Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
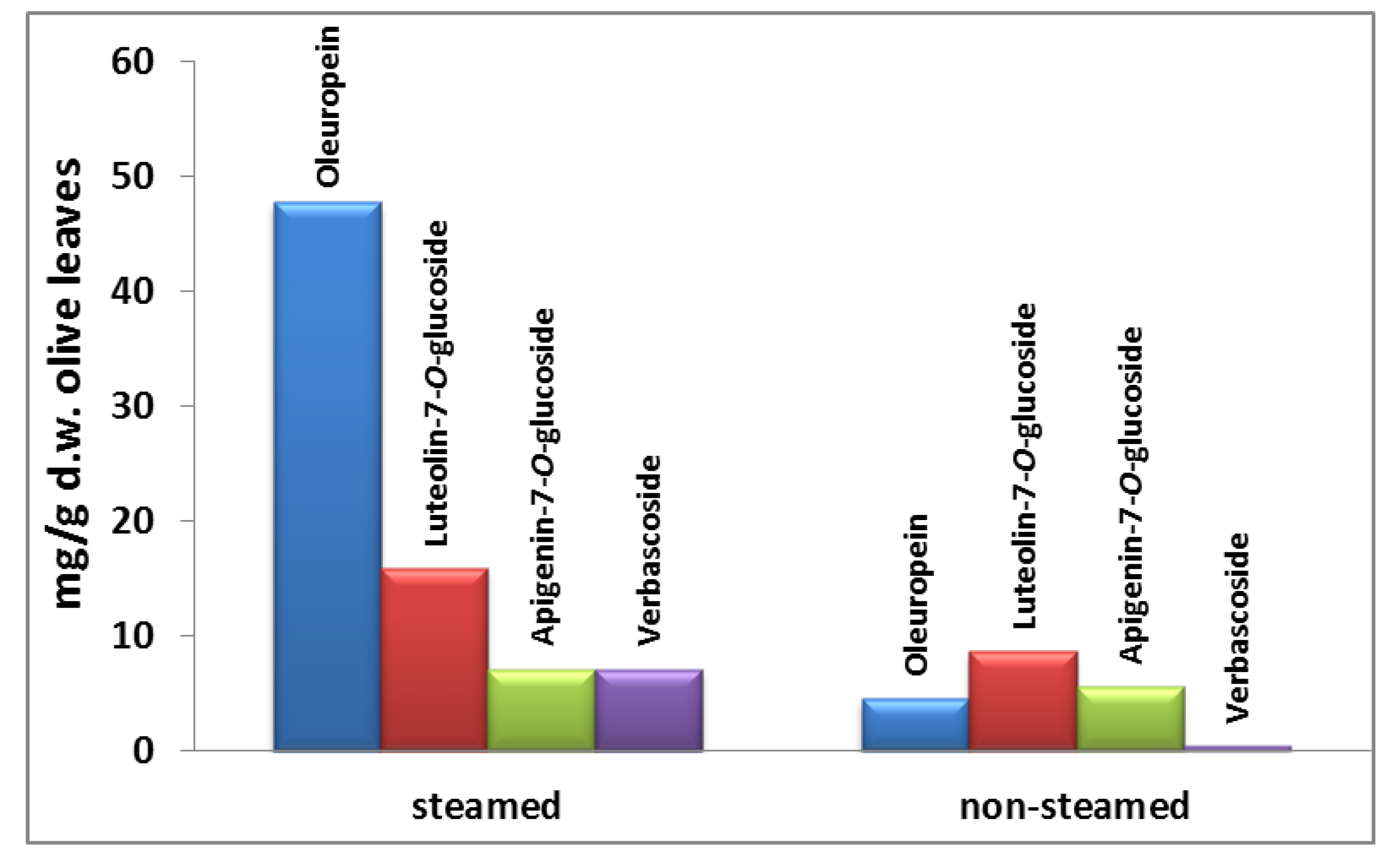
2.2. Steam Blanching of Olive Leaves
2.3. Optimization of Phenolic Compounds Extraction from Olive Leaves
2.3.1. Effect of Particle Size
2.3.2. Effect of pH
2.3.3. Effect of Solid-to-Liquid Ratio (S/L)
2.3.4. Effect of Ethanol Concentration (% EtOH, v/v)
2.4. Multistage Extraction Scheme of Olive Leaves
2.5. Conventional Extraction Protocol
2.6. Chromatographic Conditions
2.7. Determination of Antioxidant Activity

2.8. Determination of Total Phenol Content (TPC)
3. Results and Discussion
3.1. Effect of Particle Size

3.2. Effect of pH
3.3. Effect of Solid-to-Liquid Ratio (S/L)
3.4. Effect of Ethanol Concentration
3.5. Multistage Extraction of Olive Leaves
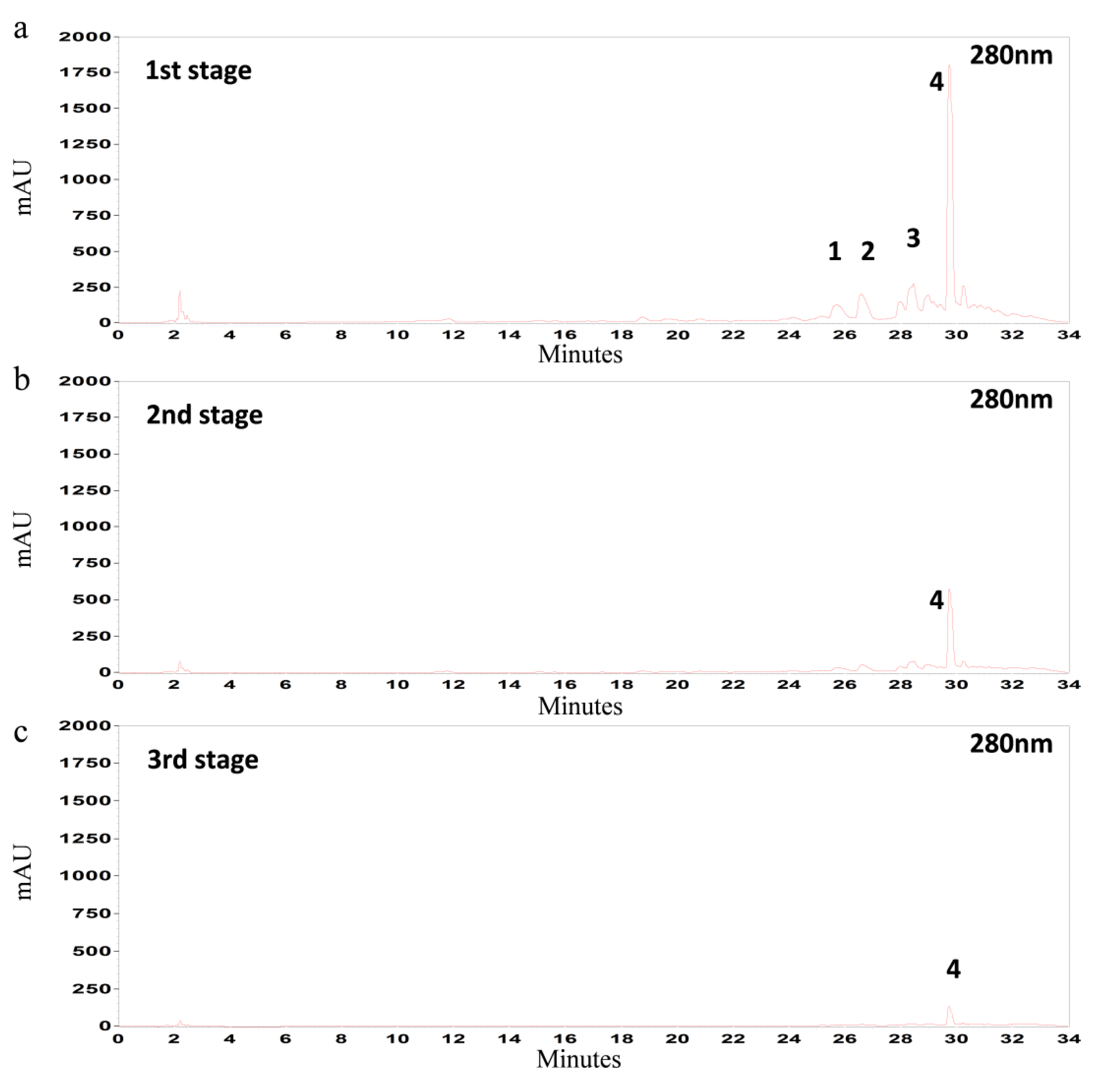

| Temperature | Extraction Stage | Total | Oleuropein * | Luteolin-7-O-glucoside * | Verbascoside * | Apigenin-7-O-glucoside * |
|---|---|---|---|---|---|---|
| 85 °C | 1st | 129.8 ± 1.8 | 78.2 ± 2.9 (60.2) | 27.0 ± 1.2 (20.7) | 13.5 ± 0.5 (10.3) | 11.1 ± 0.9 (8.5) |
| 2nd | 29.3 ± 0.9 | 19.4 ± 1.2 (66.1) | 5.5 ± 0.6 (18.8) | 2.2 ± 0.3 (7.5) | 2.2 ± 0.5 (7.5) | |
| 3rd | 7.5 ± 0.2 | 5.5 ± 0.3 (72.6) | 1.2 ± 0.2 (16.7) | 0.3 ± 0.1 (3.9) | 0.5 ± 0.1 (6.6) | |
| Total | 166.6 ± 0.9 | 103.1 ± 1.5 | 33.7 ± 0.6 | 16 ± 0.9 | 13.8 ± 0.5 | |
| 70 °C | 1st | 103.5 ± 2.0 | 65.4 ± 4.3 (63.1) | 17.1 ± 1.4 (16.5) | 9.9 ± 1.2 (9.5) | 11.1 ± 1.0 (10.7) |
| 2nd | 29.3 ± 0.7 | 16.9 ± 0.4 (57.6) | 3.1 ± 1.1 (10.8) | 2.0 ± 0.7 (6.8) | 2.2 ± 0.7 (7.5) | |
| 3rd | 7.5 ± 0.1 | 3.9 ± 0.1 (51.5) | 0.9 ± 0.1 (4.7) | 0.7 ± 0.1 (1.8) | 0.5 ± 0.1 (6.6) | |
