Bioactive Properties of the Microwave-Assisted Olive Leaf Extract and Its Incorporation into a Whey Protein Isolate Coating of Semi-Hard Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Olive Leaf Extract and WPI-Based Coating
2.1.1. Olive Leaf Extract Production
2.1.2. WPI-Based Coating Preparation
2.1.3. Antimicrobial Activity of Olive Leaf Extract and WPI-Based Coating
2.1.4. Total Phenol, Total Flavonoid Content, and Antioxidant Activity of Olive Leaf Extract
2.1.5. Cytotoxicity and Activation of Caspases
2.2. Production of Cheese Samples with WPI-Based Coating
2.2.1. Physico-Chemical Analyses of Cheese
2.2.2. Microbiological Analyses of Cheese
2.2.3. Total Phenol, Total Flavonoid Content, and Antioxidant Activity in Cheese
2.3. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant, Antimicrobial, and Cytotoxic Effects of Olive Leaf Extract
3.2. Physico-Chemical Properties of the Cheese with WPI Coating
3.3. Total Phenols, Flavonoid Content, and Antioxidant Activity in Coated Cheese
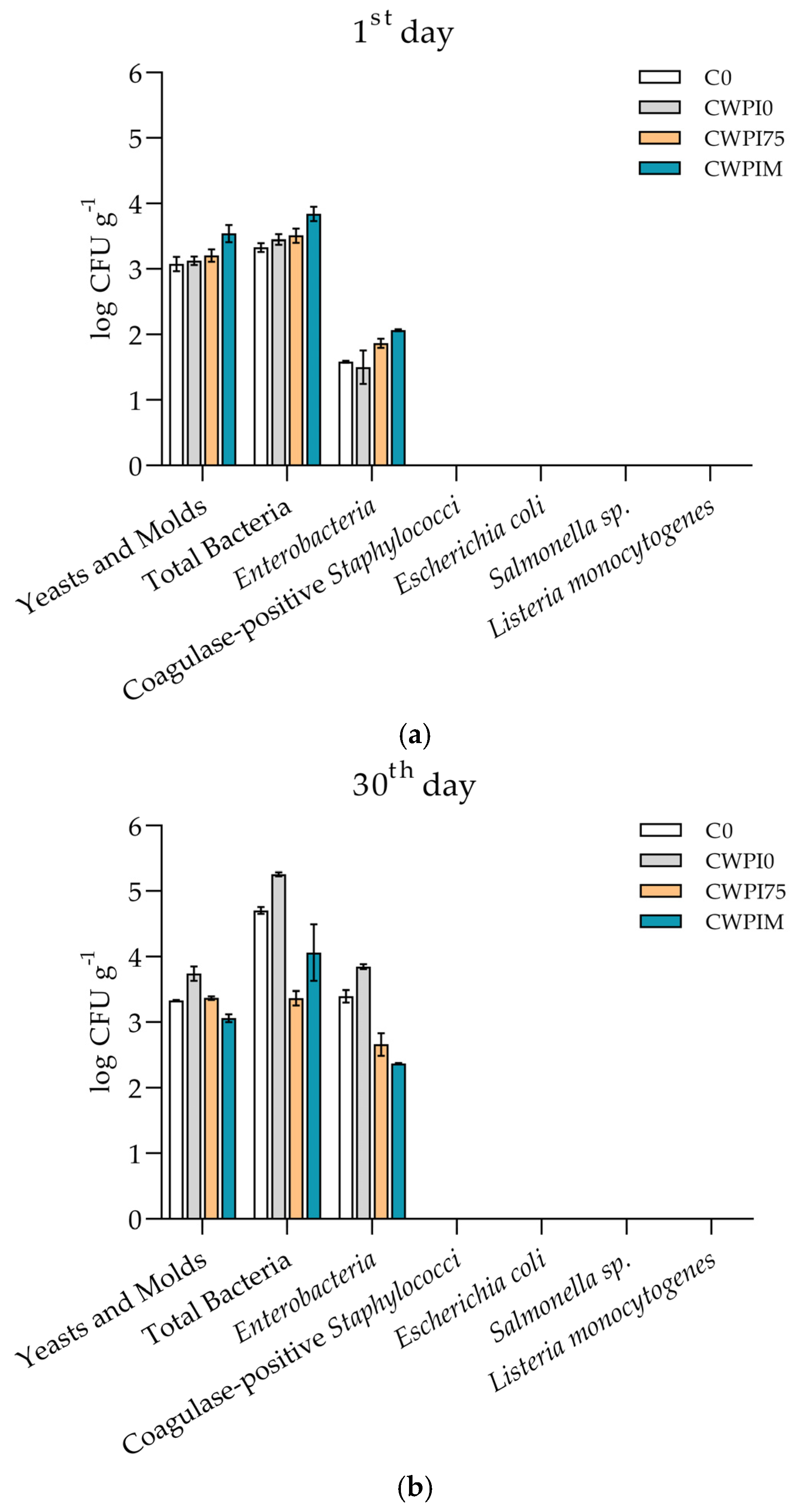
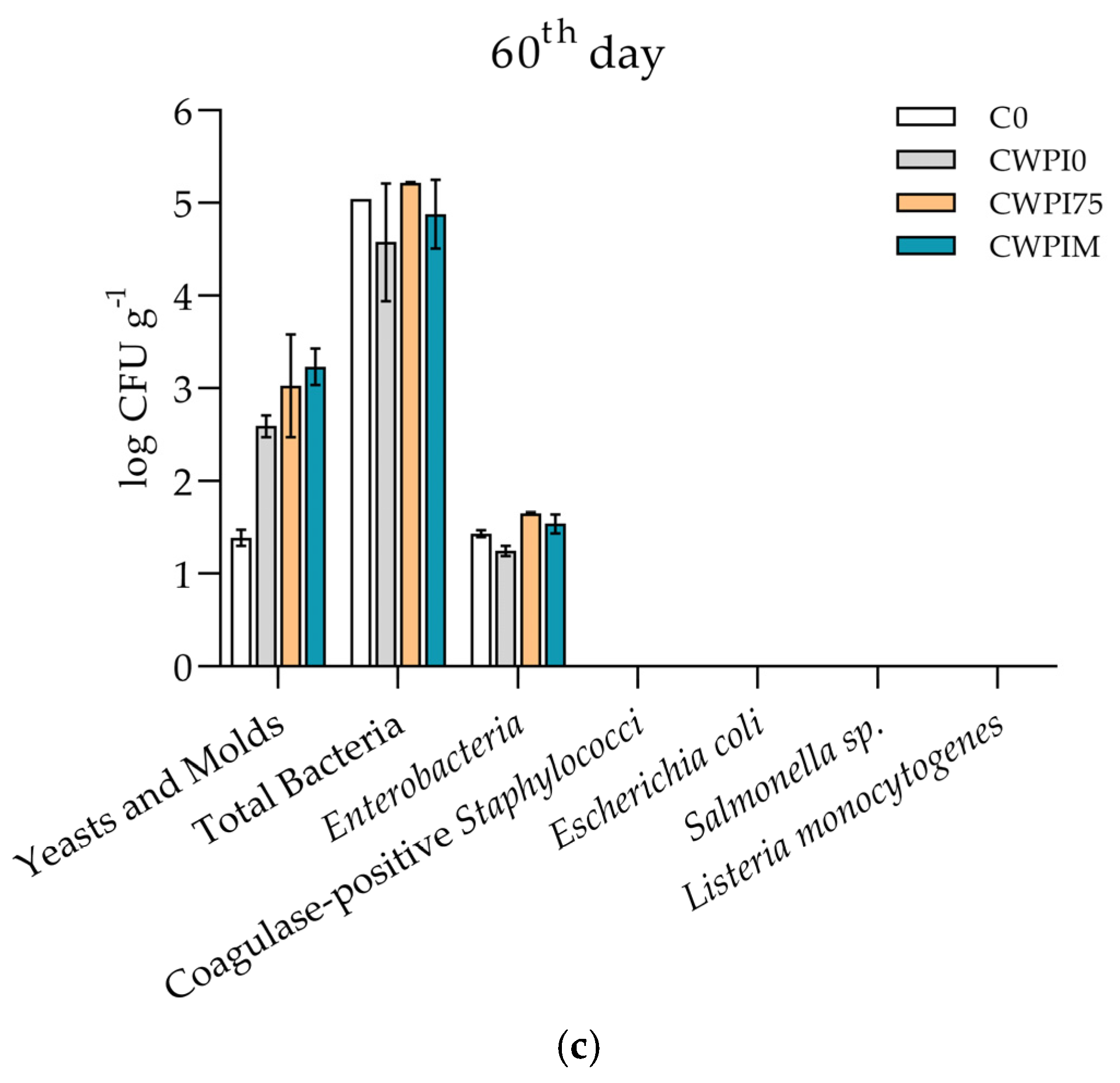
3.4. Cheese Microbiology and Antimicrobial Activity of WPI-Based Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rangel, A.H.d.N.; Bezerra, D.A.F.V.; Sales, D.C.; Araújo, E.e.O.M.; de Lucena, L.M.; Porto, A.L.F.; Véras, Í.V.U.M.; Lacerda, A.F.; Ribeiro, C.V.D.M.; Anaya, K. An overview of the occurrence of bioactive peptides in different types of cheeses. Foods 2023, 12, 4261. [Google Scholar] [CrossRef]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Tagliazucchi, D. Peptidomics profile, bioactive peptides identification and biological activities of six different cheese varieties. Biology 2023, 12, 78. [Google Scholar] [CrossRef]
