Microbiological Changes during Ripening of Chihuahua Cheese Manufactured with Raw Milk and Its Seasonal Variations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Texture and Proximate Analysis of Chihuahua Cheese
2.3. Cheese Ripenning and Sampling
2.4. Determination of Indicator Organisms
2.5. Lactic Acid Bacteria (LAB) Groups Enumeration
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chihuahua Cheese Characterization and Texture Analysis
3.2. Ripening and Seasonal Effect on LAB Microbial Counts
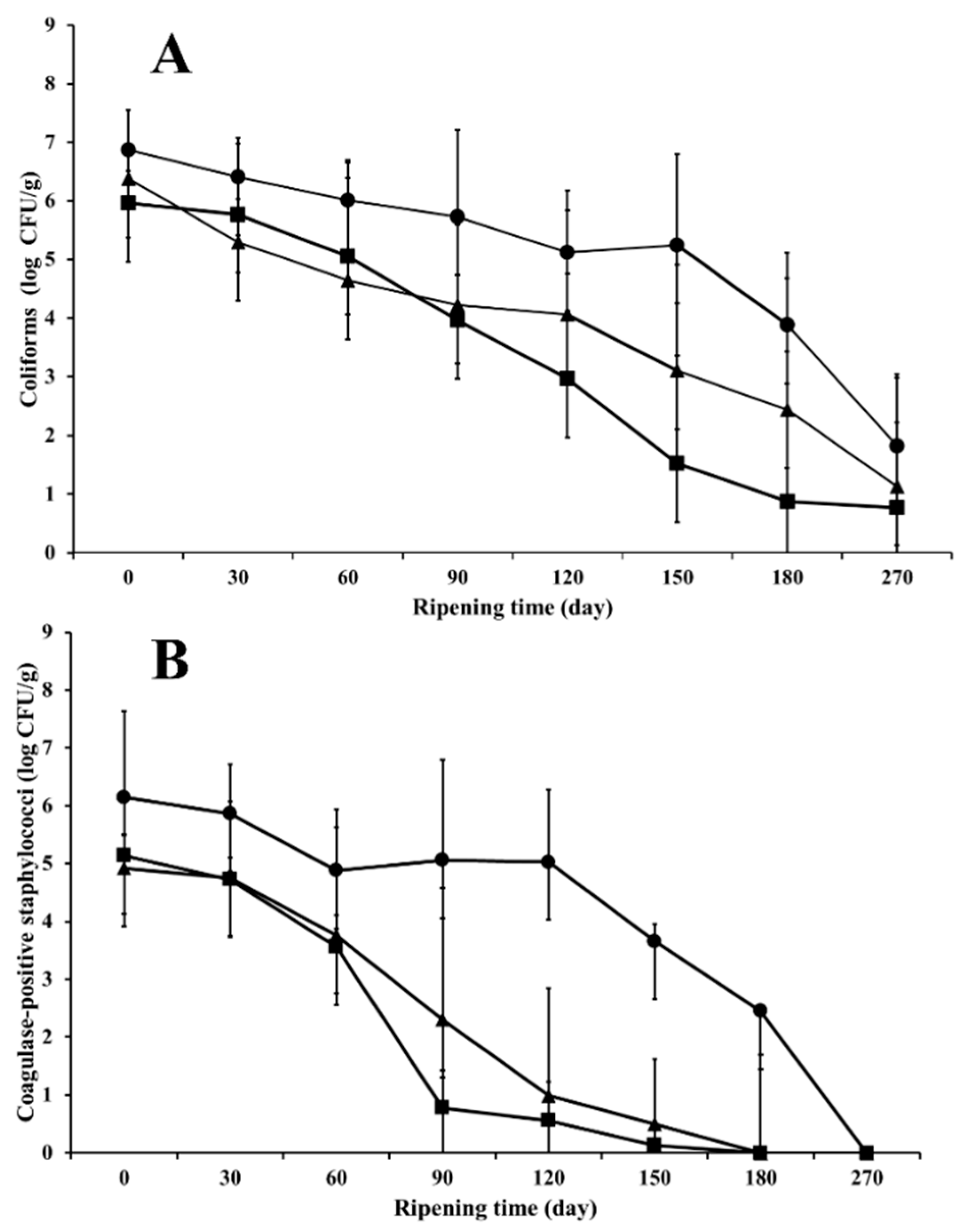
3.3. Influence of Ripening Time and Season on Microbial Counts of Indicator Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bautista-Gallego, J.; Alessandria, V.; Fontana, M.; Bisotti, S.; Taricco, S.; Dolci, P.; Cocolin, L.; Rantsiou, K. Diversity and functional characterization of Lactobacillus spp. isolated throughout the ripening of a hard cheese. Int. J. Food Microbiol. 2014, 181, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; O’sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Gaya, P.; Medina, M.; Nuñez, M. Evolution of the volatile components of raw ewes’ milk Castellano cheese: Seasonal variation. Int. Dairy J. 2004, 14, 39–46. [Google Scholar] [CrossRef]
- Wullschleger, S.; Lacroix, C.; Bonfoh, B.; Sissoko-Thiam, A.; Hugenschmidt, S.; Romanens, E.; Baumgartner, S.; Traoré, I.; Yaffee, M.; Jans, C.; et al. Analysis of lactic acid bacteria communities and their seasonal variations in a spontaneously fermented dairy product (Malian fènè) by applying a cultivation/genotype-based binary model. Int. Dairy J. 2013, 29, 28–35. [Google Scholar] [CrossRef]
- Scatassa, M.L.; Gaglio, R.; Macaluso, G.; Francesca, N.; Randazzo, W.; Cardamone, C.; Di Grigoli, A.; Moschetti, G.; Settanni, L. Transfer, composition and technological characterization of the lactic acid bacterial populations of the wooden vats used to produce traditional stretched cheeses. Food Microbiol. 2015, 52, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbent, J.R.; Steele, J.L. Cheese flavor and the genomics of lactic acid bacteria. ASM NEWS 2005, 71, 121–128. [Google Scholar]