| Total | 140.3 ± 0.9 | 86.2 ± 1.6 | 21.4 ± 0.8 | 12.6 ± 0.6 | 13.8 ± 0.6 | |
| 65 °C | 1st | 107.4 ± 2.0 | 66.5 ± 5.5 (61.9) | 19.1 ± 1.1 (17.7) | 10.9 ± 0.6 (10.1) | 10.9 ± 0.6 (10.1) |
| 2nd | 21.9 ± 0.2 | 14.6 ± 0.5 (66.6) | 3.4 ± 0.1 (15.5) | 1.8 ± 0.1 (8.2) | 2.1 ± 0.1 (9.5) | |
| 3rd | 3.7 ± 0.1 | 3.04 ± 0.2 (81.9) | 0.4 ± 0.1 (11.0) | 0.6 ± 0.1 (1.6) | 0.4 ± 0.1 (5.3) | |
| Total | 133.0 ± 0.8 | 84.1 ± 2.1 | 23.3 ± 0.4 | 13.3 ± 0.3 | 4.5 ± 0.3 | |
| 60 °C | 1st | 109.9 ± 2.6 | 73.1 ± 4.8 (66.5) | 18.2 ± 4.2 (16.5) | 7.7 ± 0.7 (7.0) | 10.9 ± 0.8 (9.9) |
| 2nd | 20.9 ± 0.2 | 13.7 ± 0.3 (65.5) | 3.3 ± 0.1 (15.7) | 1.8 ± 0.1 (8.6) | 2.1 ± 0.2 (10.0) | |
| 3rd | 2.7 ± 0.1 | 1.5 ± 0.4 (55.6) | 0.5 ± 0.1 (19.7) | 0.5 ± 0.1 (18.2) | 0.2 ± 0.1 (7.2) | |
| Total | 133.5 ± 1.0 | 29.4 ± 1.8 | 22 ± 1.5 | 10 ± 0.3 | 13.2 ± 0.4 | |
| 40 °C | 1st | 105.9 ± 1.9 | 67.3 ± 4.1 (63.5) | 17.6 ± 1.6 (16.6) | 10.5 ± 0.8 (9.9) | 10.5 ± 0.9 (9.9) |
| 2nd | 27.0 ± 0.2 | 18.3 ± 0.7 (67.7) | 3.9 ± 0.2 (14.4) | 2.3 ± 0.1 (8.5) | 2.5 ± 0.1 (9.2) | |
| 3rd | 4.6 ± 0.1 | 3.9 ± 0.1 (84.7) | 0.2 ± 0.1 (4.3) | 0.2 ± 0.1 (4.3) | 0.3 ± 0.1 (6.5) | |
| Total | 137.5 ± 0.7 | 89.5 ± 1.6 | 21.7 ± 0.6 | 13.0 ± 0.3 | 13.3 ± 0.4 |
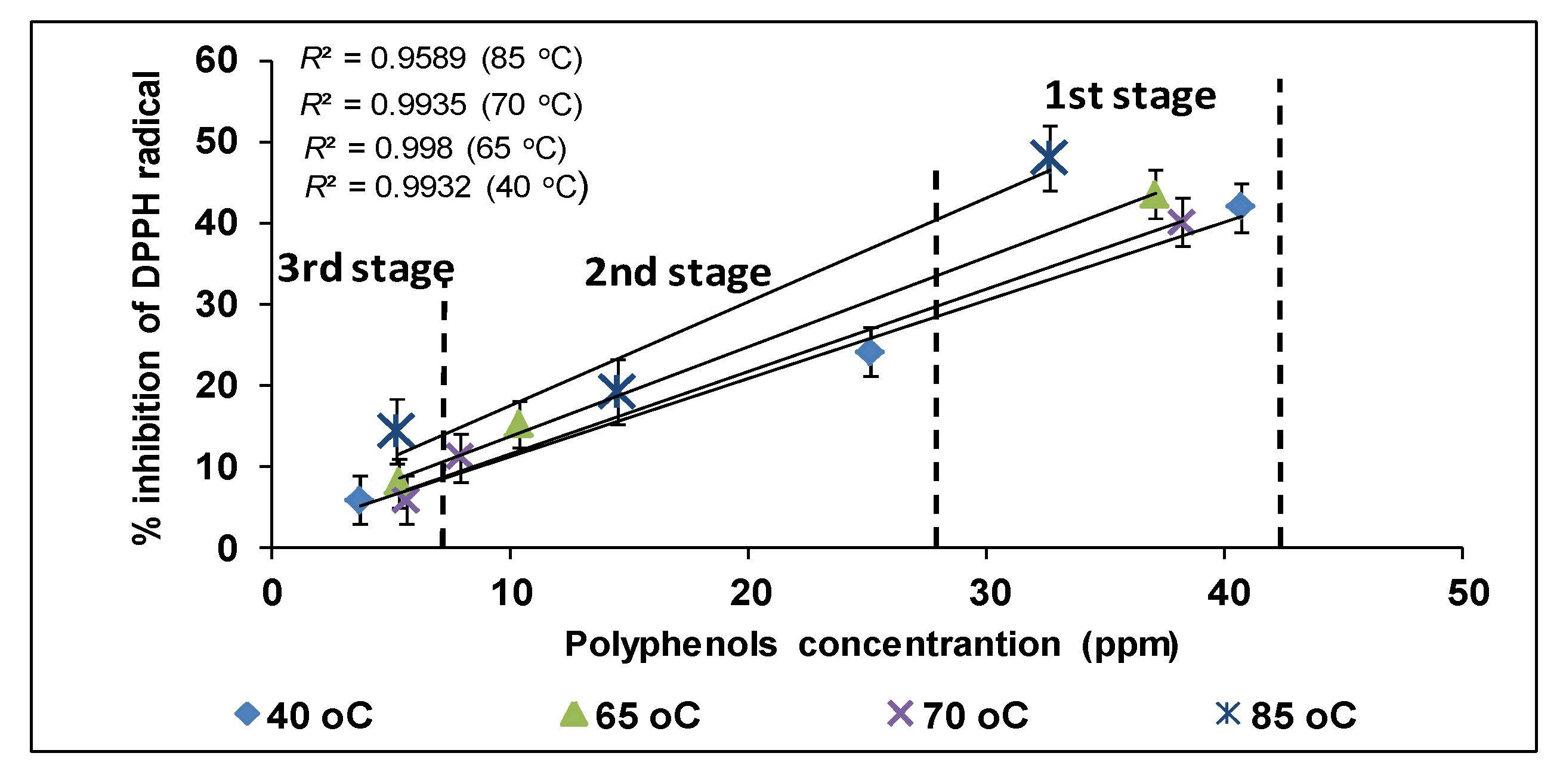
3.6. Antioxidant Activity of Ethanolic Extracts of Olive Leaves
4. Conclusions
Conflicts of Interest
References
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds-recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Shi, J.; Nawaz, H.; Pohorly, J.; Mittal, G.; Kakuda, Y.; Jiang, Y. Extraction of polyphenolics from plant material for functional foods—Engineering and technology. Food Rev. Int. 2005, 21, 139–166. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque de Castro, M.D. Superheated liquid extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1136, 185–191. [Google Scholar]
