Engineering Baker’s Yeast for Efficient cAMP Synthesis via Regulation of PKA Activity
Abstract
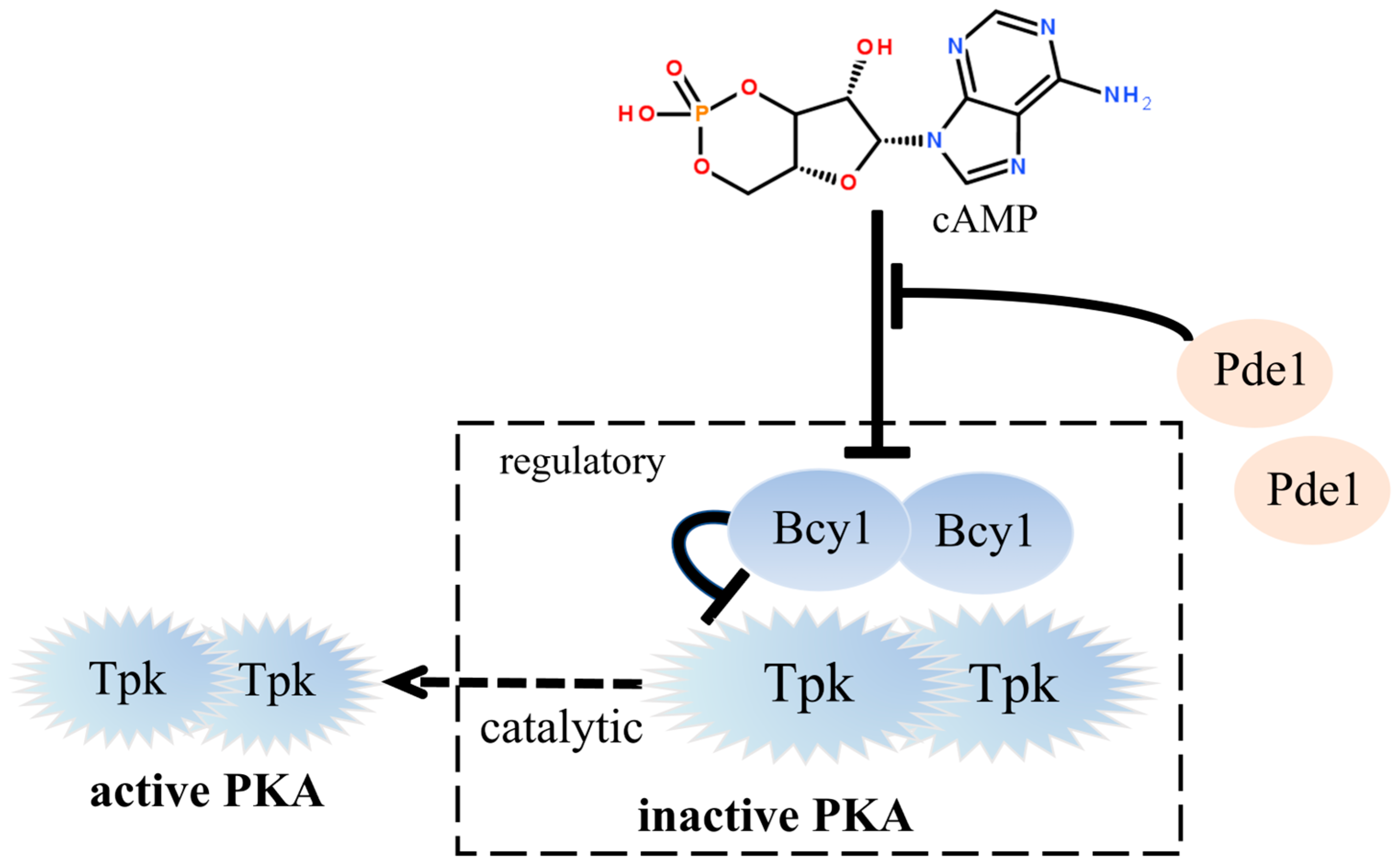
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Medium
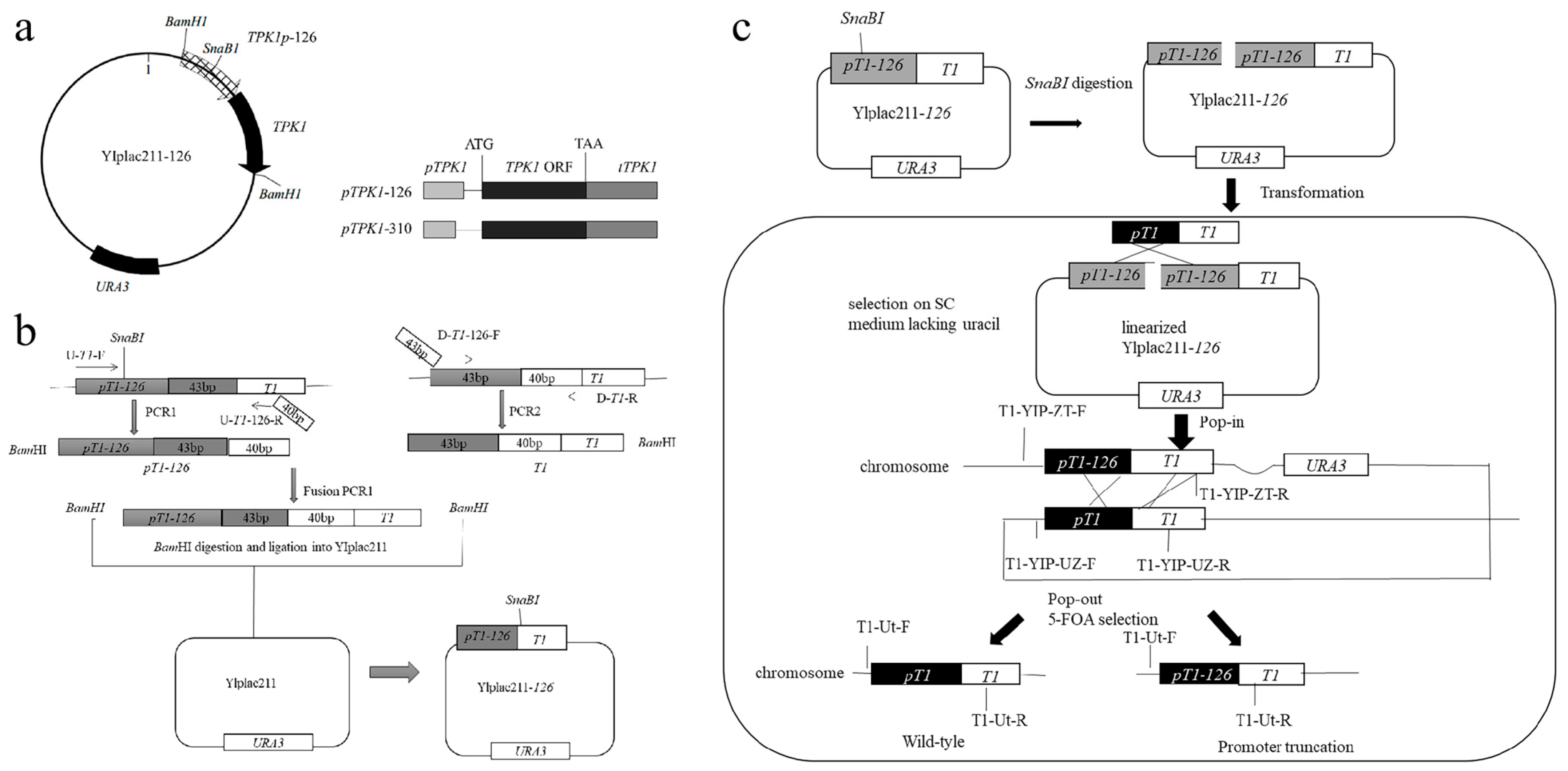
2.2. Construction of Engineered Strains
2.3. Measurements of Intracellular cAMP in Engineered Strains
2.4. RT-qPCR Assay
2.5. Determination of Heat Shock Resistance in Engineered Strains
3. Results
3.1. Construction of Engineered Strains
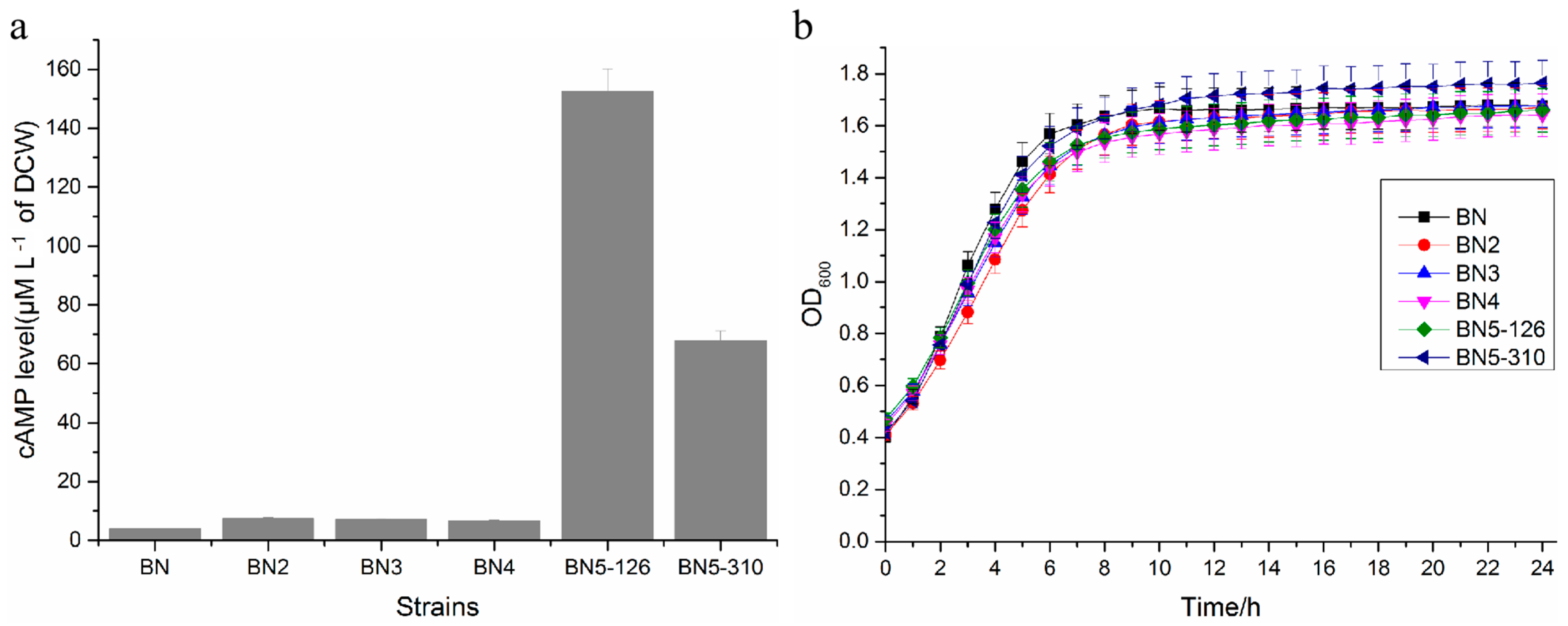
3.2. cAMP Levels and Growth Performance of Engineered Strains
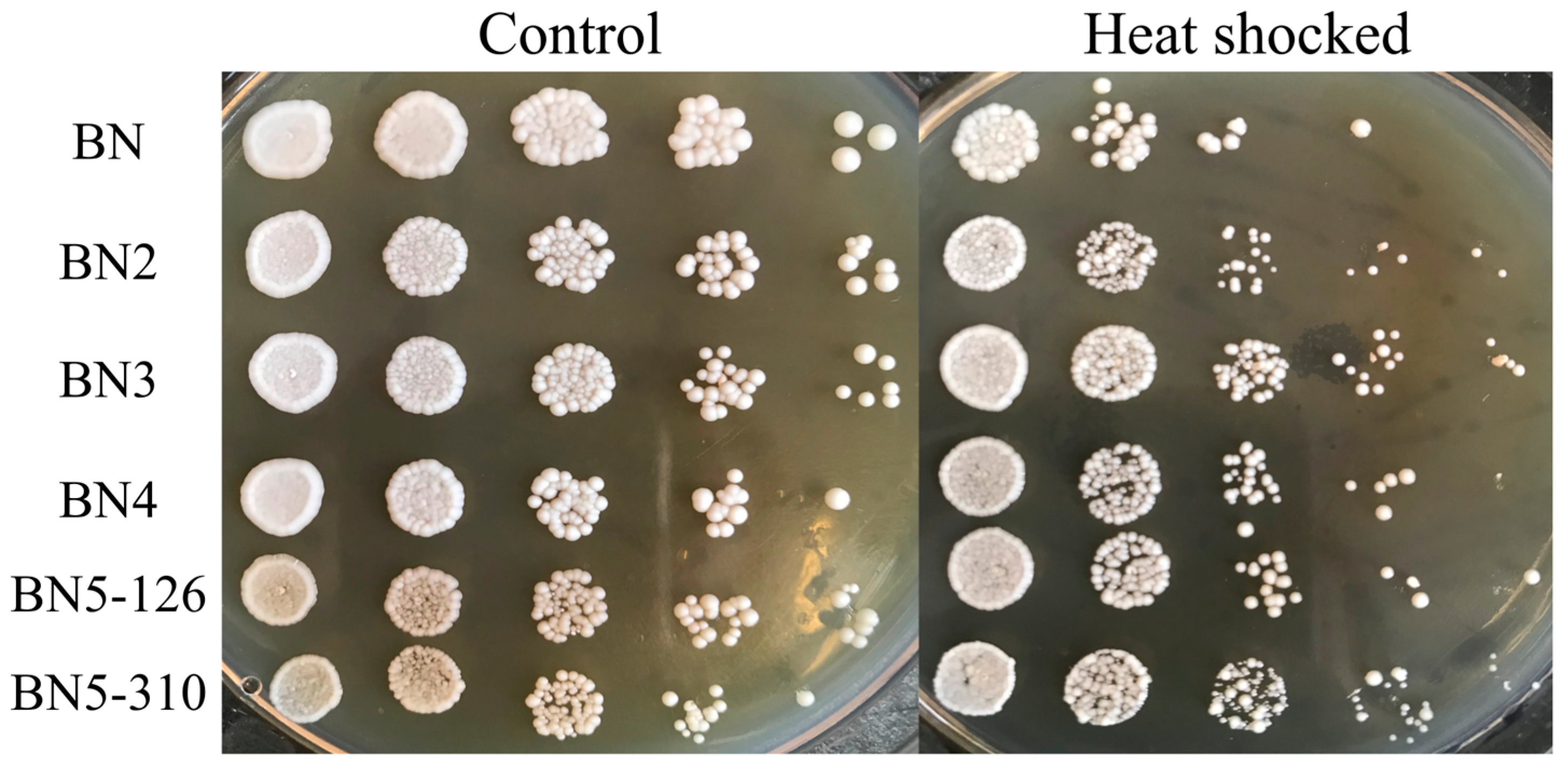
3.3. Heat Shock Resistance of the Engineered Strains
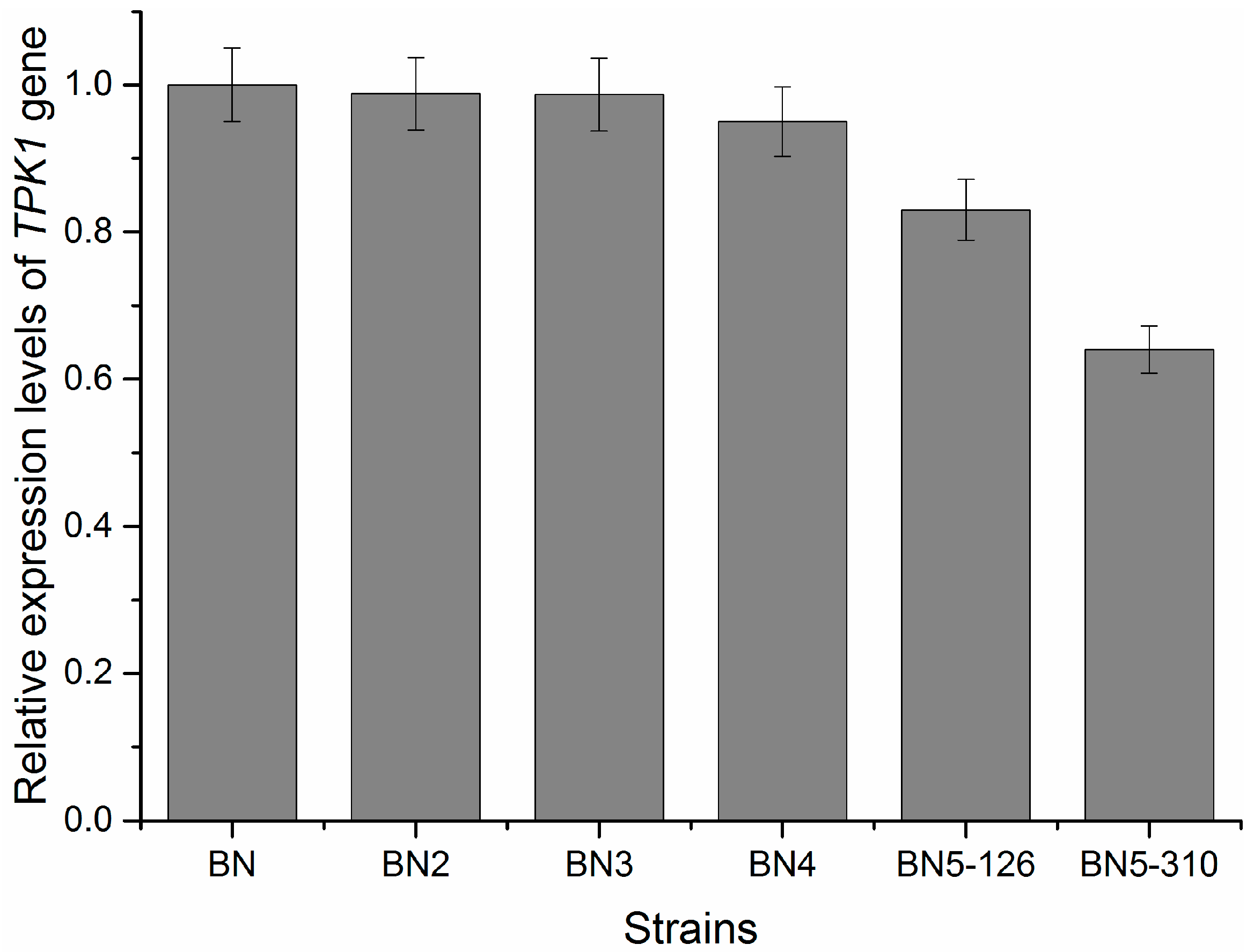
3.4. Relative Expression Level of TPK1 Gene in Engineered Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moutinho, A.; Hussey, P.J.; Trewavas, A.J.; Malhó, R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc. Natl. Acad. Sci. USA 2001, 98, 10481–10486. [Google Scholar] [CrossRef]
- Ma, J.; Staler, D.W.; Mahato, R.I. Targeting cAMP signaling and phosphodiesterase 4 for liver disease treatment. Med. Chem. Res. 2024, 33, 1339–1353. [Google Scholar] [CrossRef]
