3.25 | 65.97 ± 1.21 |
| 9 | p-coumaric acid | ND | ND | 0.74 ± 0.28 | 23.32 ± 0.32 | ND |
| 10 | hyperoside | 15.78 ± 0.36 | 26.49 ± 1.21 | 1.38 ± 0.04 | 3.48 ± 1.10 | 1.71 ± 0.32 |
| 11 | isoquercitrin | 12.09 ± 0.05 | 20.51 ± 0.24 | 1.09 ± 0.06 | 2.78 ± 0.86 | 1.36 ± 0.24 |
| 12 | ferulic acid | 3.80 ± 0.64 | 5.69 ± 0.24 | 2.46 ± 0.34 | 11.20 ± 0.55 | 0.59 ± 0.07 |
| 13 | kaempferol-3-o-rutinoside | 96.89 ± 3.29 | 66.75 ± 2.16 | ND | 5.72 ± 0.88 | 3.73 ± 0.29 |
| 14 | quercetin | 139.73 ± 8.51 | 64.43 ± 3.90 | 7.46 ± 0.89 | 2.61 ± 1.03 | 3.39 ± 0.70 |
| 15 | kaempferol | 6.81 ± 0.98 | 1.41 ± 0.31 | ND | 0.53 ± 0.03 | ND |
| 16 | quinic acid | 328.37 ± 10.37 | 336.26 ± 12.30 | 9.74 ± 1.02 | 9.68 ± 0.64 | 17.58 ± 0.65 |
| 17 | helicid | 8.91 ± 0.61 | 4.19 ± 0.50 | ND | ND | ND |
| 18 | esculetin | 3.47 ± 0.49 | 2.94 ± 0.47 | ND | ND | ND |
| 19 | vanillic acid | 28.97 ± 0.13 | 29.65 ± 0.44 | 5.28 ± 0.15 | 5.37 ± 0.73 | 3.55 ± 0.25 |
| 20 | p-hydroxycinnamic acid | ND | ND | 0.93 ± 0.12 | 21.54 ± 0.38 | 0.19 ± 0.03 |
| 21 | isoferulic acid | 24.25 ± 0.71 | 28.55 ± 1.24 | 8.33 ± 0.60 | 72.51 ± 2.37 | 4.57 ± 0.24 |
| 22 | hesperidin | 0.70 ± 0.08 | 1.39 ± 0.04 | ND | ND | ND |
| 23 | vanillin | ND | ND | 2.36 ± 0.19 | 3.62 ± 0.22 | 1.22 ± 0.05 |
| 24 | morin total | 0.54 ± 0.05 2200.33 ± 54.94 | ND 1688.25 ± 45.21 | ND 120.06 ± 12.68 | ND 484.15 ± 30.37 | ND 131.36 ± 6.49 |
| No. | Compounds | 80% Methanol (mg/kg DW) | Water (mg/kg DW) | Acid (mg/kg DW) | Base (mg/kg DW) | Enzyme (mg/kg DW) |
|---|---|---|---|---|---|---|
| 1 | salicylic acid | 11.68 ± 0.58 | 11.26 ± 0.95 | 0.74 ± 0.13 | 0.65 ± 0.12 | 0.36 ± 0.05 |
| 2 | gallic acid | ND | ND | ND | 2.44 ± 0.18 | ND |
| 3 | phloroglucinol | 1.24 ± 0.32 | 3.68 ± 0.24 | 1.62 ± 0.29 | 1.55 ± 0.29 | ND |
| 4 | 3,4-dihydroxybenzoic acid | 5.53 ± 0.90 | 5.00 ± 0.70 | 2.40 ± 0.28 | 0.85 ± 0.22 | ND |
| 5 | p-hydroxybenzoic acid | 22.03 ± 0.03 | 20.56 ± 1.33 | 0.33 ± 0.09 | ND | ND |
| 6 | caffeic acid | 0.15 ± 0.04 | 0.35 ± 0.12 | ND | ND | ND |
| 7 | syringic acid | 2.60 ± 0.11 | 2.42 ± 0.25 | 0.32 ± 0.02 | 0.52 ± 0.03 | ND |
| 8 | benzoic acid | 66.58 ± 3.43 | 68.94 ± 1.90 | 44.08 ± 2.95 | 183.52 ± 4.14 | 14.64 ± 0.99 |