- De Pasquale, I.; Calasso, M.; Mancini, L.; Ercolini, D.; La Storia, A.; De Angelis, M.; Di Cargo, R.; Gobbetti, M. Causal relationship between microbial ecology dynamics and proteolysis during manufacture and ripening of protected designation of origin (PDO) cheese Canestrato Pugliese. Appl. Environ. Microb. 2014, 80, 4085–4094. [Google Scholar] [CrossRef] [PubMed]
- Pogačić, T.; Mancini, A.; Santarelli, M.; Bottari, B.; Lazzi, C.; Neviani, E.; Gatti, M. Diversity and dynamic of lactic acid bacteria strains during aging of a long ripened hard cheese produced from raw milk and undefined natural starter. Food Microbiol. 2013, 36, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Marincola, F.C.; Savorani, F.; Engelsen, S.B.; Cosentino, S.; Viale, S.; Pisano, M.B. A NMR metabolomics study of the ripening process of the Fiore Sardo cheese produced with autochthonous adjunct cultures. Food Chem. 2013, 141, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA); Servicio de Información Agroalimentaria y Pesquera (SIAP). Boletín de leche. Enero-Marzo 2018. Available online: http://infosiap.siap.gob.mx/opt/boletlech/Bolet%C3%ADn%20de%20Leche%20enero-marzo%202018.pdf (accessed on 29 July 2018).
- Sánchez-Gamboa, C.; Hicks-Pérez, L.; Gutiérrez-Méndez, N.; Heredia, N.; García, S.; Nevárez-Moorillón, G.V. Seasonal influence on the microbial profile of Chihuahua cheese manufactured from raw milk. Int. J. Dairy Technol. 2018, 71, 81–89. [Google Scholar] [CrossRef]
- Olson, D.W.; Van Hekken, D.L.; Tunick, M.H.; Tomasula, P.M.; Molina-Corral, F.J.; Gardea, A.A. Mexican Queso Chihuahua: Functional properties of aging cheese. J. Dairy Sci. 2011, 94, 4292–4299. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Nuñez, A.; Van Hekken, D.L.; Renye, J.A. Sensory and protein profiles of Mexican Chihuahua cheese. J. Food Sci. Technol. 2014, 51, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Rockville, MD, USA, 1998; ISBN 0-93558-454-4. [Google Scholar]
- Brennan, J.G. Food texture measurement, In Developments in Food Analysis Techniques, 2nd ed.; King, R.D., Ed.; Applied Science Publishers: Essex, UK, 1980; pp. 1–78. ISBN 0-85334-755-7. [Google Scholar]
- Minitab 17 Statistical Software [Computer software]; Minitab, Inc.: State College, PA, USA, 2016.
