Secretory Production of Plant Heme-Containing Globins by Recombinant Yeast via Precision Fermentation
Abstract
:1. Introduction
2. Material and Methods
2.1. Strains and Plasmids
2.2. Media and Culture Conditions
2.3. Protein Purification and Quantification
2.4. Peroxidase Activity Assay for Plant Hemoglobin
2.5. Precipitation of Recombinant Proteins by Ammonium Sulfate
2.6. Statistical Analysis
3. Results
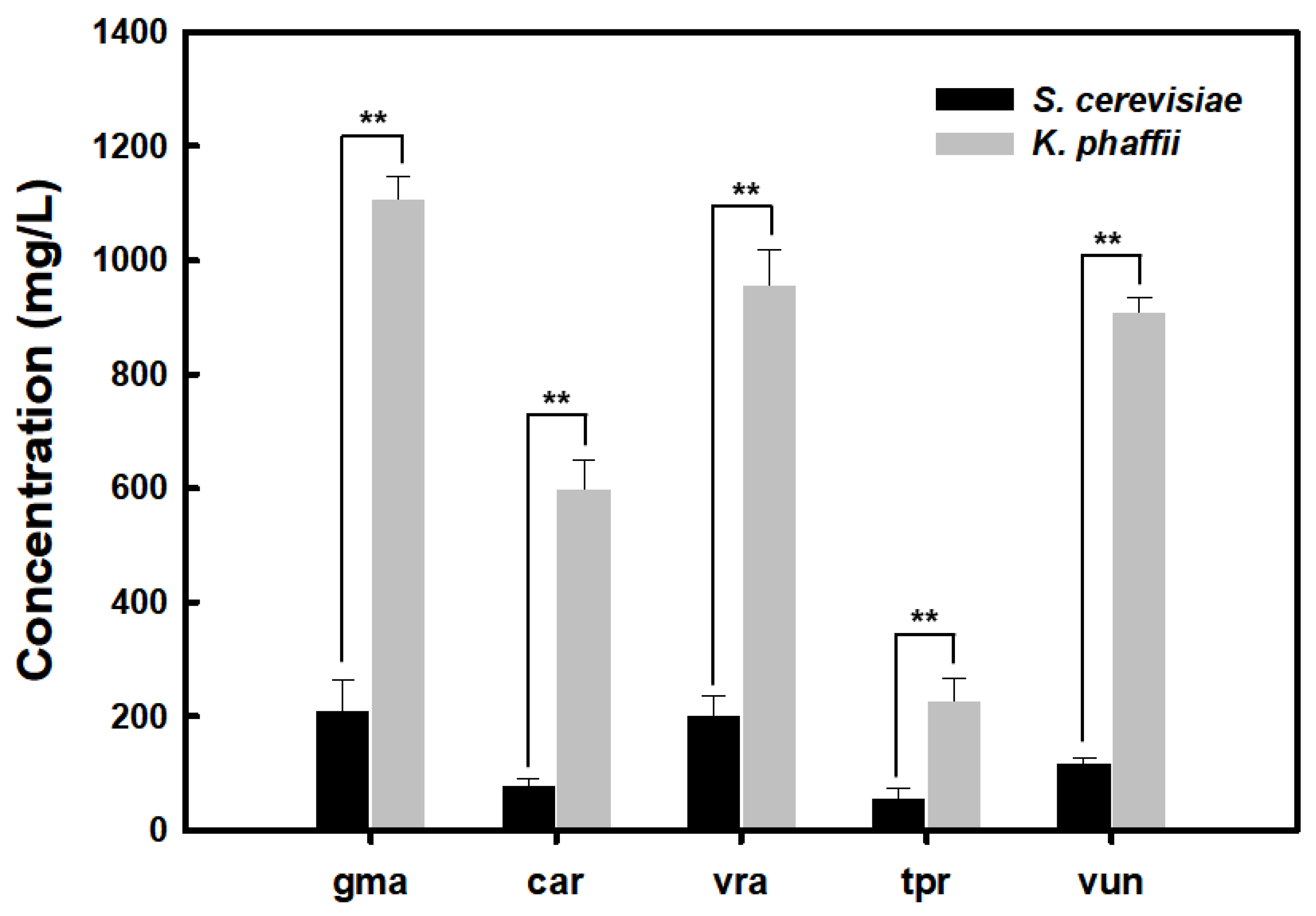
3.1. Secretory Production of Plant Leghemoglobin in Recombinant Yeast
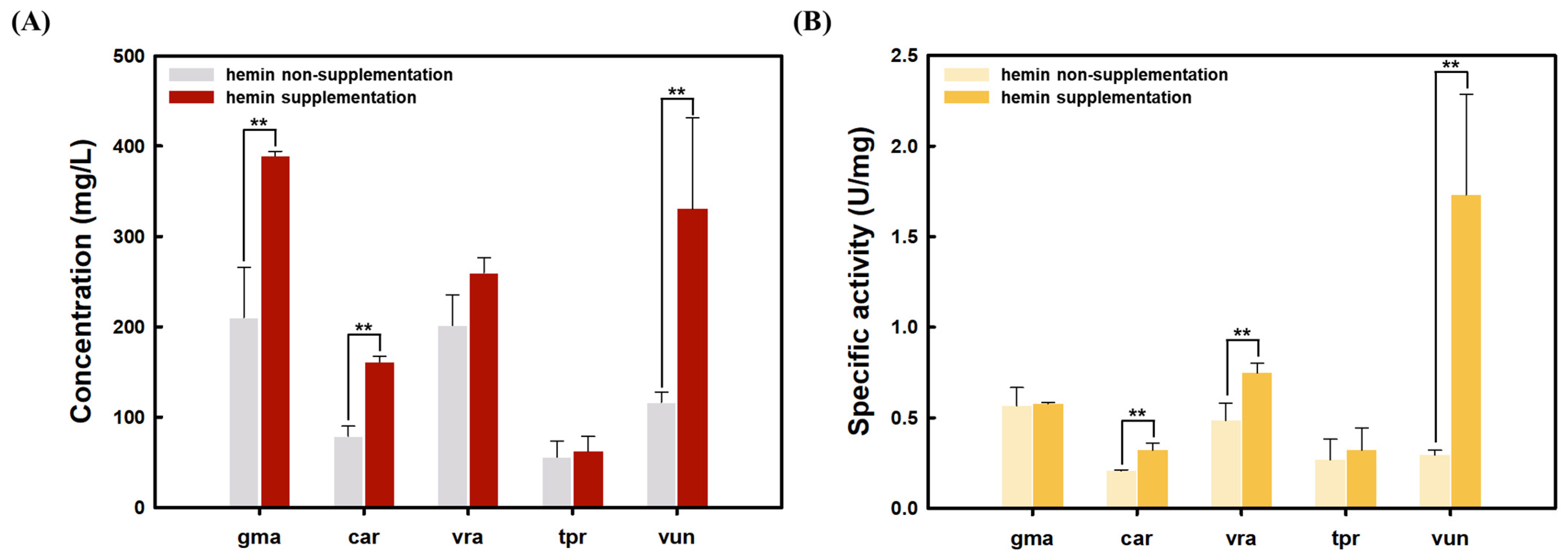
3.2. Effect of Hemin Supplementation on Plant Leghemoglobin Production
3.3. Precipitation of Plant Leghemoglobin Using Ammonium Sulfate
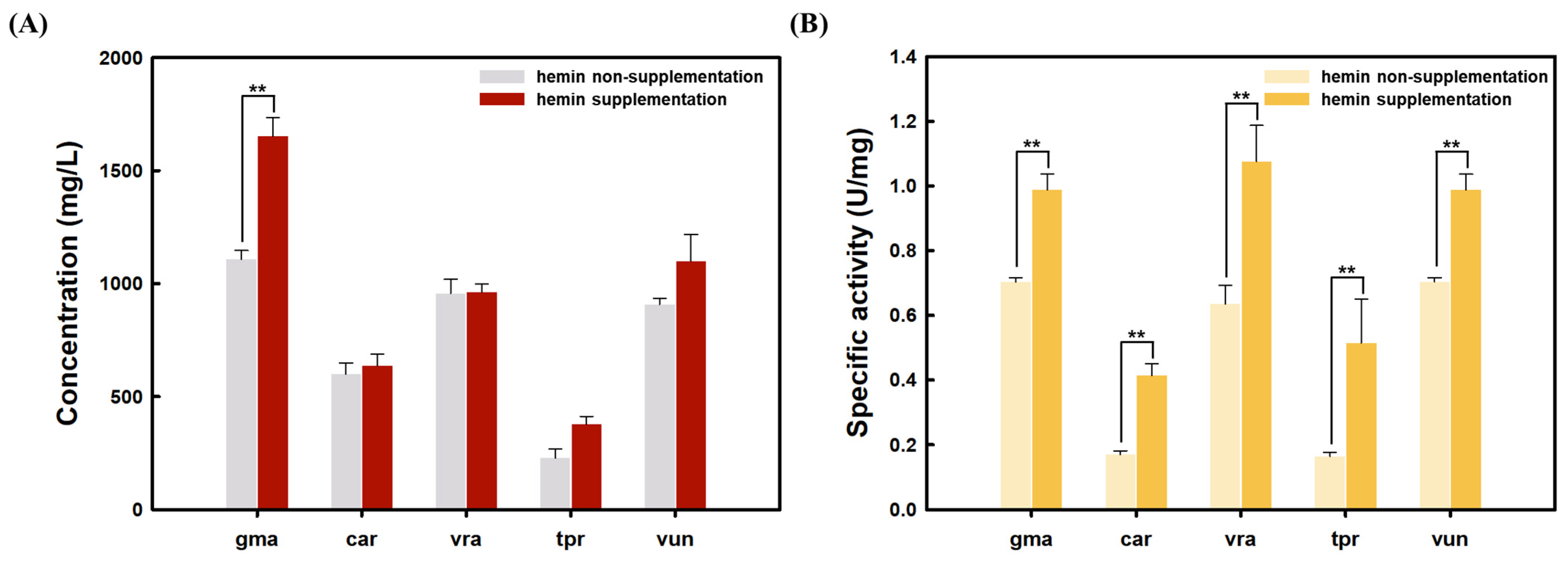
3.4. Replacement of Signal Peptide for Enhanced Secretion of Plant Leghemoglobin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Rosado, P.; Roser, M. Meat and dairy production. Our World in Data 2019. [Google Scholar]
- McLeod, A. World Livestock 2011—Livestock in Food Security; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Handral, H.K.; Hua Tay, S.; Wan Chan, W.; Choudhury, D. 3D Printing of cultured meat products. Crit. Rev. Food Sci. Nutr. 2022, 62, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef]
- Romao, B.; Botelho, R.B.A.; Torres, M.L.; Maynard, D.D.C.; de Holanda, M.E.M.; Borges, V.R.P.; Raposo, A.; Zandonadi, R.P. Nutritional Profile of Commercialized Plant-Based Meat: An Integrative Review with a Systematic Approach. Foods 2023, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Michelfelder, A.J. Soy: A complete source of protein. Am. Fam. Physician 2009, 79, 43–47. [Google Scholar]
- Costa-Catala, J.; Toro-Funes, N.; Comas-Basté, O.; Hernández-Macias, S.; Sánchez-Pérez, S.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Castell-Garralda, V.; Vidal-Carou, M.C. Comparative assessment of the nutritional profile of meat products and their plant-based analogues. Nutrients 2023, 15, 2807. [Google Scholar] [CrossRef]