| 9 | rutin | 1063.25 ± 2.38 | 1068.25 ± 19.60 | 2.00 ± 0.12 | 19.42 ± 0.45 | 7.59 ± 0.71 |
| 10 | p-coumaric acid | ND | ND | ND | 10.91 ± 0.41 | 0.18 ± 0.03 |
| 11 | hyperoside | 17.39 ± 0.90 | 22.32 ± 1.11 | 0.50 ± 0.01 | 2.20 ± 0.13 | ND |
| 12 | isoquercitrin | 28.34 ± 2.15 | 17.72 ± 1.07 | 0.34 ± 0.09 | 1.46 ± 0.39 | ND |
| 13 | ferulic acid | 1.75 ± 0.28 | 1.90 ± 0.13 | 2.02 ± 0.31 | 5.75 ± 0.73 | 0.91 ± 0.15 |
| 14 | kaempferol-3-o-rutinoside | 70.49 ± 4.31 | 73.20 ± 0.15 | ND | 0.87 ± 0.07 | 0.23 ± 0.03 |
| 15 | quercetin | 30.53 ± 4.00 | 3.34 ± 0.27 | ND | ND | ND |
| 16 | kaempferol | ND | ND | ND | 0.53 ± 0.12 | ND |
| 17 | quinic acid | 472.41 ± 6.21 | 298.64 ± 3.81 | 4.55 ± 0.79 | 6.70 ± 0.69 | 2.80 ± 0.59 |
| 18 | helicid | 1.84 ± 0.49 | ND | ND | ND | ND |
| 19 | esculetin | 2.65 ± 0.16 | 2.50 ± 0.38 | ND | ND | ND |
| 20 | vanillic acid | 23.30 ± 3.03 | 23.76 ± 1.15 | 4.94 ± 0.20 | 5.29 ± 0.31 | 1.62 ± 0.11 |
| 21 | p-hydroxycinnamic acid | ND | ND | ND | 10.31 ± 0.39 | 0.31 ± 0.10 |
| 22 | isoferulic acid | 17.44 ± 0.71 | 17.19 ± 1.07 | 6.95 ± 0.68 | 51.58 ± 1.00 | 5.33 ± 0.68 |
| 23 | hesperidin | ND | 0.03 ± 0.01 | ND | ND | ND |
| 24 | vanillin | 2.98 ± 0.02 | 2.86 ± 0.19 | 1.98 ± 0.07 | 3.08 ± 0.06 | ND |
| total | 1842.18 ± 30.05 | 1620.16 ± 34.43 | 72.77 ± 6.03 | 307.63 ± 9.73 | 33.97 ± 3.44 |
| No. | Compounds | 80% Methanol (mg/kg DW) | Water (mg/kg DW) | Acid (mg/kg DW) | Base (mg/kg DW) | Enzyme (mg/kg DW) |
|---|---|---|---|---|---|---|
| 1 | gallic acid | 1.22 ± 0.16 | 0.26 ± 0.08 | 2.66 ± 0.03 | 8.44 ± 0.28 | 0.85 ± 0.03 |
| 2 | phloroglucinol | 3.35 ± 0.48 | 0.71 ± 0.07 | 3.38 ± 0.63 | 7.49 ± 0.29 | 0.50 ± 0.18 |
| 3 | 3,4-dihydroxybenzoic acid | 1.19 ± 0.08 | 1.14 ± 0.29 | 1.00 ± 0.22 | 1.57 ± 0.13 | ND |
| 4 | p-hydroxybenzoic acid | 4.91 ± 0.93 | 4.29 ± 0.33 | 12.97 ± 1.17 | 14.27 ± 1.47 | 4.46 ± 0.24 |
| 5 | caffeic acid | 46.45 ± 1.20 | 32.21 ± 2.62 | ND | ND | ND |
| 6 | benzoic acid | 13.30 ± 0.90 | 14.54 ± 2.11 | 21.65 ± 2.00 | 118.21 ± 0.40 | 11.25 ± 1.82 |
| 7 | rutin | 609.72 ± 7.21 | 465.18 ± 11.40 | 0.24 ± 0.04 | 5.84 ± 0.08 | 4.40 ± 0.16 |
