Enabling Stable Recycling of L-Arabinose Isomerase Through Whole-Cell Immobilization for Efficient and Cost-Effective D-Tagatose Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Culture, and Reagents
2.2. Optimal Reaction Conditions of Whole-Cell ECaraAF118M/F279I
2.2.1. Preparation of Whole-Cell ECaraAF118M/F279I
2.2.2. Determination of Optimal Reaction Conditions
2.3. Preparation of Immobilized Cell Catalyst and Biocatalyst Activity
2.4. Optimization of Immobilized Cells Preparation
2.5. Optimization of Reaction Conditions and Immobilized Cells Stability Accessment
2.6. High Substrate Concentration Reaction and Recycle Measurement of Immobilized Cells
2.7. Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Whole-Cell Reaction Conditions
3.2. Optimization of Immobilized Cell Preparation Conditions
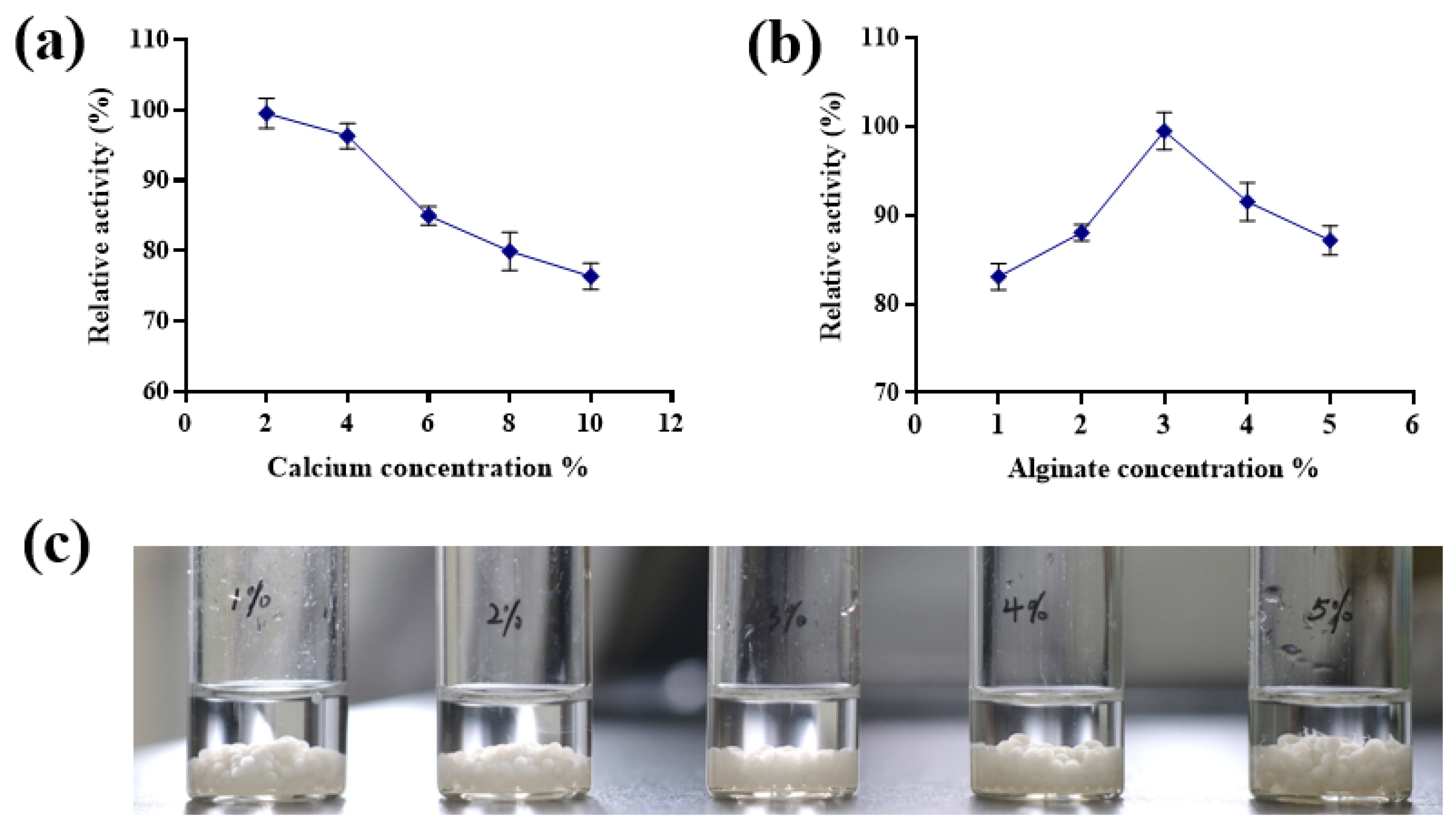
3.2.1. Effects of CaCl2 and Sodium Alginate Concentrations on Immobilized Cell Activity
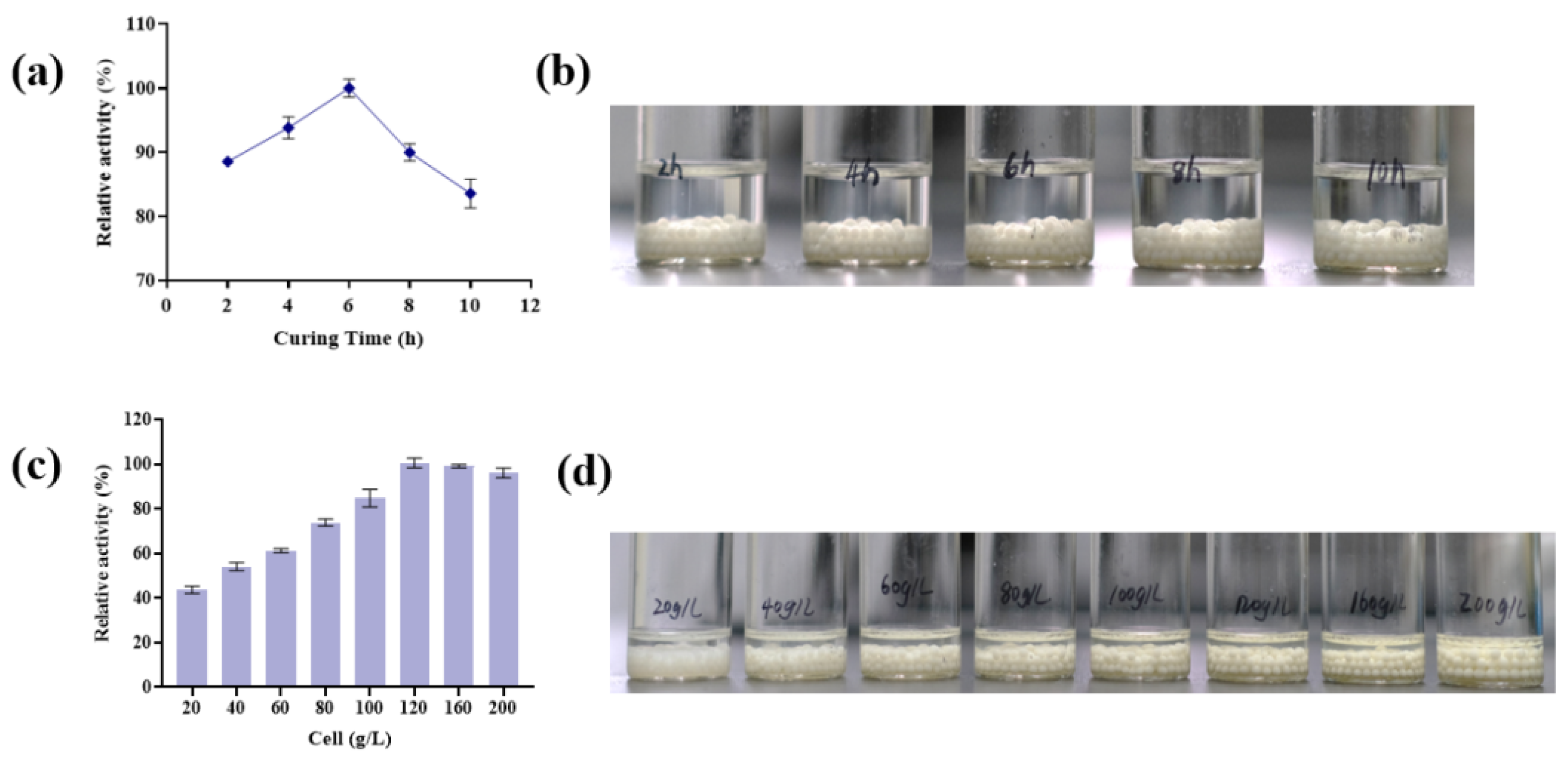
3.2.2. Effects of Hardening Time and Addition of Wet Cells on Immobilized Cell Activity
3.3. Effect of Reaction Condition and Stability of Immobilized Cells on Biocatalyst Activity
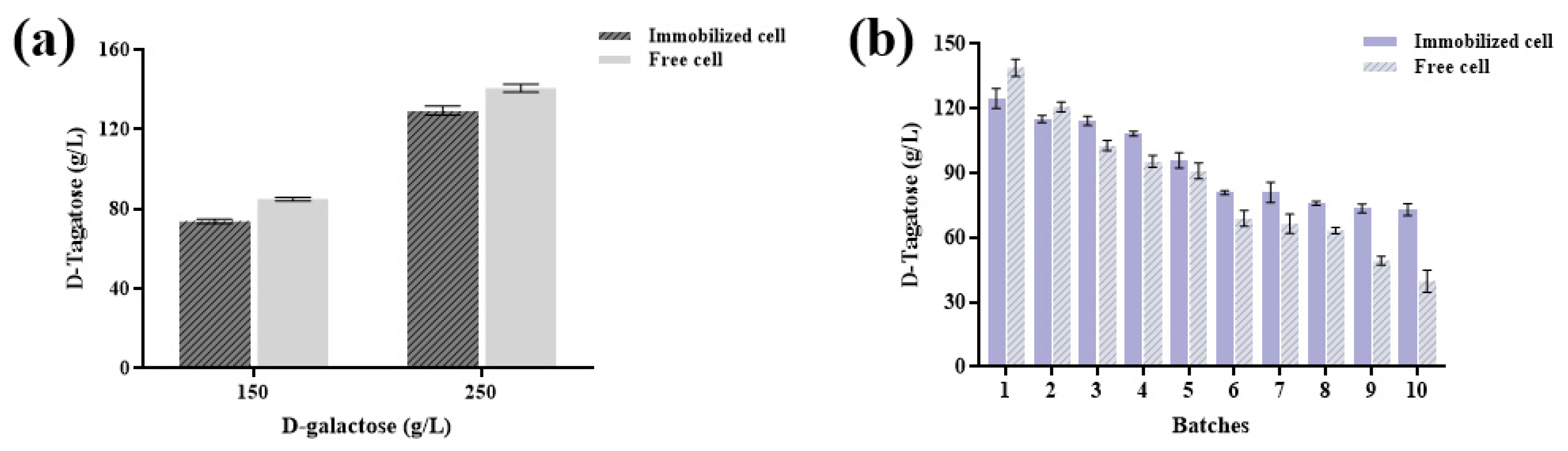
3.4. D-Tagatose Production Using Immobilized Cells Under a Higher Substrate Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Jobe, M.; Mactaggart, I.; Bell, S.; Kim, M.J.; Hydara, A.; Bascaran, C.; Njai, M.; Badjie, O.; Perel, P.; Prentice, A.M.; et al. Prevalence of hypertension, diabetes, obesity, multimorbidity, and related risk factors among adult Gambians: A cross-sectional nationwide study. Lancet Glob. Health 2024, 12, e55–e65. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Jin, Y.S. Rare sugar bioproduction: Advantages as sweeteners, enzymatic innovation, and fermentative frontiers. Curr. Opin. Food Sci. 2024, 56, 101137. [Google Scholar] [CrossRef]
- JJayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of D-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437. [Google Scholar] [CrossRef]
