Mapping the Quantitative Dose–Response Relationships Between Nutrients and Health Outcomes to Inform Food Risk–Benefit Assessment
Abstract
1. Introduction
- What are the established dose–response relationships between selected nutrients and various health outcomes, as documented in the current scientific literature?
- What are the direction and magnitude of these dose–response relationships, specifically whether they are protective or harmful and to what extent?
- Are there complexities identified within these dose–response relationships, such as nonlinear patterns, threshold effects, or variations contingent upon the nutrient source or dietary context?
- What are the principal data gaps and areas requiring further investigation to enhance the understanding of nutrient dose–response relationships for risk–benefit assessment?
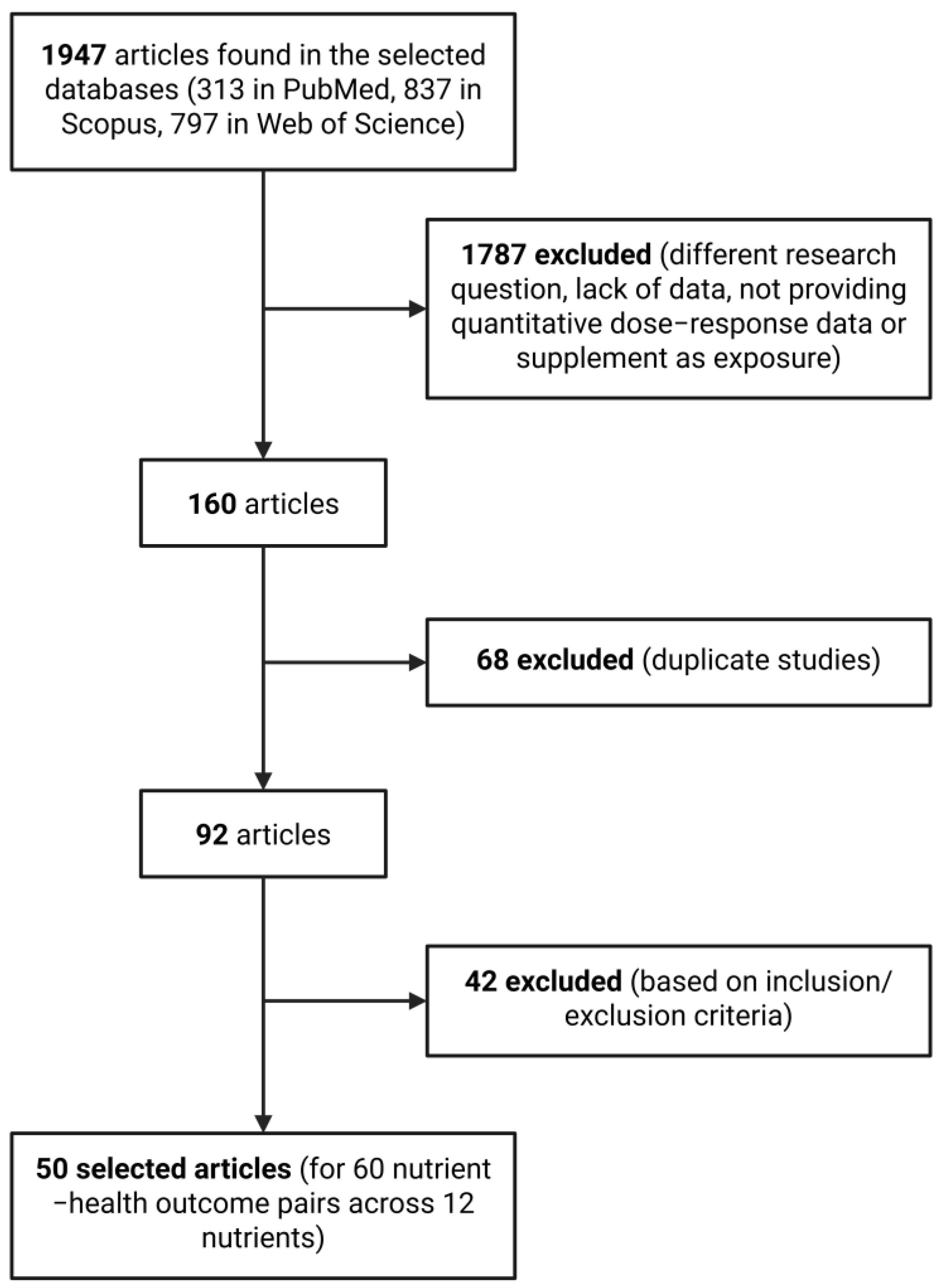
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
3. Results and Discussion
3.1. Calcium
3.2. Iron
3.3. Zinc
3.4. Magnesium
3.5. Selenium
3.6. Sodium
3.7. Vitamin B12
3.8. Vitamin D3
3.9. Fibre
3.10. Saturated Fatty Acids
3.11. n-3 Fatty Acids
3.12. n-6 Fatty Acids
3.13. Nutrients Not Included
3.14. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | Alpha-linolenic acid |
| CHD | Coronary heart disease |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular disease |
| LA | Linoleic acid |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
| RBA | Risk–benefit assessment |
| RCT | Randomised controlled trial |
| SFA | Saturated fatty acid |
| T2DM | Type 2 diabetes |
References
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A Systematic Review on Consumer Acceptance of Alternative Proteins: Pulses, Algae, Insects, Plant-Based Meat Alternatives, and Cultured Meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef] [PubMed]
- Huis, A. van Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Alvito, P.; Brazão, R.; Carmona, P.; Fernandes, P.; Jakobsen, L.S.; Lopes, C.; Martins, C.; Membré, J.-M.; Monteiro, S.; et al. Building Capacity in Risk-Benefit Assessment of Foods: Lessons Learned from the RB4EU Project. Trends Food Sci. Technol. 2019, 91, 541–548. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Ververis, E.; Ackerl, R.; Azzollini, D.; Colombo, P.A.; de Sesmaisons, A.; Dumas, C.; Fernandez-Dumont, A.; Ferreira da Costa, L.; Germini, A.; Goumperis, T.; et al. Novel Foods in the European Union: Scientific Requirements and Challenges of the Risk Assessment Process by the European Food Safety Authority. Food Res. Int. 2020, 137, 109515. [Google Scholar] [CrossRef] [PubMed]
- Boué, G.; Ververis, E.; Niforou, A.; Federighi, M.; Pires, S.M.; Poulsen, M.; Thomsen, S.T.; Naska, A. Risk-Benefit Assessment of Foods: Development of a Methodological Framework for the Harmonized Selection of Nutritional, Microbiological, and Toxicological Components. Front. Nutr. 2022, 9, 951369. [Google Scholar] [CrossRef]
- Boué, G.; Guillou, S.; Antignac, J.-P.; Bizec, B.L.; Membré, J.-M. Public Health Risk-Benefit Assessment Associated with Food Consumption–A Review. Eur. J. Nutr. Food Saf. 2015, 5, 32–58. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.J.; Benford, D.; Hougaard Bennekou, S.; Bampidis, V.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on Risk-Benefit Assessment of Foods. EFSA J. 2024, 22, e8875. [Google Scholar] [CrossRef]
- Naska, A.; Ververis, E.; Niforou, A.; Pires, S.M.; Poulsen, M.; Jakobsen, L.S.; Becker, N.; Lohmann, M.; Tesson, V.; Federighi, M.; et al. Novel Foods as Red Meat Replacers—An Insight Using Risk Benefit Assessment Methods (the NovRBA Project). EFSA Support. Publ. 2022, 19, 7316E. [Google Scholar] [CrossRef]
- Ververis, E.; Niforou, A.; Poulsen, M.; Pires, S.M.; Federighi, M.; Samoli, E.; Naska, A.; Boué, G. Substituting Red Meat with Insects in Burgers: Estimating the Public Health Impact Using Risk-Benefit Assessment. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2024, 189, 114764. [Google Scholar] [CrossRef]