- Kumari, S.; Alam, A.N.; Hossain, M.J.; Lee, E.Y.; Hwang, Y.H.; Joo, S.T. Sensory Evaluation of Plant-Based Meat: Bridging the Gap with Animal Meat, Challenges and Future Prospects. Foods 2023, 13, 108. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-based meat analogues. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. [Google Scholar]
- Fraser, R.Z.; Shitut, M.; Agrawal, P.; Mendes, O.; Klapholz, S. Safety evaluation of soy leghemoglobin protein preparation derived from Pichia pastoris, intended for use as a flavor catalyst in plant-based meat. Int. J. Toxicol. 2018, 37, 241–262. [Google Scholar] [CrossRef]
- Hargrove, M.S.; Barry, J.K.; Brucker, E.A.; Berry, M.B.; Phillips, G.N., Jr.; Olson, J.S.; Arredondo-Peter, R.; Dean, J.M.; Klucas, R.V.; Sarath, G. Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J. Mol. Biol. 1997, 266, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Bolmanis, E.; Bogans, J.; Akopjana, I.; Suleiko, A.; Kazaka, T.; Kazaks, A. Production and Purification of Soy Leghemoglobin from Pichia pastoris Cultivated in Different Expression Media. Processes 2023, 11, 3215. [Google Scholar] [CrossRef]
- Jin, Y.; He, X.; Andoh-Kumi, K.; Fraser, R.Z.; Lu, M.; Goodman, R.E. Evaluating Potential Risks of Food Allergy and Toxicity of Soy Leghemoglobin Expressed in Pichia pastoris. Mol. Nutr. Food Res. 2018, 62, 1700297. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, Y. Progress in the study of leghemoglobin. Acta Bot. Boreali-Occident. Sin. 2000, 20, 684–689. [Google Scholar]
- Wang, M.; Shi, Z.; Gao, N.; Zhou, Y.; Ni, X.; Chen, J.; Liu, J.; Zhou, W.; Guo, X.; Xin, B.; et al. Sustainable and high-level microbial production of plant hemoglobin in Corynebacterium glutamicum. Biotechnol. Biofuels Bioprod. 2023, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wu, X.; Wu, P.; Lu, X.; Wang, Q.; Lin, Y.; Liu, C.; Zhou, J.; Yu, Y.; Lu, H. High-level expression of leghemoglobin in Kluyveromyces marxianus by remodeling the heme metabolism pathway. Front. Bioeng. Biotechnol. 2023, 11, 1329016. [Google Scholar] [CrossRef]