- Zhang, G.; Zabed, H.M.; Yun, J.; Yuan, J.; Zhang, Y.; Wang, Y.; Qi, X. Two-stage biosynthesis of D-tagatose from milk whey powder by an engineered Escherichia coli strain expressing L-arabinose isomerase from Lactobacillus plantarum. Bioresour. Technol. 2020, 305, 123010. [Google Scholar] [CrossRef]
- Du, M.; Zhao, D.; Cheng, S.; Sun, D.; Chen, M.; Gao, Z.; Zhang, C. Towards efficient enzymatic conversion of D-galactose to D-tagatose: Purification and characterization of L-arabinose isomerase from Lactobacillus brevis. Bioprocess Biosyst. Eng. 2019, 42, 107–116. [Google Scholar] [CrossRef]
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. D-Tagatose is a promising sweetener to control glycaemia: A new functional food. BioMed Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef]
- Donner, T.W.; Wilber, J.F.; Ostrowski, D. D-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291. [Google Scholar] [CrossRef]
- Liang, Y.X.; Wen, P.; Wang, Y.; OuYang, D.M.; Wang, D.; Chen, Y.Z.; Song, Y.; Deng, J.; Sun, Y.M.; Wang, H. The Constipation-Relieving Property of d-Tagatose by Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5721. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, S.; Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Ishikawa, A.; Ijima, Y.; Amano, A. Potential of prebiotic D-tagatose for prevention of oral disease. Front. Cell. Infect. Microbiol. 2021, 11, 767944. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, X.; Jiang, Y.; Ban, Q.; Wang, X. Effect of Maillard reaction conditions on the gelation and thermal stability of whey protein isolate/d-tagatose conjugates. Food Chem. 2023, 405, 134928. [Google Scholar] [CrossRef]
- Nouri, M.; Agahi, A.H. The Effect of Adding D-tagatose and Lemon Essential Oil on Quality of Chocolate. Iran. J. Food Sci. Ind. 2023, 19, 149–160. [Google Scholar]
- Bautista, D.A.; Pegg, R.B.; Shand, P.J. Effect of L-glucose and D-tagatose on bacterial growth in media and a cooked cured ham product. J. Food Prot. 2000, 63, 71–77. [Google Scholar] [CrossRef]