- Servili, M.; Montedoro, G.F. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, J.H.; Jin, Y.R.; Yun, Y.P. The inhibitory effect and mechanism of luteolin 7-glucoside on rat aortic vascular smooth muscle cell proliferation. Arch. Pharm. Res. 2006, 29, 67–72. [Google Scholar] [CrossRef]
- Omar, S.H. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm. J. 2010, 18, 111–121. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Ortuno, A.; del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box-Behnken design in optimization for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Lee, W.C.; Yusof, S.; Hamid, N.S.A.; Baharin, B.S. Optimizing conditions for hot water extraction of banana juice using response surface methodology (RSM). J. Food Eng. 2006, 75, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Corsano, G.; Montagna, J.M.; Aguirre, P.A. Design and planning optimization of multiplant complexes in the food industry. Food Bioprod. Process. 2007, 85, 381–388. [Google Scholar] [CrossRef]
- Savournin, C.; Elias, B.R.; Dargouth-Kesraoui, F.; Boukef, K.; Balansard, G. Rapid high-performance liquid chromatography analysis for the quantitative determination of oleuropein in Olea europaea leaves. J. Agric. Food Chem. 2001, 49, 618–621. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Multivariate optimisation of the microwave-assisted extraction of oleuropein and related biophenols from olive leaves. Anal. Bioanal. Chem. 2006, 385, 753–759. [Google Scholar] [CrossRef]
