Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Reagents
2.3. Preparation of WPI hydrolysate
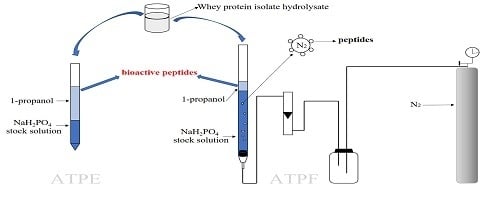
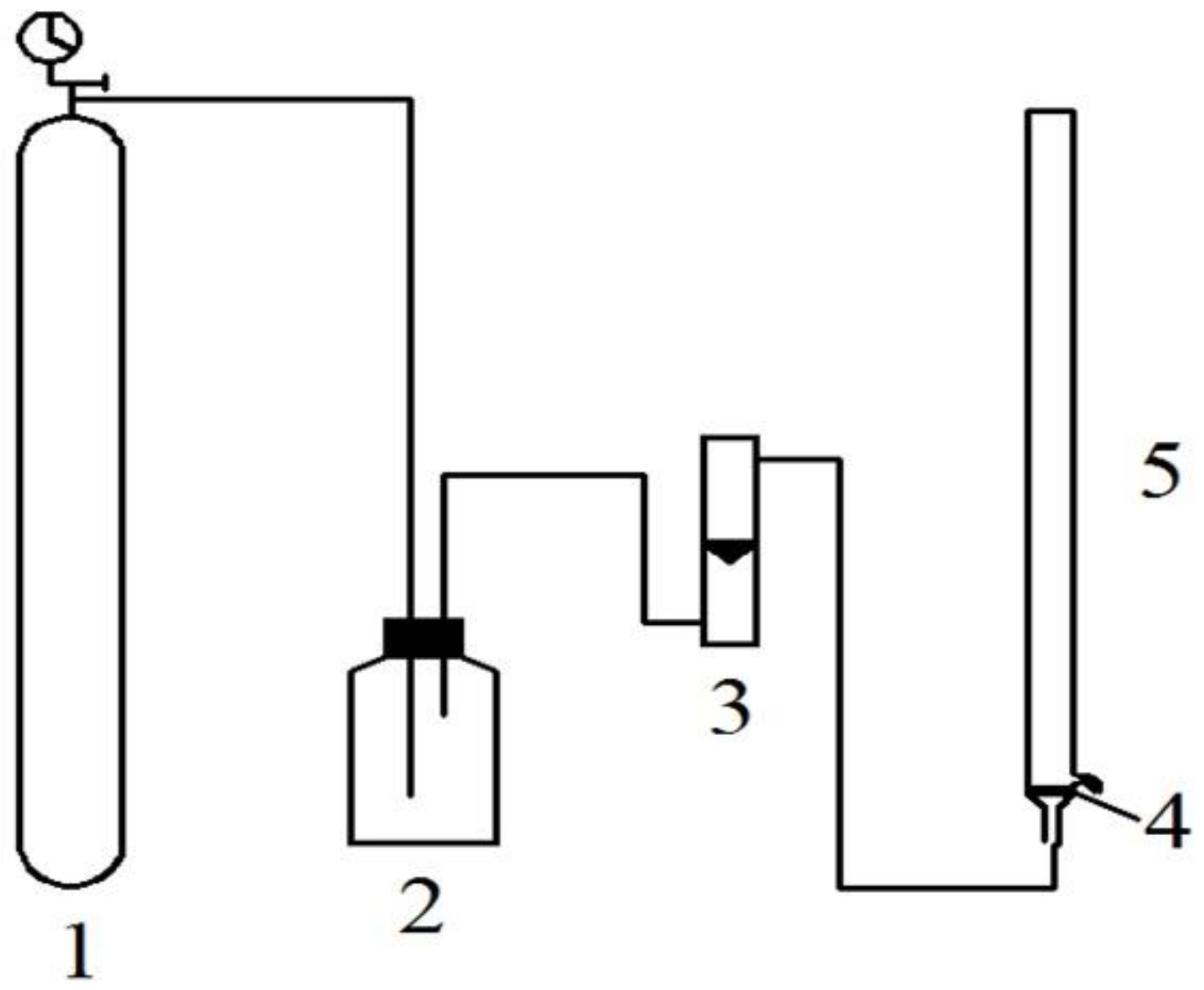
2.4. Separation of Antioxidant Peptides from Wpi Hydrolysate by ATPE and ATPF
2.5. Determination of the Distribution of Peptides in ATPE and ATPF
2.6. Purification of Peptides
2.7. Determination of Antioxidant Activity of Purified Peptides
2.8. RP-HPLC and MALDI-TOF MS Analysis for the Purified Peptides
3. Results and Discussion
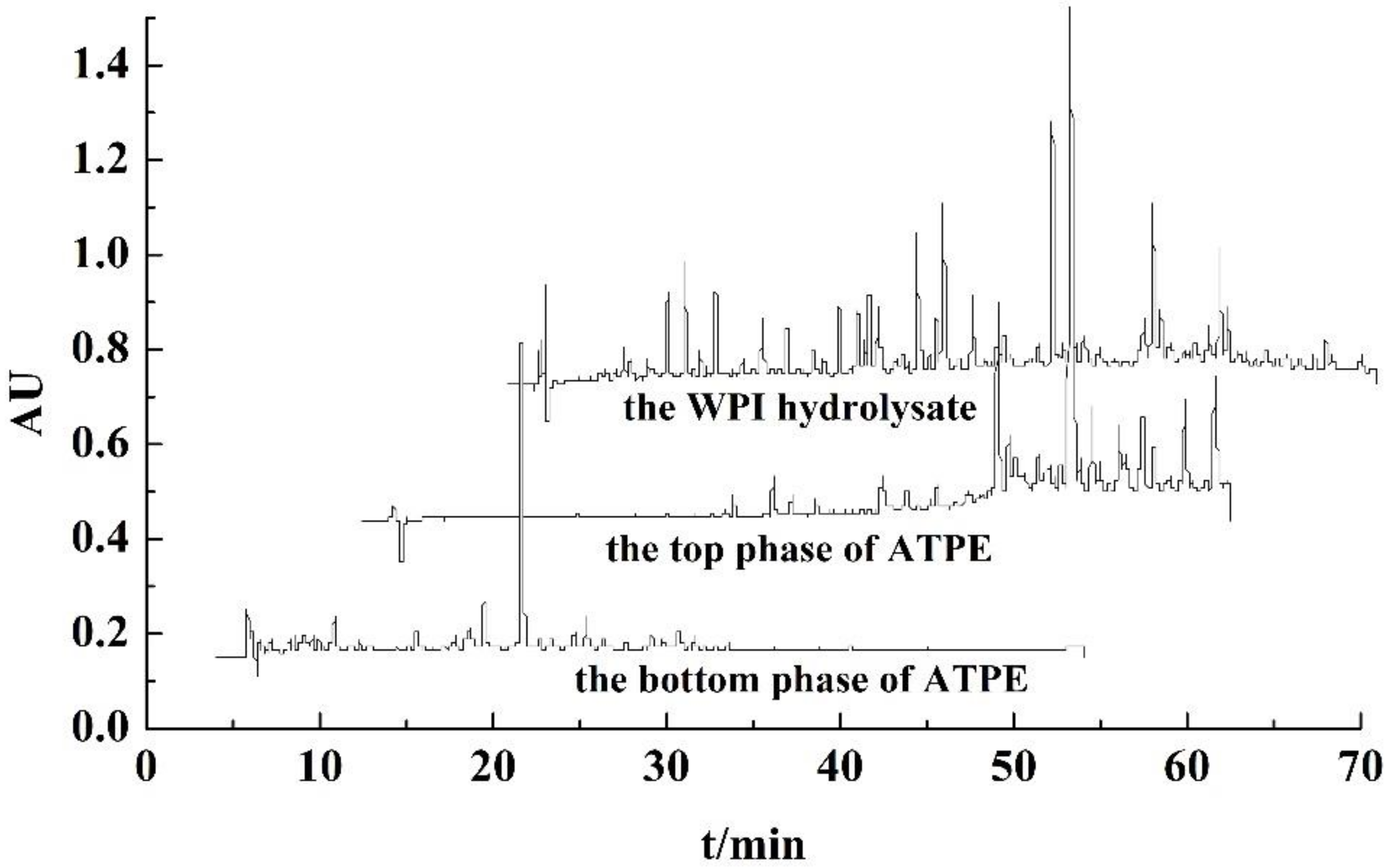
3.1. Factors on Partitioning of Antioxidant Peptides by ATPE
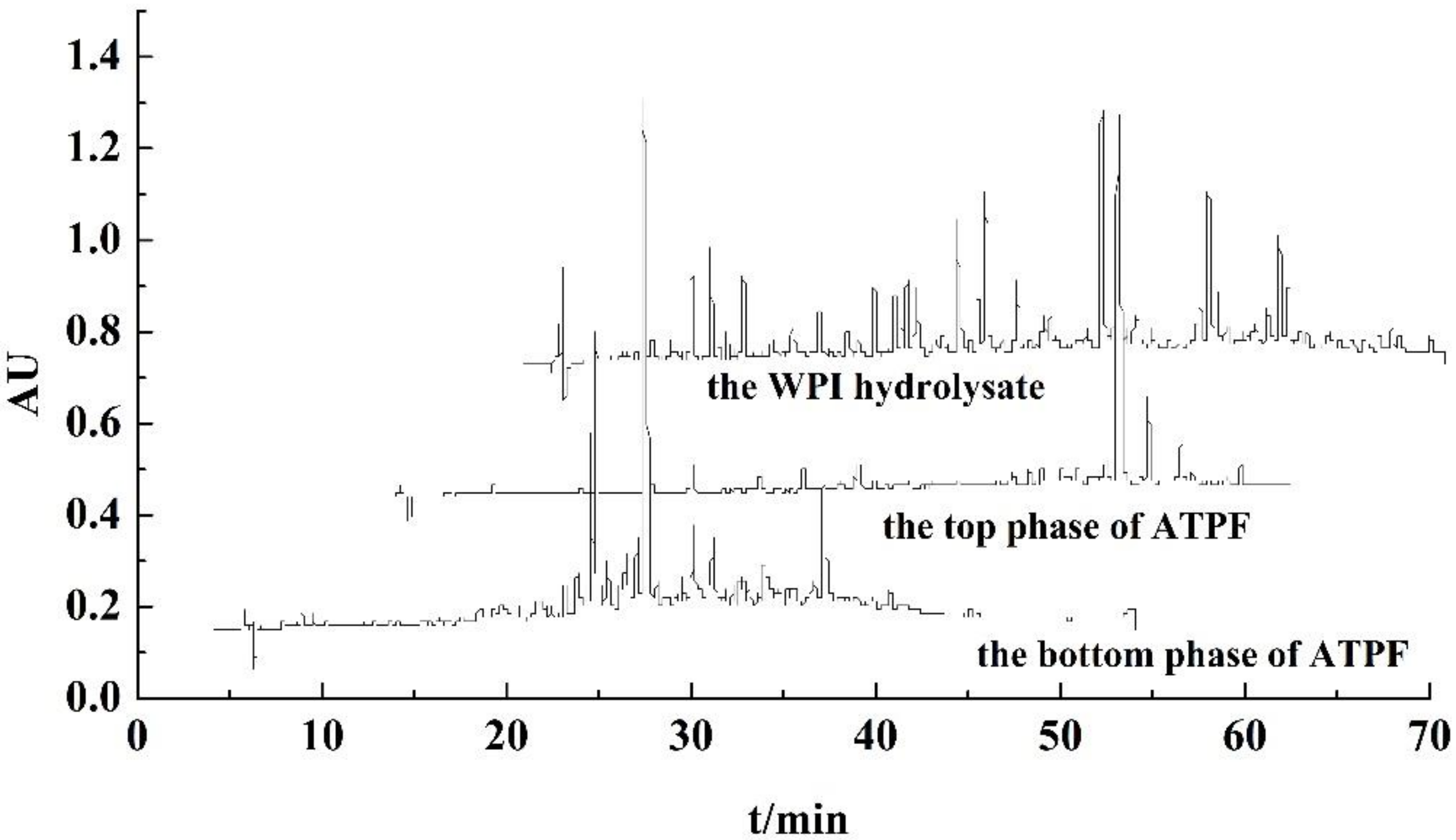
3.2. Factors on Partitioning of Antioxidant Peptides by ATPF
3.3. Determination of Antioxidant Activity of Purified Peptides
3.4. RP-HPLC Analysis of Purified Peptides
3.5. MALDI-TOF MS Analysis for Purified Peptides
3.6. Comparison of ATPE, ATPF and Other Methods of Extraction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Bovine whey proteins–Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, B.; Feng, Z.B.; Qu, Y.X.; Li, X. Separation of α-Lactalbumin and β-Lactoglobulin in Whey Protein Isolate by Aqueous Two-phase System of Polymer/Phosphate. Chin. J. Anal. Chem. 2016, 44, 754–759. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Technol. 2015, 52, 5377–5392. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.F.; Kise, F.; Rosso, A.M.; Parisi, M.G. Potential antioxidant peptides produced from whey hydrolysis with an immobilized aspartic protease from Salpichroa origanifolia fruits. Food Chem. 2017, 237, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Boudesocque, L.; Kapel, R.; Paris, C.; Dhulster, P.; Marc, I.; Renaul, J.H. Concentration and selective fractionation of an antihypertensive peptide from an alfalfa white proteins hydrolysate by mixed ion-exchange centrifugal partition chromatography. J. Chromatogr. B 2012, 905, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.M.; Winkler, R.; Garcíalara, S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteom. 2014, 111, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.; Aeld, B.; Mcconnell, M.A.; Carne, A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016, 213, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.; Azevedo, A.M.; Alstine, J.M.V.; Aires, M.R. Partitioning in aqueous two-phase systems: Analysis of strengths, weaknesses, opportunities and threats. Biotechnol. J. 2015, 10, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, X.Q.; Yuan, Y.Q.; Qu, Y.X.; Feng, Z.B. Separation of Antioxidant Peptides from Pepsin Hydrolysate of Whey Protein Isolate by ATPS of EOPO Co-polymer (UCON)/Phosphate. Sci. Rep. 2017, 7, 13320. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Khoiroh, I.; Ling, T.C.; Show, P.L. Aqueous two-phase flotation for the recovery of biomolecules. Sep. Purif. Methods 2014, 45, 81–92. [Google Scholar] [CrossRef]
- Padilha, C.E.D.A.; Dantas, P.V.F.; Júnior, F.C.S.; Júnior, S.D.O.; Nogueira, C.D.C.; Souza, D.F.D.S. Recovery and concentration of ortho-phenylphenol from biodesulfurization of 4-methyl dibenzothiophene by aqueous two-phase flotation. Sep. Purif. Technol. 2017, 176, 306–312. [Google Scholar] [CrossRef]
- Bi, P.Y.; Li, D.Q.; Dong, H.R. A novel technique for the separation and concentration of penicillin G from fermentation broth: Aqueous two-phase flotation. Sep. Purif. Technol. 2009, 69, 205–209. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.H.; Han, J.; Yan, Y.S. Separation/enrichment of trace tetracycline antibiotics in water by [Bmim]BF4–(NH4)2SO4 aqueous two-phase solvent sublation. Desalination 2011, 266, 114–118. [Google Scholar] [CrossRef]
- Ledesma, B.H.; Daavalos, A.; Bartolomea, B.; Amigo, A.L. Preparation of Antioxidant Enzymatic Hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of Active Peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, K.; Nazeer, R.A. Antioxidant Activity of Purified Protein Hydrolysates from Northern Whiting Fish (Sillago sihama) Muscle. Int. J. Pept. Res. Ther. 2014, 20, 209–219. [Google Scholar] [CrossRef]
- Jiang, B.; Yuan, Y.Q.; Zhang, X.Q.; Feng, G.B.; Liu, C.H. Separation and Enrichment of Lectin from Zihua Snap-Bean (Phaseolus vulgaris) Seeds by PEG 600–Ammonium Sulfate Aqueous Two-Phase System. Molecules 2017, 22, 1596. [Google Scholar] [CrossRef]