- Mureşan, C.C.; Marc, R.A.; Anamaria Semeniuc, C.; Ancuţa Socaci, S.; Fărcaş, A.; Fracisc, D.; Rodica Pop, C.; Rotar, A.; Dodan, A.; Mureşan, V.; et al. Changes in physicochemical and microbiological properties, fatty acid and volatile compound profiles of Apuseni cheese during ripening. Foods 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Possas, A.; Bonilla-Luque, O.M.; Valero, A. From cheese-making to consumption: Exploring the microbial safety of cheeses through predictive microbiology models. Foods 2021, 10, 355. [Google Scholar] [CrossRef]
- Alshareef, H.A.; Aly, M.M.; Bokhari, F.M.; Al-Seeni, M.N. Microbial contaminants and risk factors involved in the contamination of different types of cheese samples. An Int. J. Agric. Biol. Res. 2023, 39, 421–426. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Pereira, J.O.; Silva, S.I.; Amorim, M.M.; Fernandes, J.C.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Effect of composition of commercial whey protein preparations upon gelation at various pH values. Food Res. Int. 2012, 48, 681–689. [Google Scholar] [CrossRef]
- Robalo, J.; Lopes, M.; Cardoso, O.; Sanches Silva, A.; Ramos, F. Efficacy of Whey Protein Film Incorporated with Portuguese Green Tea (Camellia sinensis L.) Extract for the Preservation of Latin-Style Fresh Cheese. Foods 2022, 11, 1158. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Díaz, O.; Cobos, A.; Pereira, C.D. A review of recent developments in edible films and coatings-Focus on whey-based materials. Foods 2024, 13, 2638. [Google Scholar] [CrossRef]
- Otero, D.M.; Lorini, A.; Oliveira, F.M.; Antunes, B.d.F.; Oliveira, R.M.; Zambiazi, R.C. Leaves of Olea europaea L. as a source of oleuropein: Characteristics and biological aspects. Res. Soc. Dev. 2021, 10, e185101321130. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Zandona, E.; Vranković, L.; Pedisić, S.; Vukušić Pavičić, T.; Dobrinčić, A.; Marušić Radovčić, N.; Lisak Jakopović, K.; Blažić, M.; Barukčić Jurina, I. Production of Acid and Rennet-Coagulated Cheese Enriched by Olive (Olea europaea L.) Leaf Extract—Determining the Optimal Point of Supplementation and Its Effects on Curd Characteristics. Foods 2024, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. BioFactors 2017, 43, 517–528. [Google Scholar] [CrossRef]
- Talhaoui, N.; Trabelsi, N.; Taamalli, A.; Verardo, V.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Arraez-Roman, D. Olea europaea as potential source of bioactive compounds for diseases prevention. Stud. Nat. Prod. Chem. 2018, 57, 389–411. [Google Scholar]
- Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 2033. [CrossRef]
- Erdohan, Ö.; Turhan, N.K. Olive leaf extract and usage for development of antimicrobial food packaging. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2012; pp. 1094–1101. [Google Scholar]
- Ramos, Ó.L.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Suspectibility Tests, Approved Standard-Eleventh Edition. Clin. Lab. Stand. Inst. 2018, 38, 2162–2914. [Google Scholar]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Katalinić, M.; Zandona, A.; Ramić, A.; Zorbaz, T.; Primožič, I.; Kovarik, Z. New cinchona oximes evaluated as reactivators of acetylcholinesterase and butyrylcholinesterase inhibited by organophosphorus compounds. Molecules 2017, 22, 1234. [Google Scholar] [CrossRef]
- Zandona, A.; Maraković, N.; Mišetić, P.; Madunić, J.; Miš, K.; Padovan, J.; Pirkmajer, S.; Katalinić, M. Activation of (un)regulated cell death as a new perspective for bispyridinium and imidazolium oximes. Arch. Toxicol. 2021, 95, 2737–2754. [Google Scholar] [CrossRef]
- ISO 3432:2008; Cheese—Determination of Fat Content—Butyrometer for Van Gulik Method. ISO: Geneva, Switzerland, 2008.