- Sokołowska, E.; Sadowska, A.; Sawicka, D.; Kotulska-Bąblińska, I.; Car, H. A head-to-head comparison review of biological and toxicological studies of isomaltulose, D-tagatose, and trehalose on glycemic control. Crit. Rev. Food Sci. 2021, 62, 5679–5704. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Ponpandian, L.N.; Zhang, G.; Yun, J.; Qi, X. Harnessing L-arabinose isomerase for biological production of D-tagatose: Recent advances and its applications. Trends Food Sci. Technol. 2021, 107, 16–30. [Google Scholar] [CrossRef]
- Aburto, C.; Vera, C.; Arenas, F.; Illanes, A.; Guerrero, C. One-pot production of tagatose using l-arabinose isomerase from Thermotoga maritima and β-galactosidase from Aspergillus oryzae. LWT 2024, 194, 115787. [Google Scholar] [CrossRef]
- Miao, P.; Wang, Q.; Ren, K.; Zhang, Z.; Xu, T.; Xu, M.; Zhang, X.; Rao, Z. Advances and Prospects of D-Tagatose Production Based on a Biocatalytic Isomerization Pathway. Catalysts 2023, 13, 1437. [Google Scholar] [CrossRef]
- Tokuriki, N.; Stricher, F.; Serrano, L.; Tawfik, D.S. How protein stability and new functions trade off. PLoS Comput. Biol. 2008, 4, e1000002. [Google Scholar] [CrossRef]
- Agostini, F.; Völler, J.S.; Koksch, B.; Acevedo-Rocha, C.G.; Kubyshkin, V.; Budisa, N. Biocatalysis with unnatural amino acids: Enzymology meets xenobiology. Angew. Chem. Int. Ed. 2017, 56, 9680–9703. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Editorial for special issue: Enzyme immobilization and its applications. Molecules 2019, 24, 4619. [Google Scholar] [CrossRef]
- Rai, S.K.; Narnoliya, L.K.; Sangwan, R.S.; Yadav, S.K. Self-assembled hybrid nanoflowers of manganese phosphate and L-arabinose isomerase: A stable and recyclable nanobiocatalyst for equilibrium level conversion of D-galactose to D-tagatose. ACS Sustain. Chem. Eng. 2018, 6, 6296–6304. [Google Scholar] [CrossRef]
