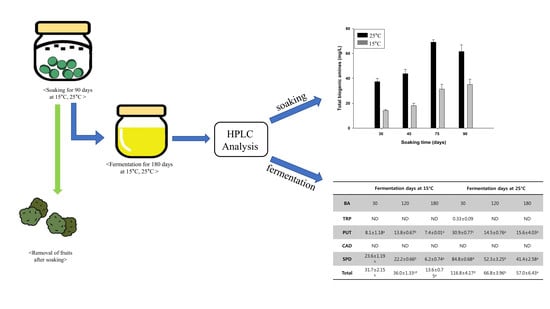
Effects of Soaking and Fermentation Time on Biogenic Amines Content of Maesil (Prunus Mume) Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Food Samples
2.3. pH Measurement
2.4. Amino Acids Analysis
2.5. Biogenic Amine Analysis
2.5.1. Extraction of Biogenic Amines
2.5.2. Derivatization of Biogenic Amines
2.5.3. HPLC Analysis of Biogenic Amines
2.6. Method Validation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
3.2. Content of Biogenic Amines in Home-Made Maesil Extract
3.3. Content of Biogenic Amines During Soaking and Fermentation
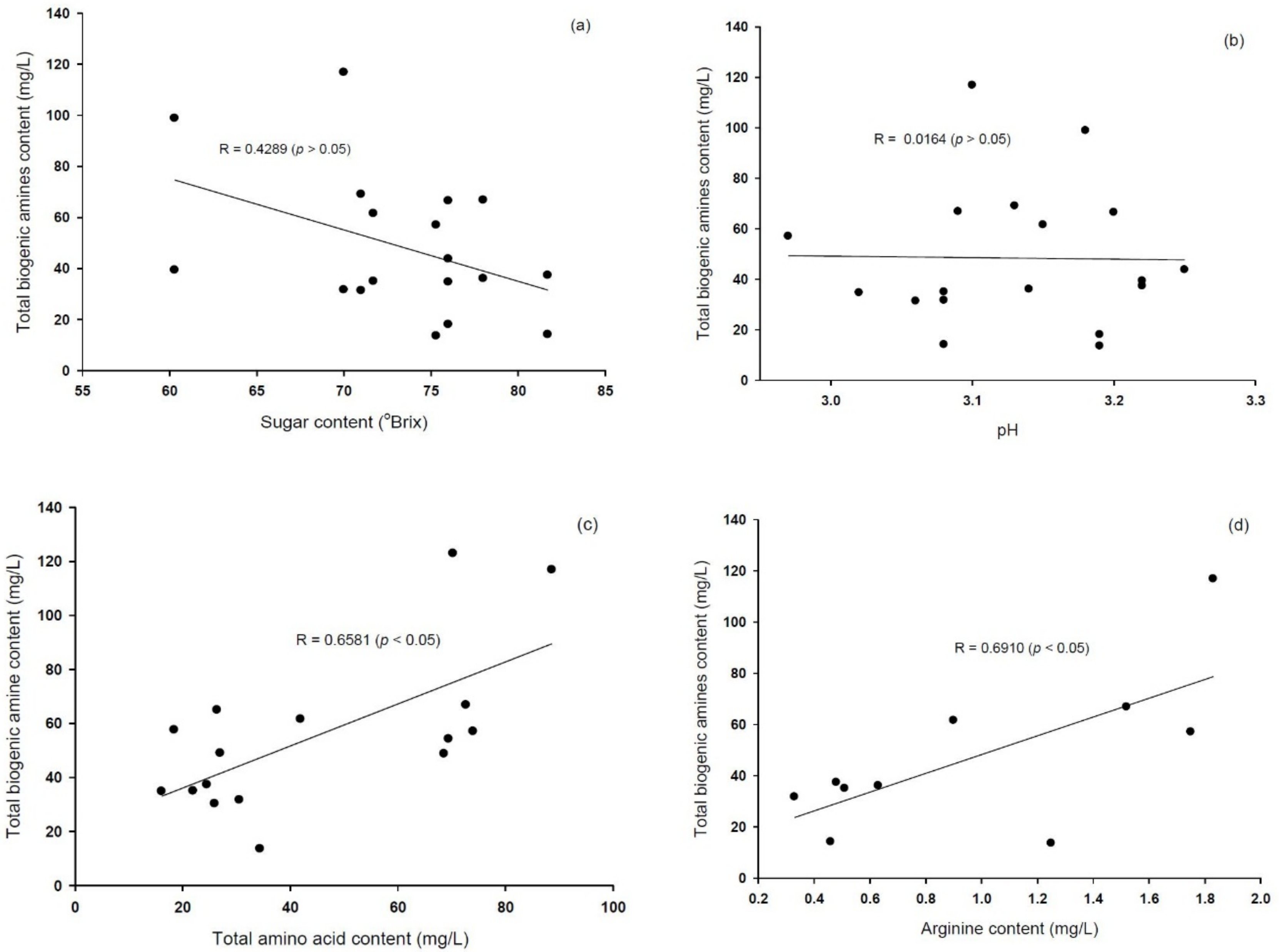
3.4. Effects of Processing Factors on Biogenic Amines Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, M.Y.; Jeong, Y.H.; Eun, J.B. Physical and chemical characteristics of flesh and pomace of Japanese Apricots (Prunus mume Sieb. et Zucc). Korean J. Food Sci. Technol. 1999, 31, 1434–1439. [Google Scholar]
- Ko, M.S.; Yang, J.B. Antimicrobial activities of extracts of Prunus mume by sugar. Korean Soc. Food Preser. 2009, 16, 759–764. [Google Scholar]
- Miyazawa, M.; Yamada, T.; Utsunomiya, H. Suppressive effect of the SOS-inducing activity of chemical mutagen by citric acid esters from Prunus mume Sieb. et Zucc. using the Salmonella typhimurium Ta1535/Psk1002 Umu test. Nat. Prod. Res. 2003, 17, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef]
- Choi, B.G.; Koh, E. Changes of ethyl carbamate and its precursors in maesil (Prunus mume) extract curing one-year fermentation. Food Chem. 2016, 209, 318–322. [Google Scholar] [CrossRef]
- Kim, H.W.; Han, S.H.; Lee, S.W.; Suh, H.J. Effect of isomaltulose used for osmotic extraction of Prunus mume fruit juice substituting sucrose. Food Sci. Technol. 2018, 27, 1599–1605. [Google Scholar] [CrossRef]
- Ko, Y.J.; Jeong, D.Y.; Lee, J.O.; Park, M.H.; Kim, E.J.; Kim, J.W.; Kim, Y.S.; Ryu, C.H. The establishment of optimum fermentation conditions for Prunus mume vinegar and its quality evaluation. Korean Soc. Food Sci. Nutr. 2007, 36, 361–365. [Google Scholar] [CrossRef]
- Yan, X.T.; Lee, S.H.; Li, W.; Sun, Y.N.; Yang, S.Y.; Jang, H.D.; Kim, Y.H. Evaluation of the antioxidant and anti-osteoporosis activities of chemical constituents of the fruits of Prunus mume. Food Chem. 2014, 156, 408–415. [Google Scholar] [CrossRef]