- AOAC Official Method 935.42Ash of Cheese. In Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: New York, NY, USA, 2023.
- AOAC Official Method 920.124Acidity of Cheese. In Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: New York, NY, USA, 2023.
- AOAC Official Method 991.23Protein Nitrogen Content of Milk. In Official Methods of Analysis of AOAC INTERNATIONAL; Oxford University Press: New York, NY, USA, 2023.
- Barukčić, I.; Filipan, K.; Lisak Jakopović, K.; Božanić, R.; Blažić, M.; Repajić, M. The Potential of Olive Leaf Extract as a Functional Ingredient in Yoghurt Production: The Effects on Fermentation, Rheology, Sensory, and Antioxidant Properties of Cow Milk Yoghurt. Foods 2022, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Wojciech, M.; Maciej, T. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Marušić Radovčić, N.; Poljanec, I.; Petričević, S.; Mora, L.; Medić, H. Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham. Foods 2021, 10, 1228. [Google Scholar] [CrossRef]
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli. ISO: Geneva, Switzerland, 2001.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- ISO 11290-2:2017; Microbiology of the Food Chain Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 2: Enumeration method; ISO: Geneva, Switzerland, 2017.
- Commission of the European Communities Commission Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32005R2073 (accessed on 29 November 2024).
- Vodič za Mikrobiološke Kriterije za Hranu [e-Dokument]/Pripremilo Ministarstvo poljoprivrede, Ribarstva i Ruralnog Razvoja—Zagreb: Ministarstvo Poljoprivrede, Ribarstva i Ruralnog Razvoja. 2011. Available online: https://sredisnjikatalogrh.gov.hr/sredisnji-katalog/dokumenti-i-publikacije/detalji?searchTerm=filterfi%255B12086%255D%3D268671&id=2704010 (accessed on 28 November 2024).
