Volatile Compounds and Fatty Acids of Mutton Carrot Filling During Dynamic Steaming Investigated Based on GC-MS and GC-IMS Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Re-Steaming Process
2.3. Methods
2.3.1. Volatile Compounds Detected by SPME-GC-MS
2.3.2. HS-GC-IMS Analysis
2.3.3. Fatty Acid Analysis
2.4. Statistical Analysis
3. Results
3.1. Comprehensive Characterization of VOC Profiles by SPME-GC-MS
3.2. OPLS-DA Analysis
3.3. GC-IMS Analysis
3.4. Fatty Acids Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Wang, Y.; Zhang, Y.; Yang, Z.; Ma, Z.; Chen, J.; Chen, X.; Qiu, Z.; Tian, J.; Pu, A.; et al. Formation and Regulation Strategies for Volatile Off-Flavor Compounds in Livestock Meat, Poultry Meat, and Their Products: A Comprehensive Review. Trends Food Sci. Technol. 2024, 152, 104689. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Ouyang, G.; Zhou, G. Changes in Volatile Compounds of Traditional Chinese Nanjing Water-Boiled Salted Duck During Processing. J. Food Sci. 2006, 71, S371–S377. [Google Scholar] [CrossRef]
- Watkins, P.J.; Kearney, G.; Rose, G.; Allen, D.; Ball, A.J.; Pethick, D.W.; Warner, R.D. Effect of Branched-Chain Fatty Acids, 3-Methylindole and 4-Methylphenol on Consumer Sensory Scores of Grilled Lamb Meat. Meat Sci. 2014, 96, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.S.; Cho, S.H.; Kim, J.O.; Kim, M.N. Lipid Classes, Fatty Acids, Flavor and Storage Stability of Washed Sheep Meat. J. Food Sci. 1998, 63, 168–172. [Google Scholar] [CrossRef]
- Liu, W.; Ding, H.; Erdene, K.; Chen, R.; Mu, Q.; Ao, C. Effects of Flavonoids from Allium Mongolicum Regel as a Dietary Additive on Meat Quality and Composition of Fatty Acids Related to Flavor in Lambs. Can. J. Anim. Sci. 2019, 99, 15–23. [Google Scholar] [CrossRef]