- Shibasaki, T.; Takahashi, H.; Miki, T.; Sunaga, Y.; Matsumura, K.; Yamanaka, M.; Zhang, C.; Tamamoto, A.; Satoh, T.; Miyazaki, J.I. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. USA 2007, 104, 19333–19338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Geng, Y.; Yuan, J.; Cui, Q.; Wang, X.; Liu, J. Determination of cAMP in Fructus jujubae by UAE-HILIC-DAD-ESI-Q-TOF/MS. Food Res. Dev. 2013, 34, 46–50. [Google Scholar]
- Liu, Y.; He, J.; Xu, Q.; Zhang, C.; Chen, N.; Xie, X. Enhanced Adenosine Production by Bacillus subtilis at Condition with Comprehensively Controlled Dissolved Oxygen and pH During Fermentation. Lect. Notes Electr. Eng. 2015, 332, 439–452. [Google Scholar]
- Yajko, D.M.; Zusman, D.R. Changes in cyclic AMP levels during development in Myxococcus xanthus. J. Bacteriol. 1978, 133, 1540–1542. [Google Scholar] [CrossRef]
- Notley-McRobb, L.; Death, A.; Ferenci, T. The relationship between external glucose concentration and cAMP levels inside Escherichia coli: Implications for models of phosphotransferase-mediated regulation of adenylate cyclase. Microbiology 1997, 143, 1909–1918. [Google Scholar] [CrossRef]
- Attaran, S.; Yokoyama, W.; Pan, J.; Berrios, J.J. Influence of extruded lentil containing high chromium nutritional yeast on the main physiological factors associated with metabolic syndrome in rodent models. Food Funct. 2018, 9, 5238–5244. [Google Scholar] [CrossRef]