- Apostolidis, E.; Kwon, Y.-I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Ayana, B.; Turhan, K.N. Use of antimicrobial methylcellulose films to control Staphylococcus aureus during storage of Kasar cheese. Packag. Technol. Sci. 2009, 22, 461–469. [Google Scholar] [CrossRef]
- Haris Omar, S. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Peterson, Q.P.; Hsu, D.C.; Goode, D.R.; Novotny, C.J.; Totten, R.K.; Hergenrother, P.J. Procaspase-3 Activation as an Anti-Cancer Strategy: Structure−Activity Relationship of Procaspase-Activating Compound 1 (PAC-1) and Its Cellular Co-Localization with Caspase-3. J. Med. Chem. 2009, 52, 5721–5731. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gur, C.; Cetin, B.; Akay, S.; Gulce-Iz, S.; Yesil-Celiktas, O. Extracts from Black Carrot Tissue Culture as Potent Anticancer Agents. Plant Foods Hum. Nutr. 2013, 68, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Singh, R.; Siddiqui, S.; Ahmad, I.; Ahmad, R.; Upadhyay, S.; Barkat, M.A.; Ali, A.M.A.; Zia, Q.; Srivastava, A.; et al. Anticancer potential of Phoenix dactylifera L. seed extract in human cancer cells and pro-apoptotic effects mediated through caspase-3 dependent pathway in human breast cancer MDA-MB-231 cells: An in vitro and in silico investigation. BMC Complement. Med. Ther. 2022, 22, 68. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Kilic, T.; Koyuncu, G. Effect of plant addition and ripening on total phenol content, antioxidant capacity, volatile compounds and sensory properties of kashar cheese. J. Food Sci. Technol. 2024, 61, 734–742. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef]


| ΔE* Value | Observer Color Difference Perception |
|---|---|
| 0–1.0 | Observer does not notice the difference |
| 1.1–2.0 | Only an experienced observer can perceive the difference |
| 2.1–3.5 | Unexperienced observer also notices the color difference |
| 3.6–5.0 | A clear distinction in color is visible |
| >5.0 | Observer notices two different colors |
| Day | Sample | aw | Lactic Acid (%) | pH |
|---|---|---|---|---|
| 1 | C0 | 0.92 ± 0.06 | 0.74 ± 0.08 | 6.66 ± 0.12 |
| CWPI0 | 0.92 ± 0.08 | 0.63 ± 0.08 | 6.65 ± 0.19 | |
| CWPI75 | 0.92 ± 0.07 | 0.67 ± 0.08 | 6.62 ± 0.11 | |
| CWPIM | 0.92 ± 0.18 | 0.72 ± 0.08 | 6.57 ± 0.21 | |
| 15 | C0 | 0.94 ± 0.19 | 2.22 ± 0.02 bc | 5.82 ± 0.15 c |