- Jiang, B.; Feng, Z.B.; Liu, C.H.; Xu, Y.C.; Li, C.M.; Ji, G. Extraction and purification of wheat-esterase using aqueous two-phase systems of ionic liquid and salt. J. Food Sci. Technol. 2015, 52, 2878–2885. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Rosa, P.A.; Ferreira, I.F.; Barros, A.M.R. Optimisation of aqueous two-phase extraction of human antibodies. J. Biotechnol. 2007, 132, 209–217. [Google Scholar] [CrossRef]
- Bi, P.Y.; Dong, H.R.; Yuan, Y.C. Application of aqueous two-phase flotation in the separation and concentration of puerarin from Puerariae extract. Sep. Purif. Technol. 2010, 75, 402–406. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Ma, H.; Huang, P.; Li, J.; Kikuchi, T. Effect of micro-bubbles on coagulation flotation process of dyeing wastewater. Sep. Purif. Technol. 2010, 71, 337–346. [Google Scholar] [CrossRef]
- Uçurum, M.; Bayat, O. Effects of operating variables on modified flotation parameters in the mineral separation. Sep. Purif. Technol. 2007, 55, 173–181. [Google Scholar] [CrossRef]
- Xie, N.; Wang, B.; Jiang, L.; Liu, C.C.; Li, B. Hydrophobicity exerts different effects on bioavailability and stability of antioxidant peptide fractions from casein during simulated gastrointestinal digestion and Caco-2 cell absorption. Food Res. Int. 2015, 76, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liang, B.; Tang, S.; Min, E. Effects of Orifice Orientation and Gas-Liquid Flow Pattern on Initial Bubble Size. Chin. J. Chem. Eng. 2013, 21, 1206–1215. [Google Scholar] [CrossRef]
- Kapust, R.B.; Tözsér, J.; Copel, T.D.; Waugh, D.S. The P10 specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun. 2002, 294, 949–955. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Lindskog, I.; Gasteiger, E.; Bairoch, A.; Sanchez, G.C.; Hochstrasser, D.F.; Appel, R.O. Detailed peptide characterization using PEPTIDEMASS-a World-Wide-Web-accessible tool. Electrophoresis 1997, 18, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Antioxidative and Antibacterial Peptides Derived from Bovine Milk Proteins. Crit. Rev. Food Sci. Nutr. 2016, 58, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Conway, V.; Gauthier, S.F.; Poulio, Y. Antioxidant Activities of Buttermilk Proteins, Whey Proteins, and Their Enzymatic Hydrolysates. J. Agric. Food Chem. 2012, 61, 364–372. [Google Scholar] [CrossRef]