- Harusekwi, S.J.; Nyamunda, B.C.; Mutonhodza, B. Development of High Protein Content Homemade Bread by Nutritional Yeast Fortification for Disadvantaged Communities. Int. J. Nutr. Food Sci. 2014, 3, 194. [Google Scholar] [CrossRef]
- Rødkær, S.V.; Færgeman, N.J. Glucose- and nitrogen sensing and regulatory mechanisms in Saccharomyces cerevisiae. FEMS Yeast Res. 2014, 14, 683–696. [Google Scholar] [CrossRef]
- Namy, O.; Duchateau-Nguyen, G.; Rousset, J.P. Translational readthrough of the PDE2 stop codon modulates cAMP levels in Saccharomyces cerevisiae. Mol. Microbiol. 2002, 43, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Crauwels, M.; Donaton, M.C.V.; Pernambuco, M.B.; Winderickx, J.; De Winde, J.H.; Thevelein, J.M. The Sch9 protein kinase in the yeast Saccharomyces cerevisiae controls cAPK activity and is required for nitrogen activation of the fermentable-growth-medium-induced (FGM) pathway. Microbiology 1997, 143 Pt 8, 2627–2637. [Google Scholar] [CrossRef]
- Toda, T.; Uno, I.; Ishikawa, T.; Powers, S.; Kataoka, T.; Broek, D.; Cameron, S.; Broach, J.; Matsumoto, K.; Wigler, M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 1985, 40, 27–36. [Google Scholar] [CrossRef]
- Mbonyi, K.; Van Aelst, L.; Argüelles, J.C.; Jans, A.W.; Thevelein, J.M. Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 1990, 10, 4518–4523. [Google Scholar] [PubMed]