- Girard, M.; Dohme-Meier, F.; Silacci, P.; Ampuero Kragten, S.; Kreuzer, M.; Bee, G. Forage Legumes Rich in Condensed Tannins May Increase N-3 Fatty Acid Levels and Sensory Quality of Lamb Meat. J. Sci. Food Agric. 2016, 96, 1923–1933. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Gao, F.; Xu, Y.; Guo, M. Effects of Polymerized Whey Protein on Goaty Flavor and Texture Properties of Fermented Goat Milk in Comparison with β-Cyclodextrin. J. Dairy Res. 2018, 85, 465–471. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–Polyphenol Conjugates: Antioxidant Property, Functionalities and Their Applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Y.; Saleh, A.S.M.; Akhtar, K.H.; Li, W.; Zhang, D.; Wang, Z. Conformational Changes Induced by Selected Flavor Compounds from Spices Regulate the Binding Ability of Myofibrillar Proteins to Aldehyde Compounds. Food Chem. 2024, 451, 139455. [Google Scholar] [CrossRef]
- Grabež, V.; Bjelanović, M.; Rohloff, J.; Martinović, A.; Berg, P.; Tomović, V.; Rogić, B.; Egelandsdal, B. The Relationship between Volatile Compounds, Metabolites and Sensory Attributes: A Case Study Using Lamb and Sheep Meat. Small Rumin. Res. 2019, 181, 12–20. [Google Scholar] [CrossRef]
- Yu, L.; Pang, Y.; Shwn, G.; Bai, B.; Yang, Y.; Zeng, M. Identification and Selection of Volatile Compounds Derived from Lipid Oxidation as Indicators for Quality Deterioration of Frozen White Meat and Red Meat Using HS-SPME-GC–MS Combined with OPLS-DA. Food Chem. 2025, 463, 141112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Han, W.; Gui, L.; Yuan, Z.; Hou, S.; Wang, Z.; Yang, B.; Raza, S.H.A.; Alowais, A.F.S.; et al. Effect of Different Heat Treatments on the Quality and Flavor Compounds of Black Tibetan Sheep Meat by HS-GC-IMS Coupled with Multivariate Analysis. Molecules 2022, 28, 165. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Cho, S.; Seong, P.; Park, B.; Kim, S.; Kim, D.; Kim, Y.; Kang, S.; Park, K. Effects of High Pressure Processing on Fatty Acid Composition and Volatile Compounds in Korean Native Black Goat Meat. Meat Sci. 2013, 94, 495–499. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, T.; Wang, S.; Sun, J.; Chen, H.; Zhang, N.; Wang, S. A Comprehensive Review on Botany, Chemical Composition and the Impacts of Heat Processing and Dehydration on the Aroma Formation of Fresh Carrot. Food Chem. X 2024, 22, 101201. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Fulgoni, V.L.; Hopfer, H.; Hayes, J.E.; Gooding, R.; Kris-Etherton, P. Using Herbs/Spices to Enhance the Flavor of Commonly Consumed Foods Reformulated to Be Lower in Overconsumed Dietary Components Is an Acceptable Strategy and Has the Potential to Lower Intake of Saturated Fat and Sodium: A National Health and Nutrition Examination Survey Analysis and Blind Tasting. J. Acad. Nutr. Diet. 2024, 124, 15–27.e1. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Huang, A. A Metabolomics-Based Approach Investigates Volatile Flavor Formation and Characteristic Compounds of the Dahe Black Pig Dry-Cured Ham. Meat Sci. 2019, 158, 107904. [Google Scholar] [CrossRef]
- Cai, L.; Li, D.; Dong, Z.; Cao, A.; Lin, H.; Li, J. Change Regularity of the Characteristics of Maillard Reaction Products Derived from Xylose and Chinese Shrimp Waste Hydrolysates. LWT Food Sci. Technol. 2016, 65, 908–916. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of Cooking Methods on Changes in Fatty Acids Contents, Lipid Oxidation and Volatile Compounds of Rabbit Meat. LWT Food Sci. Technol. 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Dermiki, M.; Phanphensophon, N.; Mottram, D.S.; Methven, L. Contributions of Non-Volatile and Volatile Compounds to the Umami Taste and Overall Flavour of Shiitake Mushroom Extracts and Their Application as Flavour Enhancers in Cooked Minced Meat. Food Chem. 2013, 141, 77–83. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Wang, R.; Li, X.; Mi, H.; Li, J. Effect of Heat Treatment on the Binding of Selected Flavor Compounds to Myofibrillar Proteins. J. Sci. Food Agric. 2019, 99, 5028–5034. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Zhao, H.; Yin, Y.; Li, X.; Yi, S.; Li, J. Effect of Heat Treatment Duration on the Interaction between Fish Myosin and Selected Flavor Compounds. J. Sci. Food Agric. 2020, 100, 4457–4463. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, J.; Nikiforov, A.; De Roose, M.; Leys, C.; De Cooman, L.; Van Durme, J. Controlled Accelerated Oxidation of Oleic Acid Using a DBD Plasma: Determination of Volatile Oxidation Compounds. Food Res. Int. 2016, 79, 54–63. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Yue, Y.; Zhu, Z.; Liao, E.; Xia, W. Modelling the Mass Transfer Kinetics of Battered and Breaded Fish Nuggets during Deep-Fat Frying at Different Frying Temperatures. J. Food Qual. 2020, 2020, 8874163. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Improving the Oxidative Stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Sun, B.; Wang, S.; Zhang, Y.; Zang, M.; Le, W.; Wang, H.; Wu, Q. Effect of Different Cooking Water on Flavor Characteristics of Mutton Soup. Food Sci. Nutr. 2021, 9, 6047–6059. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sañudo, C.; Escudero, A. Relationship Between Odour-Active Compounds and Flavour Perception in Meat from Lambs Fed Different Diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of Thermal Treatment on Formation of Volatile Compounds, Cooking Loss and Lipid Oxidation in Foal Meat. LWT Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Snel, S.J.E.; Pascu, M.; Bodnár, I.; Avison, S.; van der Goot, A.J.; Beyrer, M. Flavor-Protein Interactions for Four Plant Proteins with Ketones and Esters. Heliyon 2023, 9, e16503. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Ohshima, T. Identification and Characterisation of Headspace Volatiles of Fish Miso, a Japanese Fish Meat Based Fermented Paste, with Special Emphasis on Effect of Fish Species and Meat Washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of Volatile Flavor Compounds and Characterization of Aroma-Active Compounds of Water-Boiled Salted Duck Using GC–MS–O, GC–IMS, and E-Nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J. Evaluation of Mutton Quality Characteristics of Dongxiang Tribute Sheep Based on Membership Function and Gas Chromatography and Ion Mobility Spectrometry. Front. Nutr. 2022, 9, 852399. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Y.; Wang, Z.; Akhtar, K.H.; Saleh, A.S.M.; Li, W.; Zhang, D. Insights into Flavor Formation of Braised Chicken: Based on E-Nose, GC–MS, GC-IMS, and UPLC-Q-Exactive-MS/MS. Food Chem. 2024, 448, 138972. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, B.; Zhang, H.; Wu, Z.; Li, M.; Wang, D.; Wang, C. Combining with E-Nose, GC-MS, GC-IMS and Chemometrics to Explore Volatile Characteristics during the Different Stages of Zanthoxylum Bungeanum Maxim Fruits. Food Res. Int. 2024, 195, 114964. [Google Scholar] [CrossRef]
- Ding, W.; Kou, L.; Cao, B.; Wei, Y. Meat Quality Parameters of Descendants by Grading Hybridization of Boer Goat and Guanzhong Dairy Goat. Meat Sci. 2010, 84, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Y.; Nan, W.; Zhou, W.; Huang, J.; Li, R.; Zhou, L.; Hu, R. Interaction of TPPP3 with VDAC1 Promotes Endothelial Injury through Activation of Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2020, 202, 5950195. [Google Scholar] [CrossRef]