- Meng, Y.; Xie, L.; You, K.; Chen, W. High-level secretory production of leghemoglobin and myoglobin in Escherichia coli through inserting signal peptides. Food Biosci. 2023, 56, 103356. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Li, J.; Du, G.; Chen, J.; Zhao, X. Efficient Secretory Expression of Leghemoglobin in Saccharomyces cerevisiae. Fermentation 2024, 10, 146. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Degen, G.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety of soy leghemoglobin from genetically modified Komagataella phaffii as a food additive. EFSA J. 2024, 22, e8822. [Google Scholar] [CrossRef]
- Reyes, T.F.; Chen, Y.; Fraser, R.Z.; Chan, T.; Li, X. Assessment of the potential allergenicity and toxicity of Pichia proteins in a novel leghemoglobin preparation. Regul. Toxicol. Pharmacol. 2021, 119, 104817. [Google Scholar] [CrossRef]
- Shao, Y.; Xue, C.; Liu, W.; Zuo, S.; Wei, P.; Huang, L.; Lian, J.; Xu, Z. High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour. Technol. 2022, 363, 127884. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Nikawa, J.-I.; Kodaki, T.; Yamashita, S. A dominant mutation that alters the regulation of INO1 expression in Saccharomyces cerevisiae. J. Biochem. 1992, 111, 352–358. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.O.; Jin, Y.S. Next-Generation Genetic and Fermentation Technologies for Safe and Sustainable Production of Food Ingredients: Colors and Flavorings. Annu. Rev. Food Sci. Technol. 2022, 13, 463–488. [Google Scholar] [CrossRef]
- Obst, U.; Lu, T.K.; Sieber, V. A Modular Toolkit for Generating Pichia pastoris Secretion Libraries. ACS Synth. Biol. 2017, 6, 1016–1025. [Google Scholar] [CrossRef]
- Yesilirmak, F.; Sayers, Z. Heterelogous Expression of Plant Genes. Int. J. Plant Genom. 2009, 2009, 296482. [Google Scholar] [CrossRef] [PubMed]
- Hurlburt, B.K.; McBride, J.K.; Nesbit, J.B.; Ruan, S.; Maleki, S.J. Purification of Recombinant Peanut Allergen Ara h 1 and Comparison of IgE Binding to the Natural Protein. Foods 2014, 3, 642–657. [Google Scholar] [CrossRef]