| CWPI0 | 0.92 ± 0.13 | 2.43 ± 0.05 ac | 5.86 ± 0.03 c | |
| CWPI75 | 0.94 ± 0.17 | 2.32 ± 0.10 c | 6.03 ± 0.19 c | |
| CWPIM | 0.93 ± 0.03 | 2.41 ± 0.08 ac | 5.70 ± 0.16 c | |
| 30 | C0 | 0.90 ± 0.15 | 2.90 ± 0.07 bc | 5.59 ± 0.02 bc |
| CWPI0 | 0.89 ± 0.01 | 3.12 ± 0.03 ac | 5.31 ± 0.11 ac | |
| CWPI75 | 0.89 ± 0.04 | 3.11 ± 0.09 ac | 5.49 ± 0.11 c | |
| CWPIM | 0.90 ± 0.14 | 3.10 ± 0.05 ac | 5.46 ± 0.14 c | |
| 45 | C0 | 0.89 ± 0.02 | 3.03 ± 0.06 bc | 5.42 ± 0.05 bc |
| CWPI0 | 0.89 ± 0.13 | 2.82 ± 0.06 ac | 5.15 ± 0.08 ac | |
| CWPI75 | 0.89 ± 0.10 | 2.99 ± 0.08 bc | 5.78 ± 0.12 abc | |
| CWPIM | 0.88 ± 0.13 | 2.45 ± 0.07 abc | 5.28 ± 0.17 c | |
| 60 | C0 | 0.91 ± 0.08 | 3.06 ± 0.01 bc | 5.25 ± 0.13 c |
| CWPI0 | 0.90 ± 0.04 | 3.19 ± 0.03 ac | 5.11 ± 0.16 c | |
| CWPI75 | 0.91 ± 0.08 | 3.01 ± 0.05 bc | 5.29 ± 0.04 c | |
| CWPIM | 0.90 ± 0.08 | 2.68 ± 0.03 abc | 5.13 ± 0.14 c |
| Day | Sample | Ash (%) | Total Solids (%) | Fat (%) |
|---|---|---|---|---|
| 1 | C0 | 6.04 ± 0.12 b | 46.17 ± 0.10 b | 23.09 ± 0.18 b |
| CWPI0 | 5.34 ± 0.11 ac | 27.07 ± 0.17 a | 22.66 ± 0.08 ab | |
| CWPI75 | 5.76 ± 0.20 bc | 46.36 ± 0.14 b | 21.88 ± 0.10 ab | |
| CWPIM | 5.55 ± 0.09 ac | 62.87 ± 0.13 ab | 21.75 ± 0.13 ab | |
| 60 | C0 | 2.20 ± 0.20 | 53.41 ± 0.07 bc | 25.24± 0.18 c |
| CWPI0 | 2.15 ± 0.19 c | 44.93 ± 0.10 ac | 25.54± 0.06 c | |
| CWPI75 | 2.25 ± 0.05 c | 54.88 ± 0.08 abc | 25.29± 0.12 c | |
| CWPIM | 2.59 ± 0.14 abc | 48.32 ± 0.09 abc | 26.59± 0.17 abc |
| Day | Sample | Total Protein (%) | TCA-SN (%) | WSN (%) |
|---|---|---|---|---|
| 1 | C0 | 18.39 ± 0.10 | 0.49 ± 0.13 | 1.68 ± 0.06 b |
| CWPI0 | 18.43 ± 0.16 | 0.70 ± 0.03 | 1.19 ± 0.13 a | |
| CWPI75 | 19.19 ± 0.17 ab | 0.77 ± 0.14 a | 0.98 ± 0.15 a | |
| CWPIM | 19.87 ± 0.15 ab | 0.70 ± 0.02 | 0.98 ± 0.12 a | |
| 15 | C0 | 18.69 ± 0.08 c | 0.91 ± 0.04 c | 3.22 ± 0.18 bc |
| CWPI0 | 18.82 ± 0.14 | 0.84 ± 0.08 | 2.24 ± 0.04 ac | |
| CWPI75 | 19.37 ± 0.16 abc | 0.56 ± 0.05 abc | 2.73 ± 0.02 abc | |
| CWPIM | 19.26 ± 0.15 | 0.42 ± 0.05 | 2.87 ± 0.10 | |
| 30 | C0 | 17.78 ± 0.06 bc | 1.82 ± 0.02 bc | 3.57 ± 0.07 bc |
| CWPI0 | 17.60 ± 0.03 ac | 1.54 ± 0.12 ac | 2.80 ± 0.04 ac | |
| CWPI75 | 19.31 ± 0.09 ab | 1.75 ± 0.02 bc | 2.87 ± 0.02 ac | |
| CWPIM | 19.47 ± 0.06 abc | 1.75 ± 0.12 bc | 3.71 ± 0.05 abc | |
| 60 | C0 | 14.75 ± 0.07 bc | 1.61 ± 0.04 c | 4.48 ± 0.02 bc |