- Henriquez-Aedo, K.; Galarce-Bustos, O.; Aqueveque, P.; Garcia, A.; Aranda, M. Dynamic of biogenic amines and precursor amino acids during cabernet sauvignon vinification. LWT Food Sci. Technol. 2018, 97, 238–244. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Bai, X.; Byun, B.Y.; Mah, J.H. Formation and destruction of biogenic amines in Chunjang (a black soybean sauce) and Jajang (a blck soybean sauce). Food Chem. 2013, 141, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Garai, G.; Duenas, M.T.; Irastorza, A.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V. Biogenic amines in natural ciders. J. Food Prot. 2006, 69, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, H.J.; Kim, M.J.; Ahn, H.J.; Byun, M.W. Survey of biogenic amine contents in commercial soy sauce. Korean J. Food Sci. Technol. 2003, 35, 325–328. [Google Scholar]
- Mohan, C.O.; Ravishankar, C.N.; Srinivasa Gopal, T.K.; Ashok Kumar, K.; Lalitha, K.V. Biogenic amines formation in seer fish (Scomberomoruscommerson) steaks packed with O2 scavenger during chilled storage. Food Res. Int. 2009, 42, 411–416. [Google Scholar] [CrossRef]
- Yoon, H.; Park, J.H.; Choi, A.; Hwang, H.J.; Mah, J.H. Validation of an HPLC analytical method for determination of biogenic amines in agricultural products and monitoring of biogenic amines in Korean fermented agricultural products. Toxicol. Res. 2015, 31, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nuriez, M.; del Olmo, A.; Calzada, J. Biogenic amines. Encycl. Food Health 2016, 416–423. [Google Scholar]
- Zarei, M.; Najafzadeh, H.; Enayati, A.; Pashmforoush, M. Biogenic amines content of canned tuna fish marketed in Iran. Am. Eurasian J. Toxicol. Sci. 2011, 3, 190–193. [Google Scholar]
- Nadeem, M.; Naveed, T.; Rehman, F.; Xu, Z. Determination of histamine in fish without derivatization by indirect reverse phase-HPLC method. Microchem. J. 2019, 144, 209–214. [Google Scholar] [CrossRef]
- Carelli, D.; Centonze, D.; Palermo, C.; Quinto, M.; Rotunno, T. An interference free amperometric biosensor for the detection of biogenic amines in food products. Biosens. Bioelectron. 2007, 23, 64–647. [Google Scholar] [CrossRef]
- Nout, M.J.R.; Ruikes, M.M.W.; Bouwmeester, H.M.; Belfaars, P.R. Effect of processing conditions on the formation of biogenic amines and ethyl carbamate in soybean Tempeh. J. Food Saf. 1993, 13, 293–303. [Google Scholar] [CrossRef]