| 8 | p-coumaric acid | ND | ND | ND | 10.01 ± 0.13 | ND |
| 9 | hyperoside | 16.35 ± 0.70 | 11.00 ± 0.51 | 0.35 ± 0.05 | 0.59 ± 0.09 | 0.23 ± 0.02 |
| 10 | isoquercitrin | 22.69 ± 1.02 | 15.84 ± 1.81 | 0.53 ± 0.03 | 0.27 ± 0.12 | 0.37 ± 0.00 |
| 11 | ferulic acid | 0.80 ± 0.06 | 0.61 ± 0.06 | 0.72 ± 0.05 | 4.02 ± 0.14 | 0.22 ± 0.04 |
| 12 | quercetin | 25.90 ± 0.38 | 1.66 ± 0.09 | 0.77 ± 0.01 | ND | ND |
| 13 | cinnamic acid | ND | ND | 3.41 ± 0.17 | ND | ND |
| 14 | kaempferol | 0.47 ± 0.06 | ND | 1.03 ± 0.05 | ND | ND |
| 15 | quinic acid | 68.35 ± 0.42 | 91.20 ± 0.56 | 1.69 ± 0.10 | 2.08 ± 0.07 | 9.85 ± 0.50 |
| 16 | esculetin | 1.44 ± 0.06 | 1.08 ± 0.03 | 0.26 ± 0.02 | ND | 0.03 ± 0.01 |
| 17 | vanillic acid | 3.53 ± 0.01 | 2.78 ± 0.08 | 2.13 ± 0.16 | ND | 1.04 ± 0.01 |
| 18 | p-hydroxycinnamic acid | ND | ND | ND | 9.78 ± 0.25 | ND |
| 19 | isoferulic acid | 3.41 ± 0.52 | 2.93 ± 0.05 | 2.36 ± 0.04 | 23.22 ± 0.20 | 1.48 ± 0.18 |
| 20 | vanillin | 2.36 ± 0.00 | ND | 1.67 ± 0.10 | 3.01 ± 0.01 | 1.16 ± 0.00 |
| total | 825.44 ± 14.19 | 645.43 ± 20.09 | 55.82 ± 4.87 | 208.80 ± 3.66 | 35.84 ± 3.19 |
| No. | Compound | 80% Methanol (mg/kg DW) | Water (mg/kg DW) | Acid (mg/kg DW) | Base (mg/kg DW) | Enzyme (mg/kg DW) |
|---|---|---|---|---|---|---|
| 1 | salicylic acid | 12.67 ± 0.69 | 12.25 ± 0.79 | 1.83 ± 0.29 | 1.50 ± 0.17 | 0.44 ± 0.04 |
| 2 | phloroglucinol | 2.63 ± 0.10 | 1.39 ± 0.08 | 1.68 ± 0.32 | 9.53 ± 0.47 | ND |
| 3 | 3,4-dihydroxybenzoic acid | 0.40 ± 0.02 | 1.33 ± 0.04 | 1.85 ± 0.11 | 0.47 ± 0.06 | ND |
| 4 | p-hydroxybenzoic acid | 10.39 ± 0.34 | 15.50 ± 0.61 | 46.89 ± 1.07 | 1.43 ± 0.04 | ND |
| 5 | caffeic acid | ND | ND | ND | 2.67 ± 0.17 | ND |
| 6 | syringic acid | 1.77 ± 0.07 | 1.52 ± 0.10 | 0.40 ± 0.07 | 0.34 ± 0.08 | ND |
| 7 | benzoic acid | 35.56 ± 0.53 | 34.13 ± 0.68 | 50.71 ± 1.08 | 209.97 ± 2.34 | 28.48 ± 0.45 |
| 8 | rutin | 1809.83 ± 14.92 | 1076.00 ± 26.96 | 1.85 ± 0.02 | 34.31 ± 1.84 | 5.78 ± 0.50 |
| 9 | p-coumaric acid | ND | ND | 0.78 ± 0.02 | 20.38 ± 1.01 | ND |
| 10 | hyperoside | 124.55 ± 5.78 | 59.62 ± 2.05 | 1.16 ± 0.20 | 3.07 ± 0.76 | ND |
| 11 | isoquercitrin | 101.32 ± 2.27 | 46.79 ± 1.17 | 0.94 ± 0.10 | 2.04 ± 0.34 | 0.08 ± 0.01 |