- Toda, T.; Cameron, S.; Sass, P.; Zoller, M.; Wigler, M. Three different genes in Saccharomyces cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 1987, 50, 277–287. [Google Scholar] [CrossRef] [PubMed]
- MAZÓN, M.J.; Margaritabehrens, M.; Morgado, E.; Portillo, F. Low activity of the yeast cAMP-dependent protein kinase catalytic subunit Tpk3 is due to the poor expression of the TPK3 gene. Eur. J. Biochem. 2010, 213, 501–506. [Google Scholar] [CrossRef]
- Nikawa, J.; Cameron, S.; Toda, T.; Ferguson, K.M.; Wigler, M. Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1987, 1, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Zhao, H.; Zhao, H. DNA assembler, an in vivo genetic method for rapid construction of biochemical pathways. Nucleic Acids Res. 2009, 37, e16. [Google Scholar] [CrossRef]
- Yörük, E.; Albayrak, G. Geneticin (G418) resistance and electroporation-mediated transformation of Fusarium graminearum and F. culmorum. Biotechnol. Biotechnol. Equip. 2015, 29, 268–273. [Google Scholar] [CrossRef]
- Cameron, A.; Gaynor, E.C. Hygromycin B and Apramycin Antibiotic Resistance Cassettes for Use in Campylobacter jejuni. PLoS ONE 2014, 9, e95084. [Google Scholar] [CrossRef]
- Hong, K.-Q.; Hou, X.-Y.; Hao, A.-L.; Wang, P.-F.; Fu, X.-M.; Lv, A.; Dong, J. Truncation of CYR1 promoter in industrial ethanol yeasts for improved ethanol yield in high temperature condition. Process Biochem. 2018, 65, 37–45. [Google Scholar] [CrossRef]
