Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source
Abstract
:1. Introduction
2. Materials and Methods
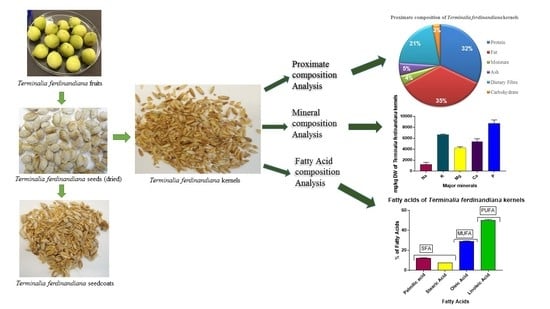
2.1. Sample Collection and Preparation
2.2. Processing of Seeds
2.3. Proximate Composition Analysis
2.4. Fatty Acid Analysis
2.5. Mineral and Trace Element Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Mineral and Trace Element Composition
3.3. Fatty Acid Composition
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.G.; Huang, W.T.; Lee, L.T.; Wang, C.C. Ellagitannins from Terminalia calamansanai induced apoptosis in HL-60 cells. Toxicol. In Vitro 2009, 23, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, M.P.; Hegarty, E.E. Food safety of Australian Plant Bushfoods. Rural Ind. Res. Dev. Corp. 2001, 28, 33–35. [Google Scholar]
- Singh, A.; Bajpai, V.; Kumar, S.; Kumar, B.; Srivastava, M.; Rameshkumar, K.B. Comparative profiling of phenolic compounds from different plant parts of six Terminalia species by liquid chromatography-tandem mass spectrometry with chemometric analysis. Ind. Crops Prod. 2016, 87, 236–246. [Google Scholar] [CrossRef]
- Chaliha, M.; Williams, D.; Edwards, D.; Pun, S.; Smyth, H.; Sultanbawa, Y. Bioactive rich extracts from Terminalia ferdinandiana by enzyme-assisted extraction: A simple food safe extraction method. J. Med. Plant Res. 2017, 11, 96–106. [Google Scholar]
- Konczak, I.; Roulle, P. Nutritional properties of commercially grown native Australian fruits: Lipophilic antioxidants and minerals. Food Res. Int. 2011, 44, 2339–2344. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Shami, A.M.M.; Philip, K.; Muniandy, S. Synergy of antibacterial and antioxidant activities from crude extracts and peptides of selected plant mixture. BMC Complement. Altern. Med. 2013, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Sultanbawa, Y.; Williams, D.; Smyth, H. Changes in Quality and Bioactivity of Native Food during Storage; Rural Industries Research and Development Corporation (RIRDC): Barton, Australia, 2015. [Google Scholar]
- Sirdaarta, J.; Matthews, B.; Cock, I.E. Kakadu plum fruit extracts inhibit growth of the bacterial triggers of rheumatoid arthritis: Identification of stilbene and tannin components. J. Funct. Foods 2015, 17, 610–620. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Zabaras, D.; Sze, D.M.Y. Potential antioxidant, antiinflammatory, and proapoptotic anticancer activities of Kakadu plum and Illawarra plum polyphenolic fractions. Nutr. Cancer 2011, 63, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 2014, 66, 100–106. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Vasconcelos, I.M.; Bezerra, L.; Silveira, S.B.; Monteiro, A.C.O.; Moreira, R.A. Composition and nutritional properties of seeds from Pachira aquatica Aubl, Sterculia striata St Hil et Naud and Terminalia catappa Linn. Food Chem. 2000, 70, 185–191. [Google Scholar] [CrossRef]
- Akpakpan, A.E.; Akpabio, U.D. Evaluation of Proximate composition, mineral element and anti-nutrient in Almond (Terminalia catappa) seeds. Res. J. Appl. Sci. 2012, 7, 489–493. [Google Scholar]
- Ladele, B.; Kpoviessi, S.; Ahissou, H.; Gbenou, J.; Kpoviessi, B.K.; Mignolet, E.; Herent, M.F.; Bero, J.; Larondelle, Y.; Leclercq, J.Q.; et al. Chemical composition and nutritional properties of Terminalia catappa L. oil and kernels from Benin. Com. Ren. Chim. 2016, 19, 876–883. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, S.; Wu, D.; Chen, J.; Liu, D.; Ye, X. Bayberry (Myrica rubra Sieb. et Zucc.) kernel: A new protein source. Food Chem. 2009, 112, 469–473. [Google Scholar] [CrossRef]
- Chivandi, E.; Mukonowenzou, N.; Berliner, D. The coastal red-milkwood (Mimusops caffra) seed: Proximate, mineral, amino acid and fatty acid composition. S. Afr. J. Bot. 2016, 102, 137–141. [Google Scholar] [CrossRef]