- Torres, P.; Batista-Viera, F. Immobilized trienzymatic system with enhanced stabilization for the biotransformation of lactose. Molecules 2017, 22, 284. [Google Scholar] [CrossRef]
- Ryu, S.A.; Kim, C.S.; Kim, H.J.; Baek, D.H.; Oh, D.K. Continuous d-tagatose production by immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor. Biotechnol. Prog. 2003, 19, 1643–1647. [Google Scholar] [CrossRef]
- Oh, D.K.; Kim, H.J.; Ryu, S.A.; Rho, H.J.; Kim, P. Development of an immobilization method of L-arabinose isomerase for industrial production of tagatose. Biotechnol. Lett. 2001, 23, 1859–1862. [Google Scholar] [CrossRef]
- Kim, P.; Yoon, S.H.; Roh, H.J.; Choi, J.H. High production of d-tagatose, a potential sugar substitute, using immobilized l-arabinose isomerase. Biotechnol. Prog. 2001, 17, 208–210. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Z.; Ravikumar, Y.; Zhang, G.; Tang, X.; Zhang, Y.; Zhao, M.; Sun, W.; Qi, X. Improving Catalytic Efficiency of L-Arabinose Isomerase from Lactobacillus plantarum CY6 towards D-Galactose by Molecular Modification. Foods 2024, 13, 1727. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, M.; Jiang, B.; Zhang, T.; Chen, J. Whole-cell biosynthesis of d-tagatose from maltodextrin by engineered Escherichia coli with multi-enzyme co-expression system. Enzym. Microb. Technol. 2021, 145, 109747. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Z.; Zhou, H.; Zhou, G.; Cheng, H. Enhanced enzymatic performance of β-Mannanase immobilized on Calcium Alginate beads for the generation of Mannan Oligosaccharides. Foods 2023, 12, 3089. [Google Scholar] [CrossRef]
- Wang, Y.; Ravikumar, Y.; Zhang, G.; Yun, J.; Zhang, Y.; Parvez, A.; Qi, X.; Sun, W. Biocatalytic Synthesis of D-Allulose Using Novel D-Tagatose 3-Epimerase from Christensenella minuta. Front. Chem. 2020, 8, 622325. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Gopal, S.R. Immobilization of Bacillus amyloliquefaciens MBL27 cells for enhanced antimicrobial protein production using calcium alginate beads. Biotechnol. Appl. Biochem. 