- Castro, R.J.S.; Sato, H.H. Biologically active peptides: Processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res. Int. 2015, 74, 185–198. [Google Scholar] [CrossRef]
- Wu, R.B.; Wu, C.L.; Liu, D.; Yang, X.H.; Huang, J.F.; Zhang, J.; Liao, B.Q.; He, H.L.; Li, H. Overview of Antioxidant Peptides Derived from Marine Resources: The Sources, Characteristic, Purification, and Evaluation Methods. Appl. Biochem. Biotechnol. 2015, 176, 1815–1833. [Google Scholar] [CrossRef]
- Re, J.; Zhao, M.M.; Shi, J.; Wang, J.S.; Jiang, Y.M.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar]
- Bougatef, A.; Arroume, N.N.; Manni, L.; Ravalle, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.B.; Ong, M.G.L.; Pang, M.J.; Wong, S.J.; The, L.K.; Chai, T.T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Anguizola, J.A.; Bi, C.; Li, R.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Vargas, J.; Zheng, X. Pharmaceutical and biomedical applications of affinity chromatography: Recent trends and developments. J. Pharm. Biomed. Anal. 2012, 69, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, P.E.A.; Xiong, Y.L.; Arteaga, G.E. Fractionation and characterisation forantioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004, 84, 1908–1918. [Google Scholar] [CrossRef]
- Maux, L.S.; Nongonierma, A.B.; Fitzgerald, R.J. Improved short peptide identification using HILIC–MS/MS: Retention time prediction model based on based on. Food Chem. 2015, 173, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Guillarme, S. Ultra-high-performance liquid chromatography for the characterization of therapeutic proteins. TrAC Trends Anal. Chem. 2014, 63, 76–84. [Google Scholar] [CrossRef]
- De Souza, E.C., Jr.; Coimbra, J.S.D.R.; Oliveira, E.B.D.; Bonomo, R.C.F. Recovery of casein-derived peptides with in vitro inhibitory activity of angiotensin converting enzyme (ACE) using aqueous two-phase systems. J. Chromatogr. B 2014, 973, 84–88. [Google Scholar] [CrossRef]




| The Source of Peptides | ABTS Radical Scavenging Activity (%) | DPPH Radical Scavenging Activity (%) | OH Radical Scavenging Activity (%) | Ferric Reducing Antioxidant Power |