- Shukla, S.; Park, H.K.; Lee, J.S.; Kim, J.K.; Kim, M. Reduction of biogenic amines and aflatoxin in Doenjang samples fermented with various Meju as starter cultures. Food Control. 2014, 42, 181–187. [Google Scholar] [CrossRef]
- Jajic, I.; Krstovic, S.; Glamocic, D.; Jaksic, S.; Abramovic, B. Validation of HPLC method for the determination of amino acids in feed. J. Serb. Chem. Soc. 2013, 78, 839–850. [Google Scholar] [CrossRef]
- Shukla, S.; Park, H.K.; Kim, J.K.; Kim, M.H. Determination of biogenic amines in Korean traditional fermented soybean paste (Doenjang). Food Chem. Toxicol. 2010, 48, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Martinez-Villaluenga, C.; Gulewicz, P.; Perez-Romero, A.; Pilarski, R.; Gulewicz, K.; Vidal-Valverde, C. Biogenic amines and HL 60 citotoxicity of alfalfa and fenugreek sprouts. Food Chem. 2007, 105, 959–967. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, Y.; Cheng, Y.; Liu, Y.; Yadav, M.P.; Yin, L. Reduction of biogenic amines in sufu by ethanol during ripening stage. Food Chem. 2018, 239, 1244–1252. [Google Scholar] [CrossRef]
- Costa, M.P.; Balthazar, C.F.; Rodrigues, B.L.; Lazaro, C.A.; Silva, A.C.O.; Cruz, A.G.; Conte Junior, C.A. Determination of biogenic amines by high-performance liquid chromatography (HPLC-DAD) in probiotic cow’s and goat’s fermented milks and acceptance. Food Sci. Nutr. 2015, 3, 172–178. [Google Scholar] [CrossRef]
- Jastrzebska, A.; Piasta, A.; Kowalska, S.; Krzeminski, M.; Szlyk, E. A new derivatization reagent for determination of biogenic amines in wines. J. Food Compos. Anal. 2016, 48, 111–119. [Google Scholar] [CrossRef]
- Poveda, J.M. Biogenic amines and free amino acids in craft beers from the Spanish market: A statistical approach. Food Control. 2019, 96, 227–233. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Polo, L.; Pardo, I. Biogenic amines in wines from three Spanish regions. J. Agric. Food Chem. 2005, 53, 1119–1124. [Google Scholar] [CrossRef]
- Marcobal, A.; Martin-Alvarez, P.J.; Polo, M.C.; Moreno-Arribas, M.V. Formation of biogenic amines throughout the industrial manufacture of red wine. J. Food Prot. 2006, 69, 397–404. [Google Scholar] [CrossRef]
- Bardocz, S. Polyamines in food and their consequences for food quality and human health. Trends Food Sci. Technol. 1995, 6, 341–346. [Google Scholar] [CrossRef]