| 12 | ferulic acid | 1.23 ± 0.13 | 1.64 ± 0.18 | 1.41 ± 0.15 | 8.92 ± 0.94 | 0.41 ± 0.05 |
| 13 | kaempferol-3-o-rutinoside | 153.53 ± 1.71 | 64.58 ± 1.08 | ND | 1.84 ± 0.11 | 0.32 ± 0.05 |
| 14 | kaempferol-3-o-glucoside | 2.84 ± 0.10 | 1.29 ± 0.07 | ND | ND | ND |
| 15 | quercetin | 13.55 ± 0.43 | 3.39 ± 0.33 | 1.08 ± 0.06 | ND | ND |
| 16 | cinnamic acid | ND | ND | ND | ND | 4.73 ± 0.34 |
| 17 | quinic acid | 198.72 ± 2.98 | 286.55 ± 1.51 | 12.90 ± 0.78 | 8.69 ± 0.30 | 7.75 ± 0.65 |
| 18 | helicid | 6.88 ± 0.14 | 4.79 ± 0.35 | ND | ND | ND |
| 19 | vanillic acid | 14.19 ± 0.60 | 13.56 ± 0.21 | 5.56 ± 0.17 | 2.36 ± 0.08 | 1.91 ± 0.15 |
| 20 | p-hydroxycinnamic acid | ND | ND | 0.82 ± 0.18 | 19.01 ± 0.86 | 0.15 ± 0.02 |
| 21 | isoferulic acid | 13.43 ± 0.39 | 11.58 ± 0.64 | 4.89 ± 0.72 | 51.03 ± 0.82 | 5.13 ± 0.37 |
| 22 | vanillin | 2.59 ± 0.07 | 2.62 ± 0.07 | 3.79 ± 0.41 | 3.77 ± 0.09 | 1.21 ± 0.05 |
| 23 | morin total | 0.48 ± 0.02 2506.56 ± 31.29 | ND 1638.53 ± 36.92 | ND 138.54 ± 5.75 | ND 390.25 ± 11.48 | ND 56.39 ± 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chai, J.; Deng, S.; Xu, J.; Feng, Y.; Yang, R.; Li, W. The Comparison of the Profile of Phenolic Compounds in Noni (Morinda citrifolia L.) Fruit by Different Drying Methods. Foods 2025, 14, 1398. https://doi.org/10.3390/foods14081398
Li Q, Chai J, Deng S, Xu J, Feng Y, Yang R, Li W. The Comparison of the Profile of Phenolic Compounds in Noni (Morinda citrifolia L.) Fruit by Different Drying Methods. Foods. 2025; 14(8):1398. https://doi.org/10.3390/foods14081398
Chicago/Turabian StyleLi, Qianxin, Juan Chai, Shenghui Deng, Jucai Xu, Yanxian Feng, Ruili Yang, and Wu Li. 2025. "The Comparison of the Profile of Phenolic Compounds in Noni (Morinda citrifolia L.) Fruit by Different Drying Methods" Foods 14, no. 8: 1398. https://doi.org/10.3390/foods14081398
APA StyleLi, Q., Chai, J., Deng, S., Xu, J., Feng, Y., Yang, R., & Li, W. (2025). The Comparison of the Profile of Phenolic Compounds in Noni (Morinda citrifolia L.) Fruit by Different Drying Methods. Foods, 14(8), 1398. https://doi.org/10.3390/foods14081398