- Tabera, J.; Guinda, A.; Ruiz Rodríguez, A.; Señoráns, F.J.; Ibáñez, E.; Albi, T. Counter-current supercritical fluid extraction and fractionation of high-added-value compounds from a hexane extract of olive leaves. J. Agric. Food Chem. 2004, 52, 4774–4779. [Google Scholar] [CrossRef]
- Tsochatzidis, N.A.; Guiraud, P.; Wilhelm, A.M.; Delmas, H. Determination of velocity, size and concentration of ultrasonic cavitation bubbles by the phase-Doppler technique. Chem. Eng. Sci. 2001, 56, 1831–1840. [Google Scholar] [CrossRef]
- Pekić, B.; Kovač, V.; Alonso, E.; Revilla, E. Study of the extraction of proanthocyanidins from grape seeds. Food Chem. 1998, 61, 201–206. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; de Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Williamson, G. Polyphenols Extraction from Foods. In Methods in Polyphenol Analysis, 1st ed.; Escribano-Balón, M.T., Santos-Buelga, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Stamatopoulos, K.; Katsoyannos, E.; Chatzilazarou, A.; Konteles, S.J. Improvement of oleuropein extractability by optimising steam blanching process as pre-treatment of olive leaf extraction via response surface methodology. Food Chem. 2012, 133, 344–351. [Google Scholar] [CrossRef]
- Braca, A.; de Tommasi, N.; di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Meireles, M.A.A. Extracting Bioactive Compounds for Food Products Theory and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Gikas, E.; Papadopoulos, N.; Tsarbopoulos, A. Kinetic study of the acid hydrolysis of oleuropein, the major bioactive metabolite of olive oil. J. Liquid Chromatogr. Relat. Technol. 2006, 29, 497–508. [Google Scholar] [CrossRef]