- Gezginc, Y.; Akyol, I.; Kuley, E.; Ozogul, F. Biogenic amines formation in Streptococcus thermophiles isolated from home-made natural yogurt. Food Chem. 2013, 138, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Karovicova, J.; Kohajdova, Z. Biogenic amines in food. Chem. Pap. 2005, 59, 70–79. [Google Scholar] [CrossRef]
- Ozogul, F.; Hamed, I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.P.L.V.O.; Mendes, E.; Oliveira, B.M.; Ferreira, M. Effect of temperature on evolution of free amino acid and biogenic amine contents during storage of Azeitao cheese. Food Chem. 2001, 75, 287–291. [Google Scholar] [CrossRef]
- Santos, M.H.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Patel, M.A.; Ou, M.S.; Harbrucker, R.; Aldrich, H.C.; Buszko, M.L.; Ingram, L.O.; Shanmugam, K.T. Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl. Environ. Micorobiol. 2006, 72, 3228–3235. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.Y.; Abu Bakar, F.; Russly, A.R.; Jamilah, B.; Mahyudin, N.A. The effects of food processing on biogenic amines formation. Int. Food Res. J. 2011, 18, 867–876. [Google Scholar]
- Kim, S.H.; Price, B.J.; Morrissey, M.T.; Field, K.G.; Wei, C.I.; An, H. Occurrence of histamine-forming bacteria in albacore and histamine accumulation in muscle at ambient temperature. J. Food Sci. 2002, 67, 1515–1521. [Google Scholar] [CrossRef]
- Arena, M.E.; Landete, J.M.; Manca de Nadra, M.C.; Pardo, I.; Ferrer, S. Factors affecting the production of putrescine from agmatine by Lactobacillus hilgardii X1B isolated from wine. J. Appl. Microbiol. 2008, 105, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Cid, B.S.; Miguelez-Arrizado, M.J.; Becker, B.; Holzapfel, W.H.; Vidal-Carou, M.C. Amino acid decarboxylation by Lactobacillus curvatus CTC 273 affected by the pH and glucose availability. Food Microbiol. 2008, 25, 269–277. [Google Scholar] [PubMed]
- Wang, Y.Q.; Ye, D.Q.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q. Rapid HPLC analysis of amino acids and biogenic amines in wine during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Soufleros, E.; Barrios, M.L.; Bertrand, A. Correlation between the content of biogenic amines and other wine compounds. Am. J. Enol. Vitic. 1998, 49, 266–278. [Google Scholar]

 ) and 15 °C (
) and 15 °C (  ).
).
 ) and 15 °C (
) and 15 °C (  ).
).