- Nauta, M.J.; Andersen, R.; Pilegaard, K.; Pires, S.M.; Ravn-Haren, G.; Tetens, I.; Poulsen, M. Meeting the Challenges in the Development of Risk-Benefit Assessment of Foods. Trends Food Sci. Technol. 2018, 76, 90–100. [Google Scholar] [CrossRef]
- Matteu Monteiro, C.D.; Membré, J.-M.; Poulsen, M.; Thomsen, S.T.; Pires, S.M. Risk–Benefit Assessment of Foods and Its Role to Inform Policy Decisions: Outcome of an International Workshop. Front. Nutr. 2024, 11, 1458531. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A New Tool to Assess Risk of Bias in Systematic Reviews Was Developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Rosenblatt, D.A.N.; Chan, D.S.; Vieira, A.R.; Vieira, R.; Greenwood, D.C.; Vatten, L.J.; Norat, T. Dairy Products, Calcium, and Prostate Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies. Am. J. Clin. Nutr. 2015, 101, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, Y.; Xin, X.; Wang, W.; Zhang, D. Dietary Fiber Intake Is Associated with a Reduced Risk of Ovarian Cancer: A Dose-Response Meta-Analysis. Nutr. Res. 2018, 57, 1–11. [Google Scholar] [CrossRef]
- Yang, W.; Li, B.; Dong, X.; Zhang, X.-Q.; Zeng, Y.; Zhou, J.-L.; Tang, Y.-H.; Xu, J.-J. Is Heme Iron Intake Associated with Risk of Coronary Heart Disease? A Meta-Analysis of Prospective Studies. Eur. J. Nutr. 2014, 53, 395–400. [Google Scholar] [CrossRef]
- Qiao, L.; Feng, Y. Intakes of Heme Iron and Zinc and Colorectal Cancer Incidence: A Meta-Analysis of Prospective Studies. Cancer Causes Control 2013, 24, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Pang, Z.; Liu, Q.-F. Magnesium Intake and Risk of Colorectal Cancer: A Meta-Analysis of Prospective Studies. Eur. J. Clin. Nutr. 2012, 66, 1182–1186. [Google Scholar] [CrossRef]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary Fat Intake and Risk of Alzheimer’s Disease and Dementia: A Meta-Analysis of Cohort Studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A Systematic Review and Dose-Response Meta-Analysis of Exposure to Environmental Selenium and the Risk of Type 2 Diabetes in Nonexperimental Studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef]
- Ghoreishy, S.M.; Bagheri, A.; Nejad, M.M.; Larijani, B.; Esmaillzadeh, A. Association between Calcium Intake and Risk of Breast Cancer: An Updated Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. Clin. Nutr. ESPEN 2023, 55, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-M.; Bu, X.-X.; Zhou, B.; Li, Y.-F.; Nie, Z.-L. Dietary Calcium Intake and the Risk of Stroke: Meta-Analysis of Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 934–946. [Google Scholar] [CrossRef]
- Guo, X.; Piao, H. A Meta-Analysis of Calcium Intake and Risk of Glioma. Nutr. Cancer 2022, 74, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Rouhani, P.; Saneei, P. Dietary Calcium Intake in Relation to Type-2 Diabetes and Hyperglycemia in Adults: A Systematic Review and Dose-Response Meta-Analysis of Epidemiologic Studies. Sci. Rep. 2022, 12, 1050. [Google Scholar] [CrossRef]
- Huang, D.; Lei, S.; Wu, Y.; Weng, M.; Zhou, Y.; Xu, J.; Xia, D.; Xu, E.; Lai, M.; Zhang, H. Additively Protective Effects of Vitamin D and Calcium against Colorectal Adenoma Incidence, Malignant Transformation and Progression: A Systematic Review and Meta-Analysis. Clin. Nutr. 2020, 39, 2525–2538. [Google Scholar] [CrossRef]
- Jayedi, A.; Zargar, M.S. Dietary Calcium Intake and Hypertension Risk: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Clin. Nutr. 2019, 73, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, H.; Jayedi, A.; Shab-Bidar, S. Dietary Iron Intake and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2022, 61, 2279–2296. [Google Scholar] [CrossRef]
- Chang, V.C.; Cotterchio, M.; Khoo, E. Iron Intake, Body Iron Status, and Risk of Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 543. [Google Scholar] [CrossRef]