| CWPI0 | 17.44 ± 0.01 ac | 1.61 ± 0.05 c | 3.22 ± 0.16 ac | |
| CWPI75 | 19.02 ± 0.12 ab | 2.17 ± 0.08 abc | 3.64 ± 0.03 abc | |
| CWPIM | 19.35 ± 0.05 abc | 2.17 ± 0.10 abc | 3.15 ± 0.09 ac |
| Day | Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| 1 | C0 | 90.71 b | −2.47 b | 14.61 b | |
| CWPI0 | 89.43 a | −2.24 | 14.78 a | 1.31 | |
| CWPI75 | 89.39 a | −2.56 | 15.62 ab | 1.66 | |
| CWPIM | 91.01 ab | −2.38 | 14.96 ab | 0.47 | |
| 15 | C0 | 91.38 bc | −2.25 bc | 16.51 bc | |
| CWPI0 | 87.5 ac | −1.56 ac | 19.4 ac | 4.89 | |
| CWPI75 | 89.38 ab | −2.03 abc | 14.55 abc | 2.81 | |
| CWPIM | 89.58 abc | −2.12 abc | 17.41 abc | 2.02 | |
| 30 | C0 | 62.5 bc | −0.79 bc | 13.59 bc | |
| CWPI0 | 59.85 ac | −0.33 ac | 12.78 ac | 2.81 | |
| CWPI75 | 74.33 abc | −1.87 abc | 13.36 abc | 11.88 | |
| CWPIM | 75.27 abc | −1.26 abc | 14.45 abc | 12.81 | |
| 45 | C0 | 80.76 bc | −1.02 bc | 16.58 bc | |
| CWPI0 | 77.07 ac | −1.63 ac | 27.48 ac | 11.52 | |
| CWPI75 | 84.37 abc | −2.46 ab | 22.23 abc | 6.86 | |
| CWPIM | 69.76 abc | −1.76 abc | 28.25 abc | 16.05 | |
| 60 | C0 | 72.48 bc | −0.79 bc | 10.67 bc | |
| CWPI0 | 67.34 ac | 0.39 ac | 9.18 ac | 5.48 | |
| CWPI75 | 68.61 abc | 0.08 ac | 8.65 abc | 4.45 | |
| CWPIM | 73.56 abc | −1.02 bc | 20.87 abc | 10.26 |
| Day | Sample | Hardness (N) | Gumminess (N) | Cohesiveness (mm) | Elasticity (mm) | Resilience |
|---|---|---|---|---|---|---|
| 1 | C0 | 7.75 ± 0.06 | 21.11 ± 0.05 b | 0.51 ± 0.13 | −1.03 ± 0.18 | 0.47 ± 0.08 |
| CWPI0 | 7.48 ± 0.12 | 23.77 ± 0.06 a | 0.60 ± 0.02 | −0.76 ± 0.01 | 0.57 ± 0.06 | |
| CWPI75 | 8.07 ± 0.14 ab | 16.93 ± 0.07 ab | 0.51 ± 0.15 | −0.52 ± 0.17 a | 0.46 ± 0.01 | |
| CWPIM | 10.54 ± 0.16 ab | 25.23 ± 0.15 ab | 0.62 ± 0.08 | −0.97 ± 0.02 | 0.57 ± 0.19 | |
| 15 | C0 | 16.95 ± 0.11 bc | 4.39 ± 0.15 bc | 0.36 ± 0.10 | −2.12 ± 0.15 c | 0.31 ± 0.16 |
| CWPI0 | 22.59 ± 0.09 ac | 7.72 ± 0.09 ac | 0.40 ± 0.19 | −2.34 ± 0.07 c | 0.32 ± 0.17 | |
| CWPI75 | 19.04 ± 0.13 abc | 2.99 ± 0.08 abc | 0.33 ± 0.02 | −3.41 ± 0.19 abc | 0.27 ± 0.18 | |
| CWPIM | 25.65 ± 0.04 abc | 11.21 ± 0.06 abc | 0.42 ± 0.12 | −1.32 ± 0.11 abc | 0.35 ± 0.16 | |
| 30 | C0 | 23.47 ± 0.17 bc | 3.74 ± 0.08 bc | 0.22 ± 0.09 | −1.23 ± 0.04 b | 0.37 ± 0.17 |
| CWPI0 | 17.05 ± 0.02 ac | 4.71 ± 0.06 ac | 0.21 ± 0.18 c | −1.54 ± 0.14 ac | 0.30 ± 0.04 | |
| CWPI75 | 23.99 ± 0.13 abc | 6.00 ± 0.18 abc | 0.32 ± 0.20 | −3.09 ± 0.12 abc | 0.31 ± 0.06 | |