- Diario Oficial de la Federación (DOF). MNX-F-738-COFOCALEC-2011. Sistema Producto Leche-Lácteos-Queso Chihuahua-Denominación, Especificaciones y Métodos de Prueba. 24 June 2011. Available online: http://www.cofocalec.org.mx/catalogo/por_clave (accessed on 16 September 2018).
- Broadbent, J.R.; Brighton, C.; McMahon, D.J.; Farkye, N.Y.; Johnson, M.E.; Steele, J.L. Microbiology of Cheddar cheese made with different fat contents using a Lactococcus lactis single-strain starter. J. Dairy Sci. 2013, 96, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Diario Oficial de la Federación (DOF). Norma Oficial Mexicana NOM-243-SSA1-2010. Productos y Servicios. Leche, Formula Láctea, Producto Lácteo Combinado y Derivados Lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba. Diciembre 2012. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5160755&fecha=27/09/2010 (accessed on 16 September 2018).
- Lucey, J.A.; Johnson, M.E.; Horne, D.S. Perspectives on the basis of the rheology and texture properties of cheese. J. Dairy Sci. 2003, 86, 2725–2743. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L.; Call, J.; Molina-Corral, F.J.; Gardea, A.A. Queso Chihuahua: Effects of seasonality of cheesemilk on rheology. Int. J. Dairy Technol. 2007, 60, 13–21. [Google Scholar] [CrossRef]
- Van Hekken, D.L.; Drake, M.A.; Tunick, M.H.; Guerrero, V.M.; Molina-Corral, F.J.; Gardea, A.A. Effect of pasteurization and season on the sensorial and rheological traits of Mexican Chihuahua cheese. Dairy Sci. Technol. 2008, 88, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Martín-Platero, A.M.; Valdivia, E.; Maqueda, M.; Martín-Sánchez, I.; Martínez-Bueno, M. Polyphasic approach to bacterial dynamics during the ripening of Spanish farmhouse cheese, using culture-dependent and-independent methods. Appl. Environ. Microb. 2008, 74, 5662–5673. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.; Fiori, M.; Riu, G.; Pes, M.; Salvatore, E.; Pirisi, A. Physico-chemical characteristics and acidic profile of PDO Pecorino Romano cheese: Seasonal variation. Small Rumin. Res. 2015, 126, 73–79. [Google Scholar] [CrossRef]
- Wouters, J.T.; Ayad, E.H.; Hugenholtz, J.; Smit, G. Microbes from raw milk for fermented dairy products. Int. Dairy J. 2002, 12, 91–109. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Deplano, M.; Corda, A.; Cosentino, S. Microbiological and chemical characterization of Fiore Sardo, a traditional Sardinian cheese made from ewe’s milk. Int. J. Dairy Technol. 2006, 59, 171–179. [Google Scholar] [CrossRef]