- Ma, J.; Li, Q.; Fang, X.; Chen, L.; Qiang, Y.; Wang, J.; Wang, Q.; Min, J.; Zhang, S.; Wang, F. Increased Total Iron and Zinc Intake and Lower Heme Iron Intake Reduce the Risk of Esophageal Cancer: A Dose-Response Meta-Analysis. Nutr. Res. 2018, 59, 16–28. [Google Scholar] [CrossRef]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary Intake of Heme Iron and Risk of Cardiovascular Disease: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35. [Google Scholar] [CrossRef]
- Talebi, S.; Ghoreishy, S.M.; Jayedi, A.; Travica, N.; Mohammadi, H. Dietary Antioxidants and Risk of Parkinson’s Disease: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2022, 13, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Shirani, F.; Askari, G. Dietary Magnesium Intake in Relation to Depression in Adults: A GRADE-Assessed Systematic Review and Dose-Response Meta-Analysis of Epidemiologic Studies. Nutr. Rev. 2025, 83, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zeng, L.; Zhao, J.; Wu, Q.; Dong, Y.; Zou, F.; Gan, L.; Wei, Y.; Zhang, W. Association of Magnesium Intake with Type 2 Diabetes and Total Stroke: An Updated Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e032240. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, M.; Yang, L.; Xu, H.; Song, W.; Qian, Y.; Zhao, M. Quantitative Association Between Serum/Dietary Magnesium and Cardiovascular Disease/Coronary Heart Disease Risk: A Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Cardiovasc. Pharmacol. 2019, 74, 516–527. [Google Scholar] [CrossRef]
- Han, H.; Fang, X.; Wei, X.; Liu, Y.; Jin, Z.; Chen, Q.; Fan, Z.; Aaseth, J.; Hiyoshi, A.; He, J.; et al. Dose-Response Relationship between Dietary Magnesium Intake, Serum Magnesium Concentration and Risk of Hypertension: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutr. J. 2017, 16, 26. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary Magnesium Intake and the Risk of Cardiovascular Disease, Type 2 Diabetes, and All-Cause Mortality: A Dose-Response Meta-Analysis of Prospective Cohort Studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Zhao, D.; Li, H.M.; Li, C.X.; Zhou, B. 24-Hour Urinary Sodium Excretion Association with Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Biomed. Environ. Sci. 2022, 35, 921–930. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Vinceti, M. Sodium Intake and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Observational Cohort Studies. Curr. Hypertens. Rep. 2022, 24, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Ghomashi, F.; Zargar, M.S.; Shab-Bidar, S. Dietary Sodium, Sodium-to-Potassium Ratio, and Risk of Stroke: A Systematic Review and Nonlinear Dose-Response Meta-Analysis. Clin. Nutr. 2019, 38, 1092–1100. [Google Scholar] [CrossRef]
- Fang, X.; Wei, J.; He, X.; An, P.; Wang, H.; Jiang, L.; Shao, D.; Liang, H.; Li, Y.; Wang, F.; et al. Landscape of Dietary Factors Associated with Risk of Gastric Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 2820–2832. [Google Scholar] [CrossRef]
- Qiang, Y.; Li, Q.; Xin, Y.; Fang, X.; Tian, Y.; Ma, J.; Wang, J.; Wang, Q.; Zhang, R.; Wang, J.; et al. Intake of Dietary One-Carbon Metabolism-Related B Vitamins and the Risk of Esophageal Cancer: A Dose-Response Meta-Analysis. Nutrients 2018, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-H.; Huang, X.-Z.; Wang, S.-B.; Li, Y.; Wang, L.-Y.; Wang, H.-C.; Zhang, C.-W.; Zhang, C.; Liu, H.-P.; Wang, Z.-N. A Dose-Response Meta-Analysis Reveals an Association between Vitamin B12 and Colorectal Cancer Risk. Public Health Nutr. 2016, 19, 1446–1456. [Google Scholar] [CrossRef]
- Shi, H.; Chen, H.; Zhang, Y.; Li, J.; Fu, K.; Xue, W.; Teng, W.; Tian, L. 25-Hydroxyvitamin D Level, Vitamin D Intake, and Risk of Stroke: A Dose-Response Meta-Analysis. Clin. Nutr. 2020, 39, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Jing, H.; Wei, Q.; Wei, G.; Heng, Z. Associations of the Risk of Lung Cancer with Serum 25-Hydroxyvitamin D Level and Dietary Vitamin D Intake: A Dose-Response PRISMA Meta-Analysis. Medicine 2018, 97, e12282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin Intake and Pancreatic Cancer Risk Reduction: A Meta-Analysis of Observational Studies. Medicine 2018, 97, e0114. [Google Scholar] [CrossRef]