|---|---|---|---|---|
| WPI hydrolysate | 53.44 ± 0.53 b | 8.34 ± 0.09 b | 79.91 ± 0.85 b | 0.297 ± 0.001 b |
| Top phase of ATPE | 68.53 ± 0.50 c | 15.98 ± 0.03 c | 85.98 ± 0.38 c | 0.361 ± 0.001 c |
| Bottom phase of ATPE | 34.81 ± 0.49 a | 5.98 ± 0.17 a | 51.26 ± 0.33 a | 0.167 ± 0.003 a |
| Top phase of ATPF | 81.88 ± 0.68 d | 23.68 ± 0.07 d | 89.72 ± 0.64 d | 0.403 ± 0.001 d |
| Peptide Source | Amino Acid Sequence | Protein Fragment | m/z | Antioxidant-Active Peptides |
|---|---|---|---|---|
| Top phase of ATPE | KILDK | α-La (113–117) | 616.249 | |
| Bottom phase of ATPE | LDQWLCEKL | α-La (115–123) | 1120.115 | |
| Top phase of ATPE | ALCSEKLDQWLCEK | α-La (109–122) | 1683.270 | |
| Bottom phase of ATPE | LSFNPTQLEEQCHI | β-Lg (149–162) | 1689.969 | |
| Bottom phase of ATPE | WENGECAQKKIIAEK | β-Lg (61–75) | 1748.036 | |
| Top phase of ATPE | KILDKVGINYWLAHK | α-La (94–108) | 1796.315 | AHK |
| Top phase of ATPE | VGINYWLAHKALCSEK | α-La (99–114) | 1846.401 | AHK |
| Bottom phase of ATPE | NDQDPHSSNICNISCDK | α-La (63–79) | 1877.905 | |
| Top phase of ATPE | TPEVDDEALEKFDKALK | β-Lg (125–141) | 1945.402 | LK |
| Bottom phase of ATPE | FLDDDLTDDIMCVKKILLDK | α-La (80–98) | 2242.638 | |
| Bottom phase of ATPE | YLLFCMENSAEPEQSLACQCLVR | β-Lg (102–124) | 2666.749 |
| Peptide Source | Amino Acid Sequences | Protein Fragment | m/z | Antioxidant-Active Peptide |
|---|---|---|---|---|
| Top phase of ATPF | IIAEKTKIPAVFK | β-Lg (71–83) | 1451.639 | IPAVF |
| Top phase of ATPF | KIIAEKTKIPAVFK | β-Lg (70–83) | 1581.305 | IPAVF |
| Top phase of ATPF | ILLDKVGINYWLAHK | α-La (95–108) | 1678.347 | AHK |
| Top phase of ATPF | KILLDKVGINYWLAHK | α-La (94–108) | 1794.545 | AHK |
| Top phase of ATPF | VGINYWLAHKALCSEK | α-La (99–114) | 1843.382 | AHK |
| Top phase of ATPF | VYVEELKPTPEGDLEILLQK | β-Lg (41–60) | 2313.923 | YVEEL |
| Top phase of ATPF | TPEVDDEALEKFDKALPMHIR | β-Lg (125–148) | 2732.832 |
| Method of Extraction | Advantage | Limitation |
|---|---|---|
| Ion-exchange chromatography [6] | The separation of highly cationic or anionic peptides. | Requires complementary steps for the separation and low selectivity. |