- Aydemir, O.; Harth, H.; Weckx, S.; Dervişoğlu, M.; De Vuyst, L. Microbial communities involved in Kaşar cheese ripening. Food Microbiol. 2015, 46, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Martín-Platero, A.M.; Maqueda, M.; Valdivia, E.; Purswani, J.; Martínez-Bueno, M. Polyphasic study of microbial communities of two Spanish farmhouse goats’ milk cheeses from Sierra de Aracena. Food Microbiol. 2009, 26, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Ordiales, E.; Martín, A.; Benito, M.J.; Hernández, A.; Ruiz-Moyano, S.; de Guía Córdoba, M. Role of the microbial population on the flavor of the soft-bodied cheese Torta del Casar. J. Dairy Sci. 2013, 96, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Verraes, C.; Vlaemynck, G.; Van Weyenberg, S.; De Zutter, L.; Daube, G.; Sindic, M.; Uyttendaele, M.; Herman, L. A review of the microbiological hazards of dairy products made from raw milk. Int. Dairy J. 2015, 50, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Schelin, J.; Wallin-Carlquist, N.; Thorup Cohn, M.; Lindqvist, R.; Barker, G.C. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence 2011, 2, 580–592. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Hunt, K.; McSweeney, S.; Jordan, K. Occurrence of foodborne pathogens in Irish farmhouse cheese. Food Microbiol. 2009, 26, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Morea, M.; Baruzzi, F.; Cocconcelli, P.S. Molecular and physiological characterization of dominant bacterial populations in traditional Mozzarella cheese processing. J. Appl. Microbiol. 1999, 87, 574–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arqués, J.L.; Rodríguez, E.; Gaya, P.; Medina, M.; Guamis, B.; Nunez, M. Inactivation of Staphylococcus aureus in raw milk cheese by combinations of high-pressure treatments and bacteriocin-producing lactic acid bacteria. J. Appl. Microbiol. 2005, 98, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.M.; Borelli, B.M.; Lara, C.A.; Soares, M.A.; Pataro, C.; Bodevan, E.C.; Rosa, C.A. The influence of seasons and ripening time on yeast communities of a traditional Brazilian cheese. Food Res. Int. 2015, 69, 331–340. [Google Scholar] [CrossRef]
- Mounier, J.; Monnet, C.; Vallaeys, T.; Arditi, R.; Sarthou, A.S.; Hélias, A.; Irlinger, F. Microbial interactions within a cheese microbial community. Appl. Environ. Microb. 2008, 74, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Cheese Factory | Moisture (%) | Fat (%) | Protein (%) | Ash (%) |
|---|---|---|---|---|
| A | 39.3 ± 1.5 ab | 22.2 ± 1.7 c | 27.2 ± 0.7 a | 4.2 ± 0.2 ab |
| B | 39.2 ± 1.2 ab | 40.1 ± 2.5 a | 25.6 ± 2.6 a | 3.7 ± 0.3 b |
| C | 37.6 ± 1.0 ab | 32.8 ± 2.6 b | 29.9 ± 2.5 a | 3.6 ± 0.2 b |
| D | 35.5 ± 1.3 b | 34.0 ± 2.1 b | 26.9 ± 0.9 a | 4.9 ± 0.4 a |
| E | 41.6 ± 3.0 a | 30.3 ± 1.6 b | 27.0 ± 0.7 a | 5.0 ± 0.3 a |
| Mexican Standard [18] | Max 45 | Min 28 | Min 23 |
| Cheese Factory | Hardness (N) | Adhesiveness (N s−2) | Springiness (mm) | Cohesiveness | Chewiness (mJ) |
|---|---|---|---|---|---|
| A | 19.9 ± 9.0 a | −0.80 ± 0.29 a | 9.5 ± 2.8 a | 0.33 ± 0.07 a | 77.3 ± 45.3 a |
| B | 15.7 ± 7.6 a | −0.40 ± 0.26 a | 11.4 ± 4.3 a | 0.54 ± 0.29 a | 53.9 ± 27.5 a |
| C | 18.6 ± 4.6 a | −1.1 ± 0.09 a | 11.0 ± 4.1 a | 0.53 ± 0.27 a | 82.4 ± 48.9 b |