- Bai, X.; Jian, D. Serine214 of Ras2p plays a role in the feedback regulation of the Ras-cAMP pathway in the yeast Saccharomyces cerevisiae. FEBS Lett. 2010, 584, 2333–2338. [Google Scholar] [CrossRef]
- Lin, P.; Lan, X.; Chen, F.; Yang, Y.; Jin, Y.; Wang, A. Reference gene selection for real-time quantitative PCR analysis of the mouse uterus in the peri-implantation period. PLoS ONE 2013, 8, e62462. [Google Scholar] [CrossRef] [PubMed]
- Fenella, S.; Zhiqiang, Z.; Griet, V.Z.; Thevelein, J.M. Multiple Transceptors for Macro- and Micro-Nutrients Control Diverse Cellular Properties Through the PKA Pathway in Yeast: A Paradigm for the Rapidly Expanding World of Eukaryotic Nutrient Transceptors Up to Those in Human Cells. Front. Pharmacol. 2018, 9, 191. [Google Scholar]
- Dong, J.; Wang, G.; Zhang, C.; Tan, H.; Sun, X.; Wu, M.; Xiao, D. A two-step integration method for seamless gene deletion in baker’s yeast. Anal. Biochem. 2013, 439, 30–36. [Google Scholar] [CrossRef]
- Peng, B.; Chen, X.; Shen, Y.; Bao, X. Effect of controlled overexpression of xylulokinase by different promoters on xylose metabolism in Saccharomyces cerevisiae. Acta Microbiol. Sin. 2011, 51, 914–922. [Google Scholar]
- Ye, L.; Kruckeberg, A.L.; Berden, J.A.; van Dam, K. Construction and expression of HXT7 promoter deletion mutants in Saccharomyces cerevisiae. Prog. Biochem. Biophys. 2001, 28, 662–669. [Google Scholar]