- Lv, Z.C.; Chen, K.; Zeng, Y.W.; Peng, Y.H. Nutritional composition of Canarium pimela L. kernels. Food Chem. 2011, 125, 692–695. [Google Scholar] [CrossRef]
- He, Z.; Xia, W. Nutritional composition of the kernels from Canarium album L. Food Chem. 2007, 102, 808–811. [Google Scholar] [CrossRef]
- Poudyal, H.; Kumar, S.A.; Iyer, A.; Waanders, J.; Ward, L.C.; Brown, L. Responses to oleic, linoleic and α-linolenic acids in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U.; Schoendorfer, N.; Scheelings, P.; Yang, X.; Jurd, S.; Robinson, A.; Smith, K.; Piispanen, J. Arsenic in rice and diets of children. Food Addit. Contam. 2015, 8, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.F.; Tinggi, U.; Yang, X.; Fry, B. Stable isotope and trace metal compositions of Australian prawns as a guide to authenticity and wholesomeness. Food Chem. 2015, 170, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chivandi, E.; Davidson, B.C.; Erlwanger, K.H. Proximate, mineral, fibre, phytate-phosphate, vitamin E, amino acid and fatty acid composition of Terminalia sericea. S. Afr. J. Bot. 2013, 88, 96–100. [Google Scholar] [CrossRef]
- Phillips, S.M. Dietary protein requirements and adaptive advantages in atheletes. Br. J. Nutr. 2012, 108, S158–S167. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Orhan, I.; Ahsan, Z.; Aslan, S.; Gulfraz, M. Fatty acid composition of seed oil of different Sorghum bicolor varieties. Food Chem. 2008, 109, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ye, X.; Chen, J.; Liu, D.; Zhou, S. Nutritional composition of underutilized bayberry (Myrica rubra Sieb. et Zucc.) kernels. Food Chem. 2008, 107, 1674–1680. [Google Scholar] [CrossRef]
- Chung, S.W.C.; Kwong, K.P.; Yau, J.C.; Wong, W.W. Dietary exposure to antimony, lead and mercury of secondary school students in Hong Kong. Food Addit. Contam. 2008, 25, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Gislerød, H.R. The importance of omega-3 fatty acids in diet. Curr. Sci. 2006, 90, 908–909. [Google Scholar]
- Iso, H.; Sato, S.; Umemura, U.; Kudo, M.; Koike, K.; Kitamura, A.; Imano, H.; Okamura, T.; Naito, Y.; Shimamoto, T. Linoleic acid, other fatty acids, and the risk of stroke. Stroke 2002, 33, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Nantapo, C.W.T.; Muchenje, V.; Nkukwana, T.T.; Hugo, A.; Descalzo, A.; Grigioni, G.; Hoffman, L.C. Socio-economic dynamics and innovative technologies affecting health-related lipid content in diets: Implications on global food and nutrition security. Food Res. Int. 2015, 76, 896–905. [Google Scholar] [CrossRef]
- Alonso, A.; Martinez-González, M.A. Olive oil consumptionand reduced incidence of hypertension: The SUN study. Lipids 2004, 39, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Balogun, A.M.; Fetuga, B.L. Fatty Acid Composition of Seed Oils of Some Members of the Meliaceae and Combretaceae Families. J. Am. Oil Chem. Soc. 1985, 62, 529–531. [Google Scholar] [CrossRef]
- Khodadoust, S.; Mohammadzadeh, A.; Mohammadi, J.; Irajie, C.; Ramezani, M. Identification and determination of the fatty acid composition of Quercus brantii growing in southwestern Iran by GC–MS. Nat. Prod. Res. 2014, 28, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Rukmini, C.; Rao, P.U. Chemical and Nutritional Studies on Terminalia bellirica Roxb. Kernel and Its Oil. J. Am. Oil Chem. Soc. 1986, 63, 360–363. [Google Scholar] [CrossRef]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- He, Z.; Zhu, H.; Li, W.; Zeng, M.; Wu, S.; Chen, S.; Qin, F.; Chen, J. Chemical components of cold pressed kernel oils from different Torreya grandis cultivars. Food Chem. 2016, 209, 196–202. [Google Scholar] [CrossRef] [PubMed]