- Krainer, F.W.; Capone, S.; Jäger, M.; Vogl, T.; Gerstmann, M.; Glieder, A.; Herwig, C.; Spadiut, O. Optimizing cofactor availability for the production of recombinant heme peroxidase in Pichia pastoris. Microb. Cell Factories 2015, 14, 4. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, X.; Liu, Y.; Wang, Y.; Li, J.; Du, G.; Zhao, X.; Chen, J. Enhancing heme import to synthesize active myoglobin and hemoglobin in Pichia pastoris. 3 Biotech 2025, 15, 115. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, X.; Wang, Z.; Wang, H.; Zhou, J.; Du, G.; Chen, J.; Li, J. Efficient Secretory Expression and Purification of Food-Grade Porcine Myoglobin in Komagataella phaffii. J. Agric. Food Chem. 2021, 69, 10235–10245. [Google Scholar] [CrossRef]
- Aza, P.; Molpeceres, G.; de Salas, F.; Camarero, S. Design of an improved universal signal peptide based on the alpha-factor mating secretion signal for enzyme production in yeast. Cell. Mol. Life Sci. 2021, 78, 3691–3707. [Google Scholar] [CrossRef]
- Rakestraw, J.A.; Sazinsky, S.L.; Piatesi, A.; Antipov, E.; Wittrup, K.D. Directed evolution of a secretory leader for the improved expression of heterologous proteins and full-length antibodies in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2009, 103, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhou, X.-T.; Guo, Q.; Xue, Y.-Z.; Xue, Y.-P.; Zheng, Y.-G. Potential of the Signal Peptide Derived from the PAS_chr3_0030 Gene Product for Secretory Expression of Valuable Enzymes in Pichia pastoris. Appl. Environ. Microbiol. 2022, 88, e00296-00222. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Idiris, A.; Tohda, H.; Kumagai, H.; Takegawa, K. Engineering of protein secretion in yeast: Strategies and impact on protein production. Appl. Microbiol. Biotechnol. 2010, 86, 403–417. [Google Scholar] [CrossRef]
- Freitas, S.S.; Santos, J.A.; Prazeres, D.M. Optimization of isopropanol and ammonium sulfate precipitation steps in the purification of plasmid DNA. Biotechnol. Prog. 2006, 22, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Varley, D.L.; Hitchcock, A.G.; Weiss, A.M.; Horler, W.A.; Cowell, R.; Peddie, L.; Sharpe, G.S.; Thatcher, D.R.; Hanak, J.A. Production of plasmid DNA for human gene therapy using modified alkaline cell lysis and expanded bed anion exchange chromatography. Bioseparation 1999, 8, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fiege, K.; Querebillo, C.J.; Hildebrandt, P.; Frankenberg-Dinkel, N. Improved method for the incorporation of heme cofactors into recombinant proteins using Escherichia coli Nissle 1917. Biochemistry 2018, 57, 2747–2755. [Google Scholar] [CrossRef]