- Watling, C.Z.; Wojt, A.; Florio, A.A.; Butera, G.; Albanes, D.; Weinstein, S.J.; Huang, W.-Y.; Parisi, D.; Zhang, X.; Graubard, B.I.; et al. Fiber and Whole Grain Intakes in Relation to Liver Cancer Risk: An Analysis in 2 Prospective Cohorts and Systematic Review and Meta-Analysis of Prospective Studies. Hepatology 2024, 80, 552–565. [Google Scholar] [CrossRef]
- Valisoltani, N.; Ghoreishy, S.M.; Imani, H.; Rajabi Harsini, A.; Jowshan, M.; Travica, N.; Mohammadi, H. Fiber Intake and Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Dose Response Meta-Analysis. Food Sci. Nutr. 2023, 11, 6775–6788. [Google Scholar] [CrossRef]
- Saghafian, F.; Hajishafiee, M.; Rouhani, P.; Saneei, P. Dietary Fiber Intake, Depression, and Anxiety: A Systematic Review and Meta-Analysis of Epidemiologic Studies. Nutr. Neurosci. 2023, 26, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, X.; Sun, C.; Lowe, S.; Su, W.; Song, Q.; Wang, H.; Liang, Q.; Liang, M.; Ding, X.; et al. Dietary Carbohydrate Intake Is Associated with a Lower Risk of Breast Cancer: A Meta-Analysis of Cohort Studies. Nutr. Res. 2022, 100, 70–92. [Google Scholar] [CrossRef]
- Yu, E.Y.W.; Wesselius, A.; Mehrkanoon, S.; Brinkman, M.; Van Den Brandt, P.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; Gunter, M.; Huybrechts, I.; et al. Grain and Dietary Fiber Intake and Bladder Cancer Risk: A Pooled Analysis of Prospective Cohort Studies. Am. J. Clin. Nutr. 2020, 112, 1252–1266. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Norat, T.; Riboli, E. Dietary Fibre Intake and the Risk of Diverticular Disease: A Systematic Review and Meta-Analysis of Prospective Studies. Eur. J. Nutr. 2020, 59, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Ebrahimi-Daryani, N.; Dieleman, L.A.; Larijani, B.; Esmaillzadeh, A. Association of Dietary Fiber, Fruit, and Vegetable Consumption with Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Lin, Y.-W.; Chen, H.; Qin, J.; Zheng, X.-Y.; Xu, X.; Xie, L.-P. Dietary Fiber Intake Is Inversely Associated with Risk of Pancreatic Cancer: A Meta-Analysis. Asia Pac. J. Clin. Nutr. 2017, 26, 89–96. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xu, J.; Xu, G.; Liu, X. Dietary Fiber Intake Reduces Risk for Barrett’s Esophagus and Esophageal Cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 2749–2757. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.; Ma, M.; Yang, J.; Liu, X. Dietary Fiber Intake Reduces Risk for Gastric Cancer: A Meta-Analysis. Gastroenterology 2013, 145, 113–120.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Deng, C.; Lin, Z.; Giovannucci, E.; Zhang, X. Dietary Fats, Serum Cholesterol and Liver Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Cancers 2021, 13, 1580. [Google Scholar] [CrossRef]
- Kang, Z.-Q.; Yang, Y.; Xiao, B. Dietary Saturated Fat Intake and Risk of Stroke: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 179–189. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in Saturated Fat Intake for Cardiovascular Disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef]
- Zhao, J.; Lyu, C.; Gao, J.; Du, L.; Shan, B.; Zhang, H.; Wang, H.-Y.; Gao, Y. Dietary Fat Intake and Endometrial Cancer Risk: A Dose Response Meta-Analysis. Medicine 2016, 95, e4121. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hou, R.; Xi, Y.; Kowalski, A.; Wang, T.; Yu, Z.; Hu, Y.; Chandrasekar, E.K.; Sun, H.; Ali, M.K. The Association and Dose-Response Relationship between Dietary Intake of α-Linolenic Acid and Risk of CHD: A Systematic Review and Meta-Analysis of Cohort Studies. Br. J. Nutr. 2018, 119, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Jalilpiran, Y.; Karimi, E.; Aune, D.; Larijani, B.; Mozaffarian, D.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Diabetes Care 2021, 44, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. High Plasma Vitamin B12 and Cancer in Human Studies: A Scoping Review to Judge Causality and Alternative Explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef]