2010, 57, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rosiak, P.; Latanska, I.; Paul, P.; Sujka, W.; Kolesinska, B. Modification of Alginates to Modulate Their Physic-Chemical Properties and Obtain Biomaterials with Different Functional Properties. Molecules 2021, 26, 7264. [Google Scholar] [CrossRef]
- Xu, Z.; Li, S.; Fu, F.; Li, G.; Feng, X.; Xu, H.; Ouyang, P. Production of D-tagatose, a functional sweetener, utilizing alginate immobilized Lactobacillus fermentum CGMCC2921 cells. Appl. Biochem. Biotechnol. 2012, 166, 961–973. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Prabhu, P.; Lee, J.K. Alginate immobilization of recombinant Escherichia coli whole cells harboring L-arabinose isomerase for L-ribulose production. Bioprocess Biosyst. Eng. 2010, 33, 741–748. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, R.; Lai, X.; Liao, W.; Liao, R.; Wu, Z.; Zhang, G.; Qi, X. Enabling Stable Recycling of L-Arabinose Isomerase Through Whole-Cell Immobilization for Efficient and Cost-Effective D-Tagatose Production. Foods 2025, 14, 1538. https://doi.org/10.3390/foods14091538
Li Z, Wang R, Lai X, Liao W, Liao R, Wu Z, Zhang G, Qi X. Enabling Stable Recycling of L-Arabinose Isomerase Through Whole-Cell Immobilization for Efficient and Cost-Effective D-Tagatose Production. Foods. 2025; 14(9):1538. https://doi.org/10.3390/foods14091538
Chicago/Turabian StyleLi, Zepeng, Runmin Wang, Xiantai Lai, Wenyi Liao, Runfeng Liao, Zhuohong Wu, Guoyan Zhang, and Xianghui Qi. 2025. "Enabling Stable Recycling of L-Arabinose Isomerase Through Whole-Cell Immobilization for Efficient and Cost-Effective D-Tagatose Production" Foods 14, no. 9: 1538. https://doi.org/10.3390/foods14091538
APA StyleLi, Z., Wang, R., Lai, X., Liao, W., Liao, R., Wu, Z., Zhang, G., & Qi, X. (2025). Enabling Stable Recycling of L-Arabinose Isomerase Through Whole-Cell Immobilization for Efficient and Cost-Effective D-Tagatose Production. Foods, 14(9), 1538. https://doi.org/10.3390/foods14091538