- Bilek, S.E. The effects of time, temperature, solvent:solid ratio and solvent composition on extraction of total phenolic compound from dried olive (Olea europaea L.) leaves. GIDA J. Food 2010, 35, 411–416. [Google Scholar]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Alonso, E.; Bourzeix, M.; Revilla, E. Suitabilitiy of water-ethanol mixtures for the extraction of catechins and proanthocyanidins from Vitis vinifera seeds contained in a winery by-product. Seed Sci. Technol. 1991, 19, 545–552. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar]
- Bouaziz, M.; Grayer, R.J.; Simmonds, M.S.J.; Damak, M.; Sayadi, S. Identification and antioxidant potential of flavonoids and low molecular weight phenols in olive cultivar chemlali growing in Tunisia. J. Agric. Food Chem. 2005, 53, 236–241. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2014, 3, 66-81. https://doi.org/10.3390/foods3010066
Stamatopoulos K, Chatzilazarou A, Katsoyannos E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods. 2014; 3(1):66-81. https://doi.org/10.3390/foods3010066
Chicago/Turabian StyleStamatopoulos, Konstantinos, Archontoula Chatzilazarou, and Evangelos Katsoyannos. 2014. "Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times" Foods 3, no. 1: 66-81. https://doi.org/10.3390/foods3010066
APA StyleStamatopoulos, K., Chatzilazarou, A., & Katsoyannos, E. (2014). Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods, 3(1), 66-81. https://doi.org/10.3390/foods3010066