- Oh, H.; Kim, H.; Lee, D.H.; Lee, A.; Giovannucci, E.L.; Kang, S.-S.; Keum, N. Different Dietary Fibre Sources and Risks of Colorectal Cancer and Adenoma: A Dose-Response Meta-Analysis of Prospective Studies. Br. J. Nutr. 2019, 122, 605–615. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Emadi, A.; Ghods, K.; Shab-Bidar, S. Dietary Intake, Biomarkers and Supplementation of Fatty Acids and Risk of Coronary Events: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials and Prospective Observational Studies. Crit. Rev. Food Sci. Nutr. 2024, 64, 12363–12382. [Google Scholar] [CrossRef]
- Mahjourian, M.; Anjom-Shoae, J.; Mohammadi, M.A.; Feinle-Bisset, C.; Sadeghi, O. Associations of Dietary Fat Types (MUFA, PUFA, SFA) and Sources (Animal, Plant) with Colorectal Cancer Risk: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Cancer Epidemiol. 2025, 95, 102768. [Google Scholar] [CrossRef]
- Fu, Y.-Q.; Zheng, J.-S.; Yang, B.; Li, D. Effect of Individual Omega-3 Fatty Acids on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Epidemiol. 2015, 25, 261–274. [Google Scholar] [CrossRef]
- Stover, P.J.; Garza, C.; Durga, J.; Field, M.S. Emerging Concepts in Nutrient Needs. J. Nutr. 2020, 150, 2593S–2601S. [Google Scholar] [CrossRef]
- Niforou, A.; Konstantinidou, V.; Naska, A. Genetic Variants Shaping Inter-Individual Differences in Response to Dietary Intakes-A Narrative Review of the Case of Vitamins. Front. Nutr. 2020, 7, 558598. [Google Scholar] [CrossRef]
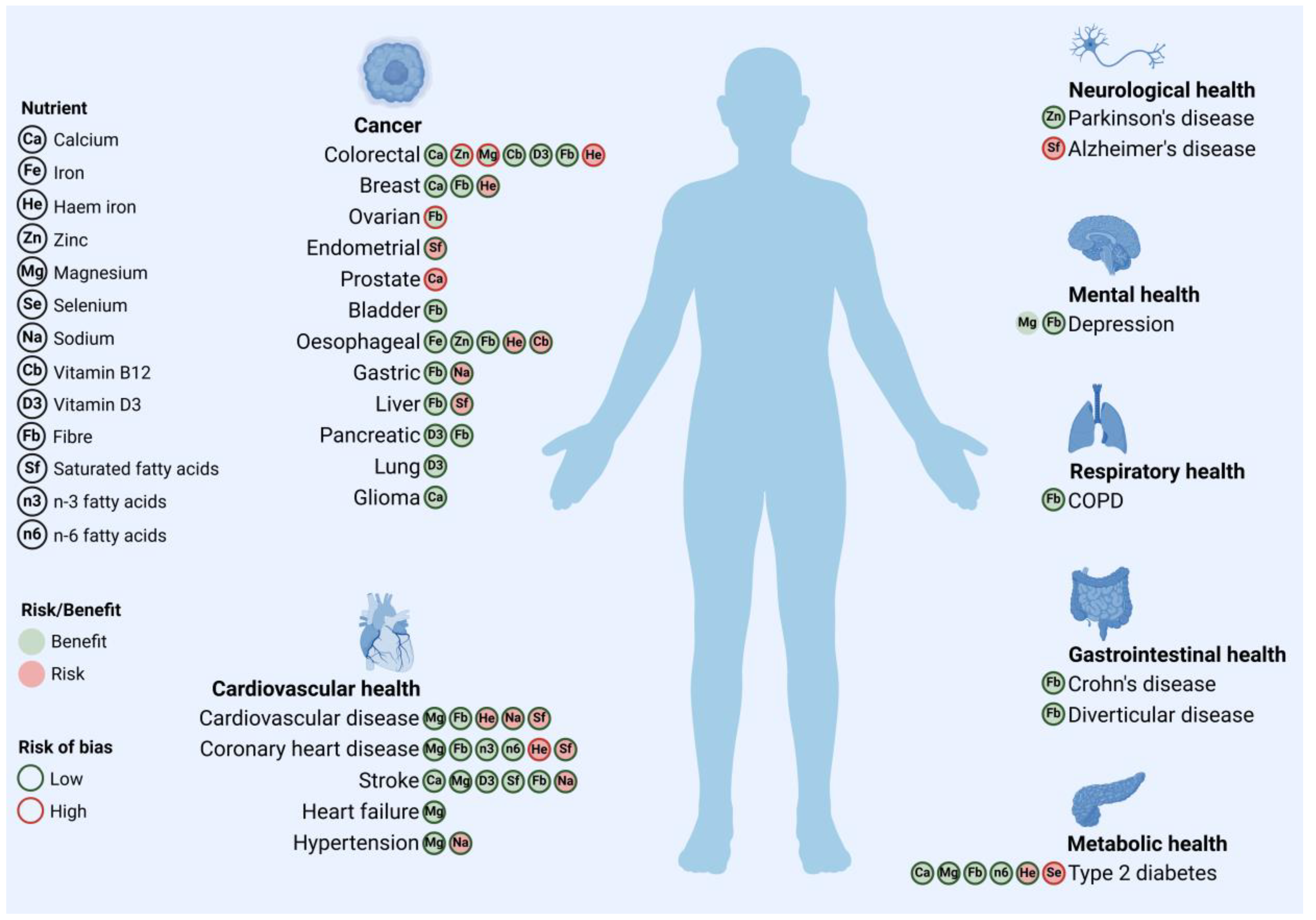
| Component | Health Outcome (Risk) | Source | Risk of Bias |
|---|---|---|---|
| Calcium | Breast cancer (↓) | [21] | Low |
| Stroke (↓) | [22] | Low | |
| Glioma (↓) | [23] | Low | |
| T2DM (↓) | [24] | Low | |
| Colorectal cancer (↓) | [25] | Low | |
| Hypertension (↓) | [26] | Low | |
| Prostate cancer (↑) | [14] | High | |
| Iron | T2DM (haem) (↑) | [27] | Low |
| Breast cancer (haem) (↑) | [28] | Low | |
| Oesophageal cancer (total) (↓); (haem) (↑) | [29] | Low | |
| CVD (haem) (↑) | [30] | Low | |
| CHD (haem) (↑) | [16] | High | |
| Colorectal cancer (haem) (↑) | [17] | High | |
| Zinc | Parkinson’s disease (↓) | [31] | Low |
| Oesophageal cancer (↓) | [29] | Low | |
| Colorectal cancer (↓) | [17] | High | |
| Magnesium | Depression (↓) | [32] | n.a. * |
| T2DM (↓) | [33] | Low | |
| Stroke (↓) | [33] | Low | |
| CVD (↓) | [34] | Low | |
| CHD (↓) | [34] | Low | |
| Hypertension (↓) | [35] | Low | |
| Heart failure (↓) | [36] | Low | |
| Colorectal cancer (↓) | [18] | High | |
| Selenium | T2DM (↑) | [20] | High |
| Sodium | CVD (↑) | [37] | Low |
| Hypertension (↑) | [38] | Low | |
| Stroke (↑) | [39] | Low | |
| Gastric cancer (↑) | [40] | Low | |
| Vitamin B12 | Oesophageal cancer (↑) | [41] | Low |
| Colorectal cancer (↓) | [42] | Low | |
| Vitamin D3 | Colorectal cancer (↓) | [25] | Low |
| Stroke (↓) | [43] | Low | |
| Lung cancer (↓) | [44] | Low | |
| Pancreatic cancer (↓) | [45] | Low | |
| Fibre | Liver cancer (↓) | [46] | Low |
| COPD (↓) | [47] | Low | |
| Depression (↓) | [48] | Low | |
| Breast cancer (↓) | [49] | Low | |
| Bladder cancer (↓) | [50] | Low | |
| T2DM (↓) | [51] | Low | |
| Colorectal cancer (↓) | [51] | Low | |
| Diverticular disease (↓) | [52] | Low | |
| CVD (↓) | [51] | Low | |
| CHD (↓) | [51] | Low | |
| Stroke (↓) | [51] | Low | |
| Ovarian cancer (↓) | [15] | High | |
| Crohn’s disease (↓) | [53] | Low | |
| Pancreatic cancer (↓) | [54] | Low | |
| Oesophageal cancer (↓) | [55] | Low | |
| Gastric cancer (↓) | [56] | Low | |
| Saturated fatty acids | Liver cancer (↑) | [57] | Low |
| Stroke (↓) | [58] | Low | |
| CVD (↑) | [59] | Low | |
| Alzheimer’s disease (↑) | [19] | High | |
| Endometrial cancer (↑) | [60] | Low | |
| CHD (↑) | [61] | Low | |
| n-3 fatty acids | CHD (ALA) (↓) | [62] | Low |