- Sañudo, C.; Enser, M.E.; Campo, M.M.; Nute, G.R.; María, G.; Sierra, I.; Wood, J.D. Fatty Acid Composition and Sensory Characteristics of Lamb Carcasses from Britain and Spain. Meat Sci. 2000, 54, 339–346. [Google Scholar] [CrossRef]
- Cameron, N.D.; Enser, M.B. Fatty Acid Composition of Lipid in Longissimus Dorsi Muscle of Duroc and British Landrace Pigs and Its Relationship with Eating Quality. Meat Sci. 1991, 29, 295–307. [Google Scholar] [CrossRef]

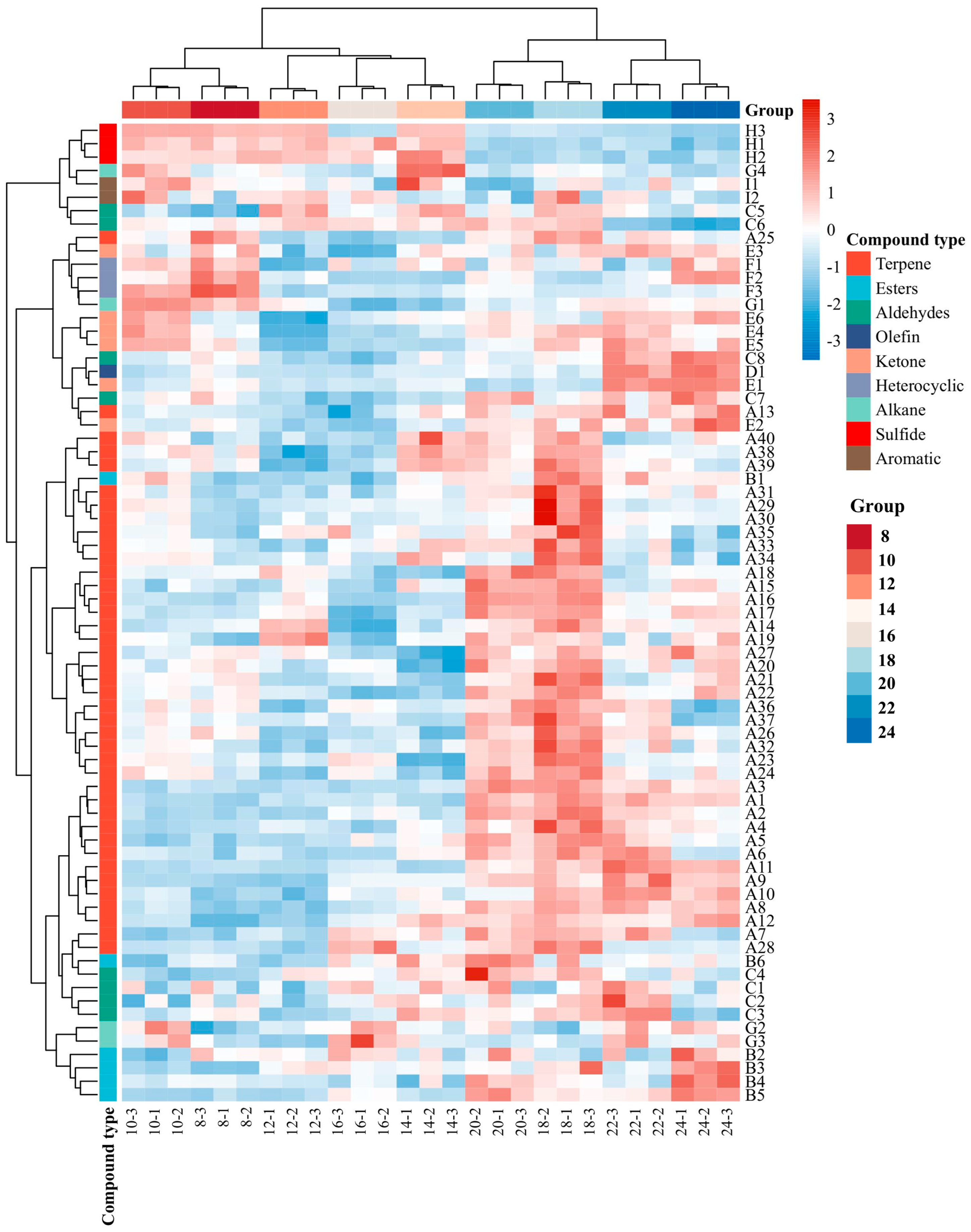
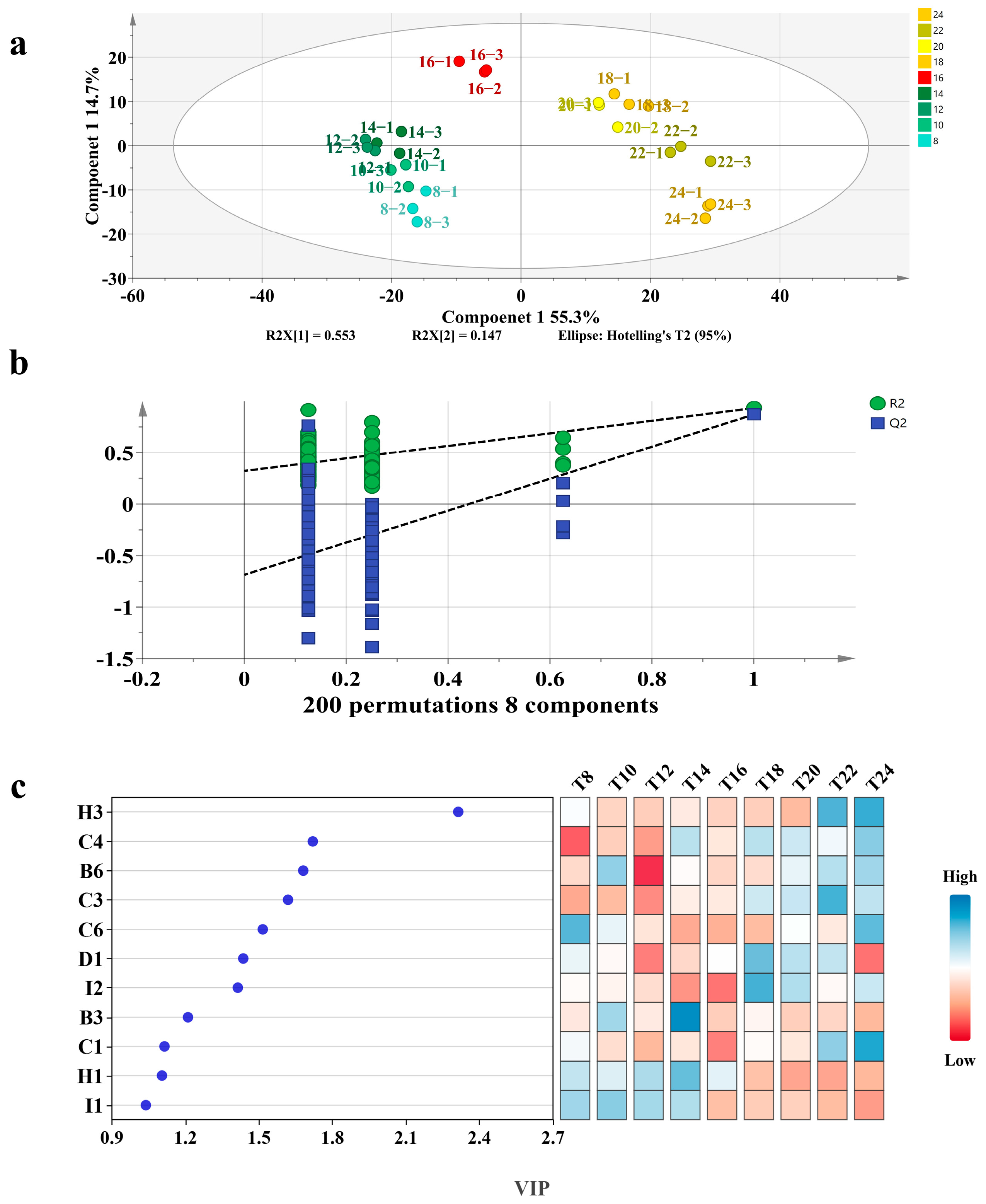
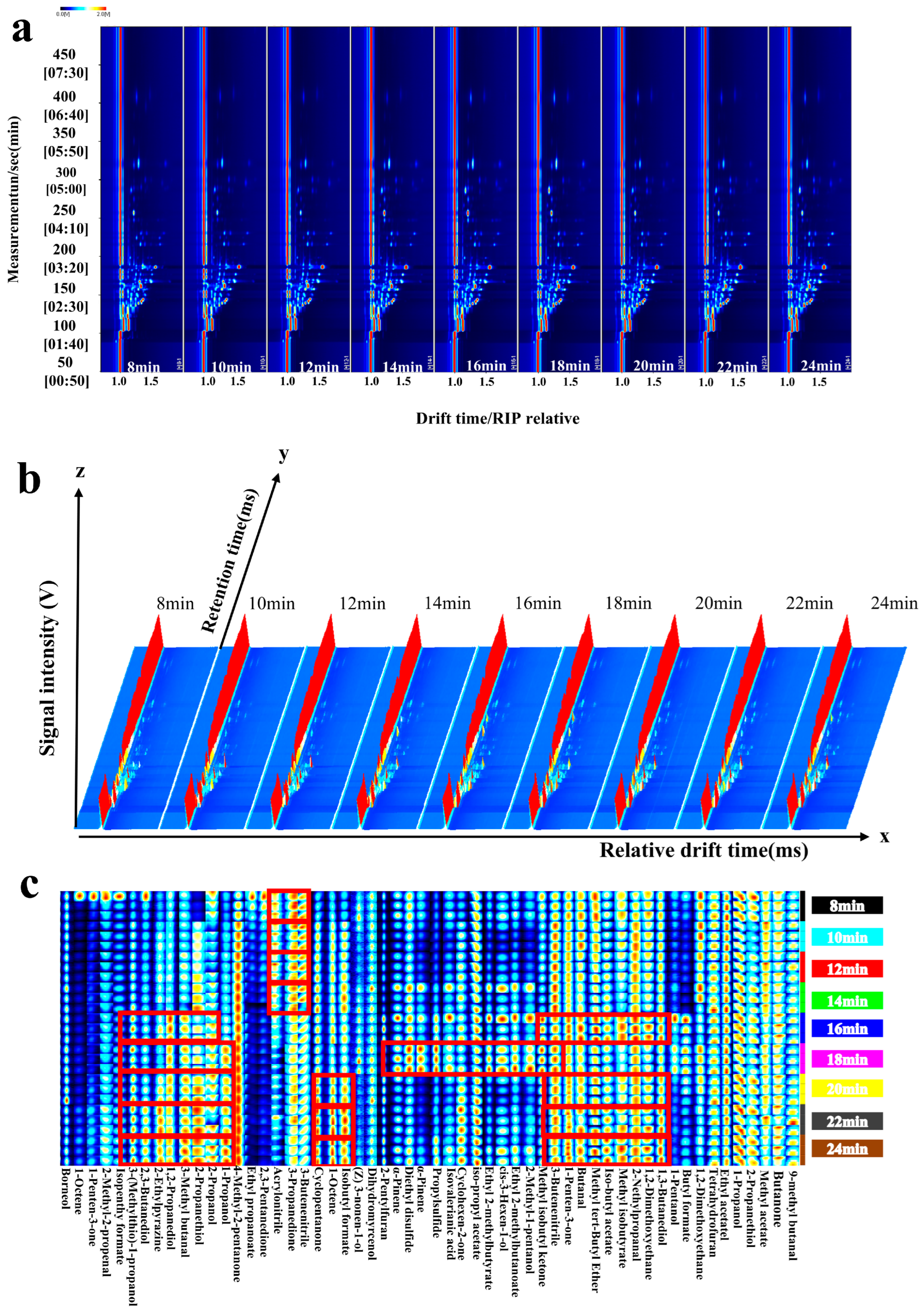
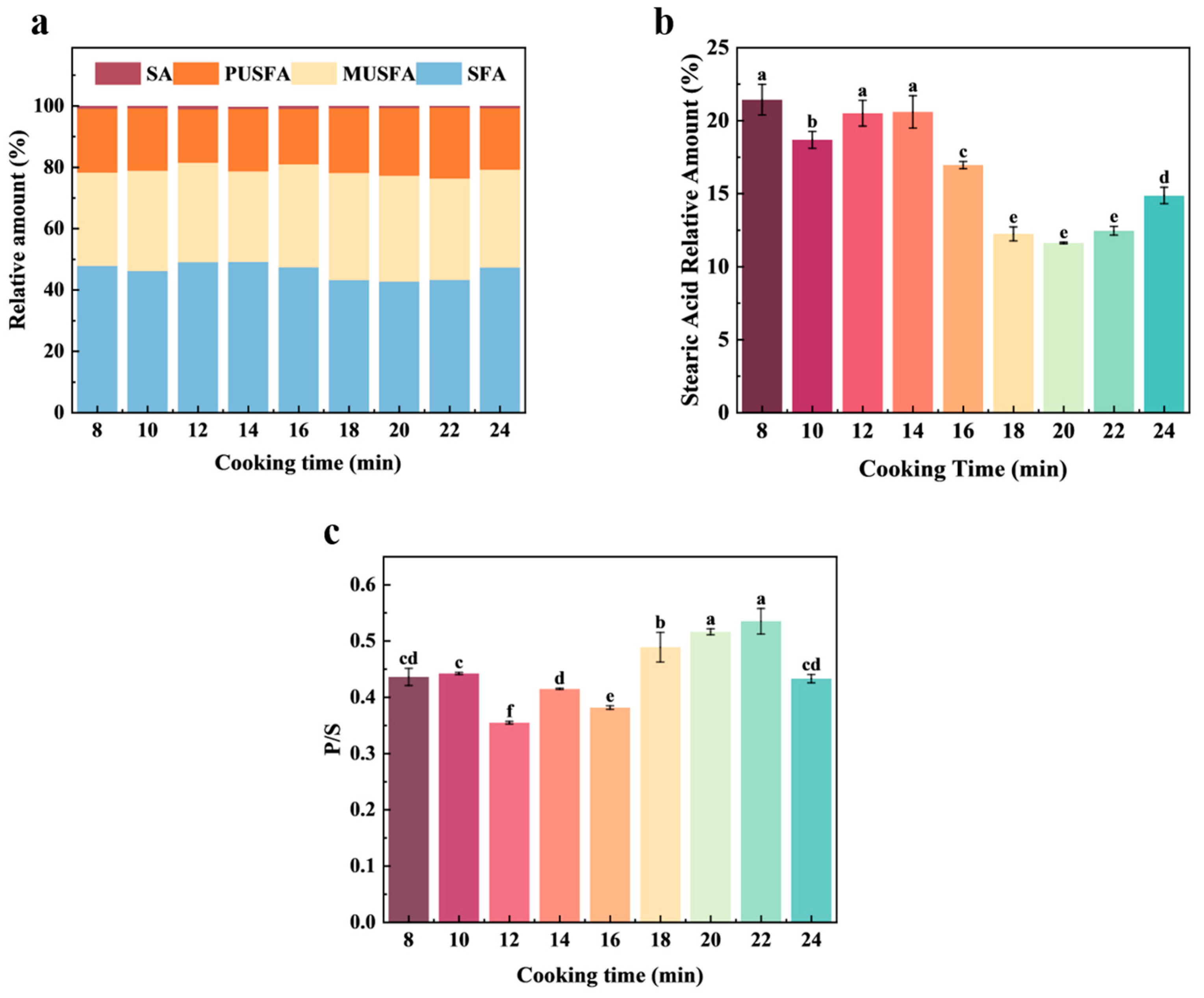
| Code | Volatile Compounds | CAS | RI Literature | RI Calculation |
|---|---|---|---|---|
| A1 | terpinolene | 586-62-9 | 1090 | 1081 |
| A2 | (+)-7-epi-sesquithujene | 159407-35-9 | 1389 | 1380 |
| A3 | 2-isopropyl-5-methylanisole | 1076-56-8 | 1235 | 1245 |
| A4 | 4-allylanisole | 140-67-0 | 1204 | 1213 |
| A5 | (+)-citronellal | 2385-77-5 | 1154 | 1154 |
| A6 | (E)-citral | 141-27-5 | 1272 | 1269 |
| A7 | β-citral | 106-26-3 | 1241 | 1243 |
| A8 | anethole | 4180-23-8 | 1280 | 1284 |
| A9 | α-pinene | 80-56-8 | 937 | 934 |
| A10 | α-phellandrene | 99-83-2 | 1002 | 1011 |
| A11 | camphene | 79-92-5 | 943 | 951 |
| A12 | myrcene | 123-35-3 | 990 | 998 |
| A13 | β-caryophyllene | 87-44-5 | 1410 | 1415 |
| A14 | α-curcumene | 644-30-4 | 1482 | 1486 |
| A15 | β-bisabolene | 495-61-4 | 1506 | 1514 |
| A16 | (-)-α-copaene | 3856-25-5 | 1375 | 1372 |
| A17 | (E)-γ-bisabolene | 53585-13-0 | 1534 | 1538 |
| A18 | (E)-α-bisabolene | 25532-79-0 | 1508 | 1504 |
| A19 | ocimene | 13877-91-3 | 1053 | 1050 |
| A20 | β-sesquiphellandrene | 20307-83-9 | 1526 | 1523 |
| A21 | α-caryophyllene | 6753-98-6 | 1460 | 1460 |
| A22 | (E)-β-farnesene | 18794-84-8 | 1459 | 1459 |