- Neiers, F.; Belloir, C.; Poirier, N.; Naumer, C.; Krohn, M.; Briand, L. Comparison of Different Signal Peptides for the Efficient Secretion of the Sweet-Tasting Plant Protein Brazzein in Pichia pastoris. Life 2021, 11, 46. [Google Scholar] [CrossRef]
- Zou, C.; Lu, L.; Wang, S.; Zhang, C.; Chen, X.; Lin, Y.; Huang, Y. The α-mating factor secretion signals and endogenous signal peptides for recombinant protein secretion in Komagataella phaffii. Biotechnol. Biofuels Bioprod. 2022, 15, 140. [Google Scholar] [CrossRef]
- Xue, S.; Liu, X.; Pan, Y.; Xiao, C.; Feng, Y.; Zheng, L.; Zhao, M.; Huang, M. Comprehensive Analysis of Signal Peptides in Saccharomyces cerevisiae Reveals Features for Efficient Secretion. Adv. Sci. 2023, 10, e2203433. [Google Scholar] [CrossRef] [PubMed]



| Name | Description | Reference |
|---|---|---|
| S. cerevisiae D452-2 | MATα, leu2, his3, ura3, and can1 | [24] |
| pESC-MFα-gmaHb | D452-2 URA3, PGAL1-MFα- Glycinin max Hb | This study |
| pESC-MFα-carHb | D452-2 URA3, PGAL1-MFα- Cicer arietinum Hb | This study |
| pESC-MFα-vraHb | D452-2 URA3, PGAL1-MFα- Vigna radiata var. radiata Hb | This study |
| pESC-MFα-tprHb | D452-2 URA3, PGAL1-MFα- Trifolium pratense Hb | This study |
| pESC-MFα-vunHb | D452-2 URA3, PGAL1-MFα- Vigna unguiculata Hb | This study |
| pESC-HS-gmaHb | D452-2 URA3, PGAL1-HS- G. max Hb | This study |
| pESC-HS-carHb | D452-2 URA3, PGAL1-HS- C. arietinum Hb | This study |
| pESC-HS-vraHb | D452-2 URA3, PGAL1-HS- V. radiata var. radiata Hb | This study |
| pESC-HS-tprHb | D452-2 URA3, PGAL1-HS- T. pratense Hb | This study |
| pESC-HS-vunHb | D452-2 URA3, PGAL1-HS- V. unguiculata Hb | This study |
| pESC-AN-gmaHb | D452-2 URA3, PGAL1-AN- G. max Hb | This study |
| pESC-AN-carLegHb | D452-2 URA3, PGAL1-AN- C. arietinum Hb | This study |
| pESC-AN-vraHb | D452-2 URA3, PGAL1-AN-V. radiata var. radiata Hb | This study |