| n-6 fatty acids | T2DM (↓) | [63] | Low |
| CHD (↓) | [61] | Low |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [21] | Breast cancer | Meta-analysis (7 cohort studies, n = 1,579,904) | Per 350 mg/day increase: BC risk ↓ 6% (RR: 0.94, 0.89–0.99). |
| [22] | Stroke * | Meta-analysis (18 cohort studies, n = 882,181) | Per 200 mg/day increase: stroke risk ↓ 5% (RR: 0.95, 0.92–0.98). Per 300 mg/day increase: stroke risk ↓ 6% (RR: 0.94, 0.90–0.98). Per 500 mg/day increase: stroke risk ↓ 5% (RR: 0.95, 0.90–0.99). |
| [23] | Glioma | Meta-analysis (4 case–control studies, n = 1942) | Per 100 mg/day increase: glioma risk ↓ 7% (OR: 0.93, 0.88–0.98). |
| [24] | T2DM | Meta-analysis (8 cohort studies, n = 255,744) | Per 300 mg/day increase: T2DM risk ↓ 7% (RR: 0.93, 0.89–0.98). Per 600 mg/day increase: T2DM risk ↓ 14% (RR: 0.87, 0.79–0.97). Per 1000 mg/day increase: T2DM risk ↓ 23% (RR: 0.80, 0.67–0.95). |
| [25] | Colorectal cancer | Meta-analysis (148 cohort studies, 18 RCTs, n = 854,195) | Per 400 mg/day increase: CRC risk ↓ 5% (RR: 0.95, 0.94–0.96). |
| [26] | Hypertension | Meta-analysis (8 cohort studies, n = 248,398) | Per 500 mg/day increase: hypertension risk ↓ 7% (RR: 0.93, 0.90–0.97). |
| [14] | Prostate cancer ** | Meta-analysis (9 cohort studies, n = 750,275) | Per 400 mg/day increase: prostate cancer risk ↑ 5% (RR: 1.05, 1.02–1.09). |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [27] | T2DM * | Meta-analysis (11 cohort studies, n = 323,788) | Per 1 mg/day (haem) increase: T2DM risk ↑ 16% (RR: 1.16, 1.03–1.30). |
| [28] | Breast cancer * | Meta-analysis (23 observational studies) | Per 1 mg/day (haem) increase: BC risk ↑ 8% (RR: 1.08, 1.002–1.17). |
| [29] | Oesophageal cancer | Meta-analysis (20 observational studies, n = 1,387,482) | Per 5 mg/day increase: OC risk ↓ 15% (OR: 0.85, 0.79–0.92). Per 1 mg/day (haem) increase: OC risk ↑ 21% (OR: 1.21, 1.02–1.45). |
| [30] | CVD * | Meta-analysis (13 observational studies, n = 252,164) | Per 1 mg/day (haem) increase: CVD risk ↑ 7% (RR: 1.07, 1.01–1.14). |
| [16] | CHD * | Meta-analysis (6 cohort studies, n = 131,553) | Per 1 mg/day (haem) increase: CHD risk ↑ 27% (RR: 1.27, 1.10–1.47). |
| [17] | Colorectal cancer * | Meta-analysis (8 cohort studies, n = 651,272) | Per 1 mg/day (haem) increase: CRC risk ↑ 11% (RR: 1.11, 1.03–1.18). |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [31] | Parkinson’s disease | Meta-analysis (6 case–control studies, 7 cohort studies, n = 467,958) | Per 1 mg/day increase: PD risk ↓ 35% (OR: 0.65, 0.49–0.86). |
| [29] | Oesophageal cancer | Meta-analysis (20 observational studies, n = 1,387,482) | Per 5 mg/day increase: OC risk ↓ 15% (OR: 0.85, 0.77–0.93). |
| [17] | Colorectal cancer | Meta-analysis (6 cohort studies, n = 350,507) | Per 5 mg/day increase: CRC risk ↓ 14% (RR: 0.86, 0.78–0.96). |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [32] | Depression | Meta-analysis (10 cross-sectional studies, 3 cohort studies, n = 63,214) | Per 100 mg/day increase: depression risk ↓ 7% (RR: 0.93, 0.90–0.96). |
| [33] | T2DM | Meta-analysis (35 cohort studies, n = 1,219,636) | Per <50 mg/day increase: T2D risk ↓ 10% (RR: 0.90, 0.88–0.93). Per ≥50 to <100 mg/day increase: T2D risk ↓ 16% (RR: 0.84, 0.82–0.87). Per ≥100 to <150 mg/day increase: T2D risk ↓ 22% (RR: 0.78, 0.74–0.83). Per ≥150 mg/day increase: T2D risk ↓ 21% (RR: 0.79, 0.74–0.84). |
| [33] | Stroke | Meta-analysis (18 cohort studies, n = 692,998) | Per ≥150 mg/day increase: total stroke risk ↓ 15% (RR: 0.85, 0.79–0.91). |
| [34] | CVD | Meta-analysis (18 cohort studies, n = 544,581) | Per 100 mg/day increase: CVD risk ↓ 10% (RR: 0.90, 0.83–0.96). |
| [34] | CHD | Meta-analysis (18 cohort studies, n = 544,581) | Per 100 mg/day increase: CHD risk ↓ 8% (RR: 0.92, 0.82–0.98). |
| [35] | Hypertension | Meta-analysis (10 cohort studies, n = 180,566) | Per 100 mg/day increase: hypertension risk ↓ 5% (RR: 0.95, 0.90–1.00). |