| Affinity chromatography [34] | The separation of different types of peptides. | physicochemical properties of the ligands yet to be discovered. |
| Size exclusion chromatography [35] | Mild elution conditions, with minimal impact on the conformational structure. | High column requires separation of mixed peptides. |
| Hydrophilic interaction liquidChromatography [36] | The method shows great potential for the separation of short peptide sequences (5 amino acids). | Limited flexibility and applicability, poorly understood problems with sample solubility and the retention mechanisms. |
| Ultra-high-pressure liquid chromatography [37] | Increased throughput, resolution, and sensitivity in separation of complex protein mixtures. | Ultra-high pressures increase chromatographic band broadening and compromise efficiency of the column. |
| Ultrafiltration | Short time, high throughput, and high recovery. | Difficult to control experimental conditions in the membrane. |
| ATPE [9,38] | Rapid, simple, and inexpensive, low in toxicity and biocompatibility separation process. | Large amounts of polymers and salts and easy to emulsify. |
| ATPF | Increased throughput in separation of complex protein mixtures. Enhanced selectivity, scale-up, process integration, continuous operation, low toxicity, and biocompatibility. | Large amounts of polymers and salts |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation. Foods 2019, 8, 34. https://doi.org/10.3390/foods8010034
Jiang B, Na J, Wang L, Li D, Liu C, Feng Z. Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation. Foods. 2019; 8(1):34. https://doi.org/10.3390/foods8010034
Chicago/Turabian StyleJiang, Bin, Jiaxin Na, Lele Wang, Dongmei Li, Chunhong Liu, and Zhibiao Feng. 2019. "Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation" Foods 8, no. 1: 34. https://doi.org/10.3390/foods8010034
APA StyleJiang, B., Na, J., Wang, L., Li, D., Liu, C., & Feng, Z. (2019). Separation and Enrichment of Antioxidant Peptides from Whey Protein Isolate Hydrolysate by Aqueous Two-Phase Extraction and Aqueous Two-Phase Flotation. Foods, 8(1), 34. https://doi.org/10.3390/foods8010034