| pESC-AN-tprHb | D452-2 URA3, PGAL1-AN-T. pratense Hb | This study |
| pESC-AN-vunHb | D452-2 URA3, PGAL1-AN-V. unguiculata Hb | This study |
| K. phaffii X-33 | Wild type | Invitrogen |
| pPICzαA-MFα-gmaHb | X-33, PAOX1-MFα-G. max Hb | This study |
| pPICzαA-MFα-carHb | X-33, PAOX1-MFα-C. arietinum Hb | This study |
| pPICzαA-MFα-vraHb | X-33, PAOX1-MFα-V. radiata var. radiata Hb | This study |
| pPICzαA-MFα-tprHb | X-33, PAOX1-MFα-T. pratense Hb | This study |
| pPICzαA-MFα-vunHb | X-33, PAOX1-MFα-V. unguiculata Hb | This study |
| pPICzαA-HS-gmaHb | X-33, PAOX1-HS-G. max Hb | This study |
| pPICzαA-HS-carHb | X-33, PAOX1-HS-C. arietinum Hb | This study |
| pPICzαA-HS-vraHb | X-33, PAOX1-HS-V. radiata var. radiata Hb | This study |
| pPICzαA-HS-tprHb | X-33, PAOX1-HS-T. pratense Hb | This study |
| pPICzαA-HS-vunHb | X-33, PAOX1-HS-V. unguiculata Hb | This study |
| pPICzαA-AN-gmaHb | X-33, PAOX1-AN-G. max Hb | This study |
| pPICzαA-AN-carHb | X-33, PAOX1-AN-C. arietinum Hb | This study |
| pPICzαA-AN-vraHb | X-33, PAOX1-AN-V. radiata var. radiata Hb | This study |
| pPICzαA-AN-tprHb | X-33, PAOX1-AN-T. pratense Hb | This study |
| pPICzαA-AN-vunHb | X-33, PAOX1-AN-V. unguiculata Hb | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.-N.; Kim, G.-H.; Seo, S.-O. Secretory Production of Plant Heme-Containing Globins by Recombinant Yeast via Precision Fermentation. Foods 2025, 14, 1422. https://doi.org/10.3390/foods14081422
Bae H-N, Kim G-H, Seo S-O. Secretory Production of Plant Heme-Containing Globins by Recombinant Yeast via Precision Fermentation. Foods. 2025; 14(8):1422. https://doi.org/10.3390/foods14081422
Chicago/Turabian StyleBae, Ha-Neul, Geun-Hyung Kim, and Seung-Oh Seo. 2025. "Secretory Production of Plant Heme-Containing Globins by Recombinant Yeast via Precision Fermentation" Foods 14, no. 8: 1422. https://doi.org/10.3390/foods14081422
APA StyleBae, H.-N., Kim, G.-H., & Seo, S.-O. (2025). Secretory Production of Plant Heme-Containing Globins by Recombinant Yeast via Precision Fermentation. Foods, 14(8), 1422. https://doi.org/10.3390/foods14081422