| [36] | Heart Failure | Meta-analysis (40 cohort studies, n > 1,000,000) | Per 100 mg/day increase: heart failure risk ↓ 22% (RR: 0.78, 0.69–0.89). |
| [18] | Colorectal cancer | Meta-analysis (8 cohort studies, n = 338,979) | Per 50 mg/day increase: CRC risk ↓ 5% (RR: 0.95, 0.89–1.00); colon cancer risk ↓ 7% (RR: 0.93, 0.88–0.99). |
| Source | Health Outcome | Study | Results |
|---|---|---|---|
| [20] | T2DM | Meta-analysis (7 case–control studies, 9 cohort studies, 18 cross-sectional studies) | Compared to 55 μg/day selenium intake: At 80 μg/day, T2DM risk ↑ 23% (RR: 1.23, 1.14–1.33). At 120 μg/day, T2DM risk ↑ 55% (RR: 1.55, 1.27–1.90). |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [37] | CVD | Meta-analysis (9 observational studies, n = 645,006) | Per 1 g/day increase: CVD risk ↑ up to 4% (RR: 1.04; 1.01, 1.07). |
| [38] | Hypertension | Meta-analysis (11 cohort studies) | Per 4 g/day increase (vs. 2 g/day): hypertension risk ↑ 4% (RR: 1.04, 0.96–1.13). Per 6 g/day increase (vs. 2 g/day): hypertension risk ↑ 21% (RR: 1.21, 1.06–1.37). |
| [39] | Stroke | Meta-analysis (14 cohort studies, 1 case–cohort study, 1 case–control study, n = 261,732) | Per 1 g/day increase: stroke risk ↑ 6% (RR: 1.06, 1.02–1.10). |
| [40] | Gastric cancer | Meta-analysis (76 cohort studies, n = 6,316,385) | Per 5 g/day increase: gastric cancer risk ↑ 12% (RR: 1.12, 95% CI: 1.02 to 1.23). |
| Source | Health Outcome | Study | Results |
|---|---|---|---|
| [41] | Oesophageal cancer | Meta-analysis (26 observational studies, n = 510,954) | Per 1 µg/day increase: OC risk ↑ 2% (OR: 1.02 (1.00–1.03). |
| [42] | Colorectal cancer | Meta-analysis (17 observational studies, n = 10,601) | Per 4.5 µg/day increase: CRC risk ↓ 8.6% (RR: 0.914, 0.856–0.977). |
| Source | Health Outcome | Study | Results |
|---|---|---|---|
| [25] | Colorectal cancer | Meta-analysis (148 observational studies, 18 RCTs) | Per 200 IU/day increase: CRC risk ↓ 5% (RR: 0.95, 0.92–0.98). |
| [43] | Stroke | Meta-analysis (20 cohort studies, n = 217,235) | High vs. low vitamin D intake: stroke risk ↓ 25% (RR: 0.75, 0.57–0.98). Optimal intake: 12 µg/day for max 20% reduction. |
| [44] | Lung cancer | Meta-analysis (5 case–control studies, 11 cohort studies, n = 280,127) | Per 100 IU/day increase: lung cancer risk ↓ 2.4% (RR: 0.976, 0.957–0.995). |
| [45] | Pancreatic cancer | Meta-analysis (14 case–control studies, 9 cohort studies, 2 RCTs, n = 1,213,821) | Per 10μg/day intake: pancreatic cancer risk ↓ 25% (RR: 0.75, 0.60–0.93). |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [46] | Liver cancer | Meta-analysis (7 cohort studies, n = 137,481) | Per 10 g/day increase: liver cancer risk ↓ 17% (HR: 0.83, 0.76–0.91). |
| [47] | COPD | Meta-analysis (5 cohort studies, n = 213,912) | Per 10 g/day increase (total dietary fibre): COPD risk ↓ 26% (RR: 0.74, 0.67–0.82). Per 10 g/day increase (cereal fibre): COPD risk ↓ 21% (RR: 0.79, 0.74–0.84). Per 10 g/day increase (fruit fibre): COPD risk ↓ 37% (RR: 0.63, 0.53–0.75). |
| [48] | Depression | Meta-analysis (12 cross-sectional studies, 5 cohort studies, 1 case–control study) | Per 5 g/day increase: depression risk ↓ 5% (OR: 0.95, 0.94–0.97). |
| [49] | Breast cancer | Meta-analysis (51 cohort studies, n = 2,725,657) | Per 10 g/day increase: BC risk ↓ 3%; premenopausal BC risk ↓ 14%. |
| [50] | Bladder cancer | Meta-analysis (13 cohort studies, n = 574,726) | Per 5 g/day increase: bladder cancer risk ↓ 4% (HR: 0.96, 0.94–0.98). |
| [52] | Diverticular disease | Meta-analysis (5 cohort studies, n = 865,829) | Per 10 g/day increase: diverticular disease risk ↓ 26% (RR: 0.74, 0.71–0.78). |
| [51] | T2DM | Meta-analysis (185 cohort studies, 58 RCTs, n = 865,829) | Per 8 g/day increase: T2DM risk ↓ 15% (HR: 0.85, 0.82–0.89). |
| [51] | CVD | Meta-analysis (185 cohort studies, 58 RCTs, n = 865,829) | Per 8 g/day increase: CVD risk ↓ 22% (HR: 0.78, 0.68–0.90). |
| [51] | CHD | Meta-analysis (185 cohort studies, 58 RCTs, n = 865,829) | Per 8 g/day increase: CHD risk ↓ 19% (HR: 0.81, 0.73–0.90). |