| Nutrition Information (Servings per Package: 1) (Serving Size: 100 g) | ||||
|---|---|---|---|---|
| T. ferdinandiana Kernels | Quantity per Serving | % Daily Intake */Serving | ||
| Protein | % (w/w) | 32.0 | 32 g | 64% |
| Fat | % (w/w) | 35.1 | 35.1 g | 50% |
| Saturated Fat | % (w/w) | 5.8 | 5.8 g | 24% |
| Mono-unsaturated Fat | % (w/w) | 9.8 | 9.8 g | |
| Poly-unsaturated Fat | % (w/w) | 19.4 | 19.4 g | |
| Trans Fat | % (w/w) | <0.01 | <0.1 g | |
| Moisture (air) | % (w/w) | 4.0 | ||
| Ash | % (w/w) | 4.5 | ||
| Dietary Fibre (Total) | % (w/w) | 21.2 | 21.2 g | 71% |
| Dry Matter | % (w/w) | 96.0 | ||
| Crude Fibre | % (w/w) | 11.6 | ||
| Energy | kJ/100 g | 2065 | 24% | |
| Total Sugar | g/100 g | 0.49 | <1 g | <1% |
| Available Carbohydrate | % | 3.2 | 3.2 g | 1% |
| Sodium (Na) | mg/100 g | 8.6 | 8.6 mg | <1% |
| Fatty Acid | Percentage (%) ± SD |
|---|---|
| Saturated | |
| C14:0 Methyl myristate | 0.1 ± 0.01 |
| C16:0 Methyl palmitate | 12 ± 0.53 |
| C18:0 Methyl stearate | 7.2 ± 0.13 |
| C20:0 Methyl arachidate | 0.7 ± 0.06 |
| C22:0 Methyl behenate | 0.4 ± 0.13 |
| TSFA | 20.4 |
| Monounsaturated | |
| C16:1 (cis-9) Methyl palmitoleate | 0.1 ± 0.06 |
| C18:1 (cis-9) Methyl oleate | 29.2 ± 0.68 |
| C20:1 (cis-11) Methyl eicosenoate | 0.1 ± 0.04 |
| TMUFA | 29.4 |
| Polyunsaturated | |
| C18:2 (all-cis-9,12) Methyl linoleate | 50.2 ± 1.1 |
| PUFA | 50.2 |
| SFA vs. UFA | 0.25:1 |
| MUFA vs. PUFA | 0.6:1 |
| Mineral Composition | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major Elements | Micro/Trace Elements | ||||||||||||||
| Ca | Mg | Na | K | P | Fe | Zn | Mn | Cu | Co | Ni | Mo | Se | Sr | B | |
| Kernels (mg/100 g DW) | 538.5 | 421.1 | 120.3 | 669.3 | 872.8 | 6.1 | 6.0 | 9.1 | 2.5 | 0.02 | 0.17 | <0.01 | 0.02 | 5.0 | 0.83 |
| DRI | 1200 AI a | 350 EAR a | 1.3 AI a | 4.7 AI a | 700 RDA a | 8 RDA a | 11 RDA a | 2.3 AI a | 700 RDA a | 0.12AI a | 1.0 UL a | 34 EAR a | 45 EAR a | 1–5 RDA a | 20 UL a |
| Units | mg | mg | g | g | mg | mg | mg | mg | µg | µg | mg | µg | µg | mg | mg |
| Non-Essential Elements | Heavy Metals | ||||
|---|---|---|---|---|---|
| Ba | As | Hg | Pb | Cd | |
| Kernels (mg/100 g DW) | 0.31 | <0.01 | <0.01 | 0.013 | <0.01 |
| DRI | 0.02 UL a | 12.5–25 UL b | 5 UL c | 25 UL c | 2.5 UL d |
| Units | mg/kg BW | µg/kg BW/week | µg/kg BW/week | µg/kg BW/week | µg/kg BW/week |
| Type of Oil/Fat | Fatty Acid Composition | P/S Index | Reference | ||
|---|---|---|---|---|---|
| SFA | MUFA | PUFA | |||
| T. ferdinandiana Kernels | 20.4 | 29.6 | 50.0 | 2.45 | Current study |
| Coconut | 90.5 | 8.8 | 0.5 | 0.005 | [36] |
| Corn | 25.1 | 26.8 | 48 | 1.91 | |
| Cottonseed | 22.4 | 35.4 | 42 | 1.87 | |
| Soybean | 13.5 | 28.5 | 57.5 | 4.26 | |
| Peanut | 19.2 | 58.5 | 20 | 1.04 | |
| Safflower | 7.2 | 16.6 | 76 | 10.55 | |
| Linseed | 9.65 | 22.1 | 68 | 7.05 | |
| Palm kernel | 76 | 22.5 | 1.25 | 0.016 | |
| Sunflower seed | 8.8 | 31.5 | 59.5 | 6.76 | |
| Canola | 9.6 | 59.5 | 30.7 | 3.2 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, S.; Netzel, M.E.; Fletcher, M.T.; Tinggi, U.; Sultanbawa, Y. Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods 2018, 7, 60. https://doi.org/10.3390/foods7040060
Akter S, Netzel ME, Fletcher MT, Tinggi U, Sultanbawa Y. Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods. 2018; 7(4):60. https://doi.org/10.3390/foods7040060
Chicago/Turabian StyleAkter, Saleha, Michael E. Netzel, Mary T. Fletcher, Ujang Tinggi, and Yasmina Sultanbawa. 2018. "Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source" Foods 7, no. 4: 60. https://doi.org/10.3390/foods7040060
APA StyleAkter, S., Netzel, M. E., Fletcher, M. T., Tinggi, U., & Sultanbawa, Y. (2018). Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods, 7(4), 60. https://doi.org/10.3390/foods7040060