| A23 | β-pinene | 18172-67-3 | 973 | 978 |
| A24 | D-camphor | 464-49-3 | 1154 | 1154 |
| A25 | α-bergamotene | 17699-05-7 | 1442 | 1430 |
| A26 | (+)-cyclosativene | 22469-52-9 | 1370 | 1371 |
| A27 | dihydrocurcumene | 1461-02-5 | 250 | 250 |
| A28 | (E)-germacrene D | 23986-74-5 | 1480 | 1480 |
| A29 | carvone | 6485-40-1 | 1246 | 1249 |
| A30 | fenchone | 1195-79-5 | 1091 | 1086 |
| A31 | (+)-Δ-cadinene | 483-76-1 | 1528 | 1519 |
| A32 | α-ylangene | 14912-44-8 | 1372 | 1372 |
| A33 | γ-terpinene | 99-85-4 | 1065 | 1061 |
| A34 | (±)-β-copaene | 18252-44-3 | 1433 | 1433 |
| A35 | sabinene | 3387-41-5 | 973 | 980 |
| A36 | (E, E)-3,6-α-farnesene | 502-61-4 | 1508 | 1514 |
| A37 | 5-(1,5-Dimethyl-4-hexenyl)-2-methyl-1,3-cyclohexadiene | 495-60-3 | 1493 | 1493 |
| A38 | γ-curcumene | 451-55-8 | 1485 | 1479 |
| A39 | β-(Z)-ocimene | 3338-55-4 | 1106 | 1109 |
| A40 | 1-methyl-4-isopropyl-1-cyclohexen-3-one | 89-81-6 | 1259 | 1250 |
| B1 | phthalic acid dibutyl ester | 84-74-2 | 1963 | 1960 |
| B2 | ethyl hexanoate | 123-66-0 | 996 | 996 |
| B3 | ethyl caprylate | 106-32-1 | 1194 | 1191 |
| B4 | L-bornyl acetate | 20347-65-3 | 1300 | 1304 |
| B5 | terpinyl acetate | 80-26-2 | 1352 | 1358 |
| B6 | linalyl acetate | 115-95-7 | 1253 | 1251 |
| C1 | 1-nonanal | 124-19-6 | 1104 | 1109 |
| C2 | octanal | 124-13-0 | 1123 | 1106 |
| C3 | benzaldehyde | 100-52-7 | 967 | 969 |
| C4 | 1-decylaldehyde | 112-31-2 | 1205 | 1201 |
| C5 | heptanal | 111-71-7 | 903 | 909 |
| C6 | hexanal | 66-25-1 | 805 | 803 |
| C7 | 3,7-dimethyl-3,6-octadienal | 55722-59-3 | 1191 | 1185 |
| C8 | phenylacetaldehyde | 122-78-1 | 1041 | 1046 |
| D1 | (Z)-2-thia-3-pentene | 52195-40-1 | 731 | 731 |
| E1 | 2-nonanone | 821-55-6 | 1090 | 1095 |
| E2 | 3-heptanone | 106-35-4 | 894 | 894 |
| E3 | 5-nonanone | 502-56-7 | 1059 | 1051 |