| [51] | Stroke | Meta-analysis (185 cohort studies, 58 RCTs, n = 865,829) | Per 8 g/day increase: stroke risk ↓ 10% (HR: 0.90, 0.85–0.95). |
| [51] | Colorectal cancer | Meta-analysis (185 cohort studies, 58 RCTs, n = 865,829) | Per 8 g/day increase: CRC risk ↓ 8% (HR: 0.92, 0.89–0.95). |
| [15] | Ovarian cancer | Meta-analysis (14 case–control studies, 5 cohort studies, n = 567,742) | Per 5 g/day increase: ovarian cancer risk ↓ 3% (RR: 0.97, 0.95–0.99). |
| [54] | Pancreatic cancer | Meta-analysis (13 case–control studies, 1 cohort study) | Per 10 g/day increase: pancreatic cancer risk ↓ 12% (OR: 0.88, 0.84–0.92). |
| [55] | Oesophageal cancer | Meta-analysis (15 case–control studies, n = 16,885) | Per 10 g/day increase: oesophageal cancer risk ↓ 31% (OR: 0.69, 0.61–0.79. |
| [53] | Crohn’s disease | Meta-analysis (6 case–control studies, 2 cohort studies, n = 478,604) | Per 10 g/d increase: CD risk ↓ 13% (RR: 0.87, 0.76–0.98). |
| [56] | Gastric cancer | Meta-analysis (19 case–control studies, 2 cohort studies, n = 580,064) | Per 10 g/day increase: gastric cancer risk ↓ 44%. |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [57] | Liver cancer | Meta-analysis (14 cohort studies) | Per 1% energy increase: liver cancer risk ↑ 4% (RR: 1.04, 1.01–1.07). |
| [58] | Stroke | Meta-analysis (12 cohort studies, n = 462,268) | Per 10 g/day increase: stroke risk ↓ 6% (RR: 0.94, 0.89–0.98). |
| [59] | CVD * | Meta-analysis (15 RCTs, n = 56,675) | Reduced SFA intake vs. higher SFA intake: cardiovascular event risk potentially ↓ 17% (RR 0.79, 0.66–0.93), with greater reductions possibly yielding larger benefits, especially when considering less than 10% of energy. PUFA replacement vs. SFA: cardiovascular event risk ↓ 21%. |
| [19] | Alzheimer’s disease | Meta-analysis (4 cohort studies, n = 8630) | Per 4 g/day increase: AD risk ↑ 15% (RR: 1.15, 1.01–1.31). |
| [60] | Endometrial cancer | Meta-analysis (14 case–control studies, 7 cohort studies, n = 524,583) | Per 10 g/1000 kcal increase: endometrial cancer risk ↑ 17%. |
| [61] | CHD | Meta-analysis (13 cohort studies, n = 310,602) | Per 5% energy intake increase when LA substitutes SFAs: CHD risk ↓ 9% (RR: 0.91, 0.87–0.96). |
| Citation | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [62] | CHD | Meta-analysis (13 cohort studies, n = 345,202) | Per 1 g/day (ALA) increase: fatal CHD risk ↓ 12% (RR: 0.88, 0.81–0.96). Only ALA intake <1.4 g/d had composite CHD risk ↓. |
| Source | Health Outcome | Study | Results (Dose–Response) |
|---|---|---|---|
| [63] | T2DM | Meta-analysis (31 cohort studies, n = 297,685) | Per 5% energy from LA increase: T2DM risk ↓ 10% (RR: 0.90, 0.84–0.98). |
| [61] | CHD | Meta-analysis (13 cohort studies, n = 310,602) | Per 5% energy from LA increase: CHD risk ↓ 10% (RR: 0.90, 0.85–0.94). Per 5% energy intake increase when LA substitutes SFAs: CHD risk ↓ 9% (RR: 0.91, 0.87–0.96). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateus, G.; Ferreira-Pêgo, C.; Assunção, R. Mapping the Quantitative Dose–Response Relationships Between Nutrients and Health Outcomes to Inform Food Risk–Benefit Assessment. Foods 2025, 14, 1420. https://doi.org/10.3390/foods14081420
Mateus G, Ferreira-Pêgo C, Assunção R. Mapping the Quantitative Dose–Response Relationships Between Nutrients and Health Outcomes to Inform Food Risk–Benefit Assessment. Foods. 2025; 14(8):1420. https://doi.org/10.3390/foods14081420
Chicago/Turabian StyleMateus, Gabriel, Cíntia Ferreira-Pêgo, and Ricardo Assunção. 2025. "Mapping the Quantitative Dose–Response Relationships Between Nutrients and Health Outcomes to Inform Food Risk–Benefit Assessment" Foods 14, no. 8: 1420. https://doi.org/10.3390/foods14081420
APA StyleMateus, G., Ferreira-Pêgo, C., & Assunção, R. (2025). Mapping the Quantitative Dose–Response Relationships Between Nutrients and Health Outcomes to Inform Food Risk–Benefit Assessment. Foods, 14(8), 1420. https://doi.org/10.3390/foods14081420