- Dong, J.; Hong, K.Q.; Hao, A.L.; Zhang, C.Y.; Fu, X.M.; Wang, P.F.; Xiao, D.G. Gradual Enhancement of Ethyl Acetate Production Through Promoter Engineering in Chinese Liquor Yeast Strains. Biotechnol. Prog. 2018, 34, 328–336. [Google Scholar] [CrossRef]
- Portela, P.; Rossi, S. cAMP-PKA signal transduction specificity in Saccharomyces cerevisiae. Curr. Genet. 2020, 66, 1093–1099. [Google Scholar] [CrossRef]
- Li, N.; He, Y.; Chen, Y.; Chen, X.; Bai, J.; Wu, J.; Xie, J.; Ying, H. Production of cyclic adenosine-3′,5′-monophosphate by whole cell catalysis using recombinant Escherichia coli overexpressing adenylate cyclase. Korean J. Chem. Eng. 2013, 30, 913–917. [Google Scholar] [CrossRef]
- Pastan, I.; Perlman, R. Cyclic Adenosine Monophosphate in Bacteria. Science 1970, 169, 339–344. [Google Scholar] [CrossRef]
- Mach, H.; Hecker, M.; Mach, F. Evidence for the presence of cyclic adenosine monophosphate in Bacillus subtilis. FEMS Microbiol. Lett. 1984, 22, 27–30. [Google Scholar] [CrossRef]
- Pereira, F.B.; Guimares, P.M.R.; Teixeira, J.A.; Domingues, L. Selection of Saccharomyces cerevisiae strains for efficient very high gravity bio-ethanol fermentation processes. Biotechnol. Lett. 2010, 32, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.T.; Schneper, L.; Russo, M.; Fernandes, A.A.R.; Broach, J.R.; Fernandes, P.M.B. Comparative Transcriptome Analysis of Industrial and Laboratory Saccharomyces cerevisiae Strains after Sequential Stresses. Fermentation 2024, 10, 395. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, P.; Wang, H.; Zhang, J.X.; Li, H.; Liu, J.Z. Simultaneous HPLC Determination of cAMP and cGMP in Commercial Jujube Juice Concentrate. Food Sci. 2009, 30, 321–322. [Google Scholar]
- Han, K.; Hong, J.; Lim, H.C. cAMP, ethanol, and CO2 production with the addition of D-glucose anomer to starved yeast cells. Biochem. Biophys. Res. Commun. 1994, 203, 640–645. [Google Scholar] [CrossRef]
- Ding, M.; Su, M.; Xiao, D. Studies on screening of edible nutrition yeast. Ind. Microbiol. 2003, 33, 27–29. [Google Scholar]
- Branduardi, P.; Fossati, T.; Sauer, M.; Pagani, R.; Porro, D. Biosynthesis of Vitamin C by Yeast Leads to Increased Stress Resistance. PLoS ONE 2007, 2, e1092. [Google Scholar] [CrossRef]
| Strain | Genotype | Source |
|---|---|---|
| BYN | Industrial baker’s yeast | Our lab |
| BN | baker’s yeast, MATa | This study |
| BN1 | MATa, ura3 was inserted in the URA3 site | This study |
| BN2 | MATa, TPK3 was deleted and URA3 was inserted | This study |
| BN3 | BN2, BCY1 was deleted and KanMX was inserted | This study |
| BN4 | BN3, TPK2 was deleted and HPH was inserted | This study |