| E4 | 3-methyl-2-heptaone | 2371-19-9 | 930 | 931 |
| E5 | 2-heptanone | 110-43-0 | 892 | 892 |
| E6 | acetophenone | 98-86-2 | 1330 | 1312 |
| F1 | 2-(3-methyl-2-butenyl)-3-methylfuran | 15186-51-3 | 1116 | 1104 |
| F2 | 3-(4-methyl-3-amyl) furan | 539-52-6 | 1118 | 1102 |
| F3 | 3-ethyl-2,5-dimethylpyrazine | 13360-65-1 | 1079 | 1084 |
| G1 | tetradecane | 629-59-4 | 236 | 236 |
| G2 | n-hendecane | 1120-21-4 | 1027 | 1022 |
| G3 | decane | 124-18-5 | 1039 | 1031 |
| G4 | 2-methyldecane | 6975-98-0 | 1088 | 1076 |
| H1 | propyl disulfide | 629-19-6 | 1103 | 1105 |
| H2 | 1-(methylsulfanyl)propane | 3877-15-4 | 752 | 752 |
| H3 | (E)-1-propenyl propyl disulfid | 23838-21-3 | 1117 | 1119 |
| I1 | benzene | 71-43-2 | 662 | 662 |
| I2 | 4-isopropyltoluene | 99-87-6 | 1033 | 1027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, K.; Li, Q.; Wang, Y.; Cao, X. Volatile Compounds and Fatty Acids of Mutton Carrot Filling During Dynamic Steaming Investigated Based on GC-MS and GC-IMS Analyses. Foods 2025, 14, 1535. https://doi.org/10.3390/foods14091535
You K, Li Q, Wang Y, Cao X. Volatile Compounds and Fatty Acids of Mutton Carrot Filling During Dynamic Steaming Investigated Based on GC-MS and GC-IMS Analyses. Foods. 2025; 14(9):1535. https://doi.org/10.3390/foods14091535
Chicago/Turabian StyleYou, Kaiyan, Qianyu Li, Ya Wang, and Xuehui Cao. 2025. "Volatile Compounds and Fatty Acids of Mutton Carrot Filling During Dynamic Steaming Investigated Based on GC-MS and GC-IMS Analyses" Foods 14, no. 9: 1535. https://doi.org/10.3390/foods14091535
APA StyleYou, K., Li, Q., Wang, Y., & Cao, X. (2025). Volatile Compounds and Fatty Acids of Mutton Carrot Filling During Dynamic Steaming Investigated Based on GC-MS and GC-IMS Analyses. Foods, 14(9), 1535. https://doi.org/10.3390/foods14091535