| D | 26.3 ± 7.4 a | −0.40 ± 0.46 a | 11.1 ± 2.4 a | 0.60 ± 0.22 a | 150.6 ± 44.7 a |
| E | 24.7 ± 12.0 a | −0.91 ± 0.76 a | 10.9 ± 2.9 a | 0.41 ± 0.14 a | 102.3 ± 25.0 ab |
| Microbial Group/Season | Autumn | Winter | Summer |
|---|---|---|---|
| Coliforms | 3.3 ± 2.3 b | 3.9 ± 2.1 b | 5.1 ± 2.2 a |
| Mold | 3.1 ±1.6 ab | 2.7 ± 1.7 b | 3.6 ± 1.7 a |
| Yeast | 4.7 ± 1.2 a | 5.6 ± 1.3 a | 5.5 ± 1.6 a |
| Presumptive staphylococci | 1.9 ± 1.9 b | 2.2 ± 2.2 b | 4.1 ± 2.0 a |
| Mesophilic lactobacilli | 7.3 ±1.4 b | 7.7 ± 1.6 a | 7.9 ± 1.2 a |
| Thermophilic lactobacilli | 7.0 ± 1.3 b | 6.9 ± 0.6 b | 7.5 ± 0.5 a |
| Presumptive lactococci. | 5.6 ± 2.3 b | 6.9 ± 1.4 a | 7.1 ± 1.6 a |
| Thermophilic cocci | 5.0 ± 2.6 c | 6.0 ± 1.2 b | 6.8 ± 1. 5 a |
| Presumptive enterococci. | 6.4 ± 1.5 b | 5.8 ± 1.2 c | 7.5 ± 0.5 a |
| Microbial Group/Cheese Factory | A | B | C | D | E |
|---|---|---|---|---|---|
| Coliforms | 4.6 ±1.9 ab | 4.2 ±2.5 b | 4.0 ± 2.0 b | 5.3 ± 1.9 a | 2.6 ± 2.4 c |
| Mold | 2.2 ± 1.6 c | 3.1 ± 1.6 bc | 4.3 ± 1.2 a | 2.8 ± 1.8 bc | 3.4 ± 1.5 ab |
| Yeast | 5.0 ± 1.5 a | 5.0 ± 1.1 a | 5.4 ± 0.9 a | 5.4 ± 1.2 a | 5.5 ± 1.2 a |
| S. aureus | 2.6 ±2.4 b | 2.6 ± 2.6 b | 2.1 ± 2.4 b | 2.6 ± 2.4 b | 3.7 ± 2.3 a |
| Mesophilic lactobacilli | 7.6 ± 1.0 a | 7.7 ±0.6 a | 7.4 ± 0.7 a | 7.7 ± 0.4 a | 7.7 ± 0.4 a |
| Thermophilic lactobacilli | 7.0 ± 0.6 b | 7.2 ± 0.7 ab | 6.8 ± 0.8 b | 7.6 ± 0.5 a | 7.2 ± 0.6 ab |
| Lactococcus sp. | 6.4 ± 2.1 a | 6.5 ± 1.2 a | 6.0 ± 2.1 a | 7.1 ± 0.5 a | 6.7 ± 0.8 a |
| Thermophilic cocci | 5.5 ± 2.3 a | 5.8 ± 2.0 a | 5.8 ± 2.0 a | 6.4 ± 1.1 a | 6.3 ± 0.6 a |
| Enterococcus sp. | 6.9 ± 0.7 a | 6.3 ± 1.0 bc | 5.8 ± 1.5 c | 6.8 ± 1.1 ab | 6.9 ± 0.8 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gamboa, C.; Hicks-Pérez, L.; Gutiérrez-Méndez, N.; Heredia, N.; García, S.; Nevárez-Moorillón, G.V. Microbiological Changes during Ripening of Chihuahua Cheese Manufactured with Raw Milk and Its Seasonal Variations. Foods 2018, 7, 153. https://doi.org/10.3390/foods7090153
Sánchez-Gamboa C, Hicks-Pérez L, Gutiérrez-Méndez N, Heredia N, García S, Nevárez-Moorillón GV. Microbiological Changes during Ripening of Chihuahua Cheese Manufactured with Raw Milk and Its Seasonal Variations. Foods. 2018; 7(9):153. https://doi.org/10.3390/foods7090153
Chicago/Turabian StyleSánchez-Gamboa, Cristina, Liliana Hicks-Pérez, Néstor Gutiérrez-Méndez, Norma Heredia, Santos García, and Guadalupe Virginia Nevárez-Moorillón. 2018. "Microbiological Changes during Ripening of Chihuahua Cheese Manufactured with Raw Milk and Its Seasonal Variations" Foods 7, no. 9: 153. https://doi.org/10.3390/foods7090153
APA StyleSánchez-Gamboa, C., Hicks-Pérez, L., Gutiérrez-Méndez, N., Heredia, N., García, S., & Nevárez-Moorillón, G. V. (2018). Microbiological Changes during Ripening of Chihuahua Cheese Manufactured with Raw Milk and Its Seasonal Variations. Foods, 7(9), 153. https://doi.org/10.3390/foods7090153