| BN5 | BN4, ura3 was inserted in the URA3 site | This study |
| BN5-126 | BN5, URA3 and TPK1p−126 were inserted in the ura3 site | This study |
| BN5-310 | BN5, URA3 and TPK1p−310 were inserted in the ura3 site | This study |
| Plasmid | Genotype | Source |
| YIplac211 | Ampr, URA3 | Our lab |
| YIplac211-126 | YIplac211, pTPK1−126-TPK1-tTPK1 | This study |
| YIplac211-310 | YIplac211, pTPK1−310-TPK1-tTPK1 | This study |
| DH5ɑ | supE44 DlacU169(u 80lacZDM15) hsdR17 recAl endAl gyrA96 thi-1 relA | Beyotime Biotech Ltd. |
| pUG6 | Ampr, KanMX | Our lab |
| pDH25 | Ampr, HPH | Our lab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Hong, K. Engineering Baker’s Yeast for Efficient cAMP Synthesis via Regulation of PKA Activity. Foods 2025, 14, 1533. https://doi.org/10.3390/foods14091533
Fu X, Hong K. Engineering Baker’s Yeast for Efficient cAMP Synthesis via Regulation of PKA Activity. Foods. 2025; 14(9):1533. https://doi.org/10.3390/foods14091533
Chicago/Turabian StyleFu, Xiaomeng, and Kunqiang Hong. 2025. "Engineering Baker’s Yeast for Efficient cAMP Synthesis via Regulation of PKA Activity" Foods 14, no. 9: 1533. https://doi.org/10.3390/foods14091533
APA StyleFu, X., & Hong, K. (2025). Engineering Baker’s Yeast for Efficient cAMP Synthesis via Regulation of PKA Activity. Foods, 14(9), 1533. https://doi.org/10.3390/foods14091533