| CWPIM | 24.45 ± 0.03 abc | 8.76 ± 0.20 abc | 0.34 ± 0.08 c | −3.46 ± 0.15 abc | 0.25 ± 0.12 | |
| 45 | C0 | 22.20 ± 0.16 bc | 6.21 ± 0.15 bc | 0.26 ± 0.18 | −1.18 ± 0.11 b | 0.39 ± 0.14 |
| CWPI0 | 33.07 ± 0.06 ac | 5.72 ± 0.09 ac | 0.34 ± 0.06 | −3.10 ± 0.15 ac | 0.25 ± 0.15 c | |
| CWPI75 | 23.27 ± 0.09 abc | 8.90 ± 0.06 abc | 0.37 ± 0.17 | −3.35 ± 0.11 ac | 0.30 ± 0.08 | |
| CWPIM | 31.66 ± 0.01 abc | 9.65 ± 0.12 abc | 0.39 ± 0.14 | −2.90 ± 0.10 ac | 0.28 ± 0.14 | |
| 60 | C0 | 41.46 ± 0.14 bc | 4.99 ± 0.20 bc | 0.22 ± 0.16 | −5.59 ± 0.01 bc | 0.15 ± 0.19 |
| CWPI0 | 39.63 ± 0.05 ac | 9.14 ± 0.08 ac | 0.28 ± 0.12 c | −4.56 ± 0.16 ac | 0.21 ± 0.17 c | |
| CWPI75 | 33.07 ± 0.16 abc | 6.29 ± 0.08 abc | 0.27 ± 0.04 | −3.17 ± 0.06 abc | 0.20 ± 0.05 c | |
| CWPIM | 40.65 ± 0.14 abc | 9.63 ± 0.11 abc | 0.30 ± 0.08 c | −3.11 ± 0.06 abc | 0.23 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zandona, E.; Vukelić, M.; Hanousek Čiča, K.; Zandona, A.; Mrvčić, J.; Katalinić, M.; Cindrić, I.; Abdurramani, A.; Jurina, I.B. Bioactive Properties of the Microwave-Assisted Olive Leaf Extract and Its Incorporation into a Whey Protein Isolate Coating of Semi-Hard Cheese. Foods 2025, 14, 1496. https://doi.org/10.3390/foods14091496
Zandona E, Vukelić M, Hanousek Čiča K, Zandona A, Mrvčić J, Katalinić M, Cindrić I, Abdurramani A, Jurina IB. Bioactive Properties of the Microwave-Assisted Olive Leaf Extract and Its Incorporation into a Whey Protein Isolate Coating of Semi-Hard Cheese. Foods. 2025; 14(9):1496. https://doi.org/10.3390/foods14091496
Chicago/Turabian StyleZandona, Elizabeta, Maja Vukelić, Karla Hanousek Čiča, Antonio Zandona, Jasna Mrvčić, Maja Katalinić, Ines Cindrić, Almir Abdurramani, and Irena Barukčić Jurina. 2025. "Bioactive Properties of the Microwave-Assisted Olive Leaf Extract and Its Incorporation into a Whey Protein Isolate Coating of Semi-Hard Cheese" Foods 14, no. 9: 1496. https://doi.org/10.3390/foods14091496
APA StyleZandona, E., Vukelić, M., Hanousek Čiča, K., Zandona, A., Mrvčić, J., Katalinić, M., Cindrić, I., Abdurramani, A., & Jurina, I. B. (2025). Bioactive Properties of the Microwave-Assisted Olive Leaf Extract and Its Incorporation into a Whey Protein Isolate Coating of Semi-Hard Cheese. Foods, 14(9), 1496. https://doi.org/10.3390/foods14091496