| Compound | R2 | LOD (mg/L) | LOQ (mg/L) | Precision (%RSD) | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|
| Inter-Day | Intra-Day | Recovery (%) | |||||||
| Low | High | Low | High | Low | High | ||||
| HIS | 1.0000 | 0.02 | 0.04 | 3.82 | 5.20 | 0.49 | 6.46 | 97.9 | 89.4 |
| TRP | 1.0000 | 0.01 | 0.02 | 0.66 | 0.19 | 0.10 | 0.09 | 101.8 | 105.3 |
| 2-PHE | 1.0000 | 0.16 | 0.22 | 0.22 | 0.74 | 0.07 | 0.64 | 101.3 | 97.9 |
| PUT | 1.0000 | 0.01 | 0.02 | 0.45 | 0.34 | 0.25 | 0.76 | 99.8 | 104.0 |
| CAD | 1.0000 | 0.01 | 0.02 | 0.14 | 0.19 | 0.25 | 0.49 | 100.1 | 100.0 |
| TYR | 1.0000 | 0.07 | 0.10 | 0.27 | 0.17 | 0.09 | 0.22 | 99.7 | 99.3 |
| SPM | 0.9981 | 0.20 | 0.61 | 2.27 | 1.86 | 1.07 | 2.21 | 95.1 | 110.8 |
| SPD | 1.0000 | 0.04 | 0.08 | 0.14 | 0.40 | 0.16 | 0.11 | 107.1 | 99.6 |
| Sample | HIS | TRP | 2-PHE | PUT | CAD | TYR | SPM | SPD | Total |
|---|---|---|---|---|---|---|---|---|---|
| A | ND | ND | ND | 19.7 ± 0.53 | 2.9 ± 1.01 | ND | ND | 219.2 ± 6.30 | 241.8 ± 4.89 |
| B | ND | 3.0 ± 0.53 | ND | 12.4 ± 0.67 | ND | ND | ND | 44.5 ± 1.96 | 60.0 ± 1.64 |
| C | ND | ND | ND | 15.3 ± 0.74 | ND | ND | ND | 14.9 ± 0.91 | 30.2 ± 1.64 |
| D | ND | 1.9 ± 0.36 | ND | 8.8 ± 0.42 | ND | ND | ND | 25.6 ± 0.74 | 36.2 ± 0.18 |
| E | ND | 3.2 ± 0.47 | ND | 15.9 ± 1.69 | ND | ND | ND | 44.3 ± 2.31 | 63.4 ± 4.07 |
| F | ND | ND | ND | 13.1 ± 0.99 | ND | ND | ND | 83.5 ± 6.28 | 96.5 ± 6.21 |
| G | ND | 2.9 ± 0.65 | ND | 25.8 ± 2.33 | 17.3 ± 3.79 | ND | ND | 44.6 ± 3.11 | 90.6 ± 4.93 |
| H | ND | 5.7 ± 1.32 | ND | ND | 12.7 ± 0.42 | ND | ND | 35.9 ± 1.22 | 54.3 ± 2.09 |
| I | ND | 2.5 ± 0.48 | ND | ND | ND | ND | ND | ND | 2.5 ± 0.48 |
| J | ND | 3.5 ± 0.35 | 5.26 ± 1.47 | ND | ND | ND | ND | ND | 8.8 ± 1.19 |
| K | ND | 5.3 ± 0.45 | ND | 21.8 ± 0.70 | ND | 0.8 ± 0.02 | ND | 77.9 ± 1.81 | 105.8 ± 1.38 |
| L | ND | ND | ND | ND | ND | 1.0 ± 0.12 | ND | ND | 1.0 ± 0.12 |
| M | ND | 5.4 ± 0.38 | ND | ND | ND | ND | ND | ND | 5.4 ± 0.38 |
| N | ND | ND | ND | 17.5 ± 0.15 | ND | ND | ND | 71.8 ± 4.60 | 89.3 ± 4.45 |
| O | ND | 3.2 ± 035 | ND | 6.8 ± 0.26 | ND | ND | ND | ND | 9.9 ± 0.46 |
| P | ND | ND | ND | 80.8 ± 4.72 | ND | ND | ND | 17.1 ± 1.76 | 97.9 ± 4.29 |
| Q | ND | 4.1 ± 0.83 | ND | 5.9 ± 0.70 | ND | 0.7 ± 0.07 | ND | 10.1 ± 1.44 | 20.8 ± 3.10 |
| R | ND | 3.4 ± 0.45 | ND | 8.4 ± 0.97 | ND | ND | ND | 41.3 ± 1.41 | 53.1 ± 2.01 |
| Fermentation Days at 15 °C | Fermentation Days at 25 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biogenic amine | 30 | 60 | 120 | 150 | 180 | 30 | 60 | 120 | 150 | 180 |
| TRP | ND | ND | ND | ND | ND | 0.33 ± 0.09 | ND | ND | ND | ND |
| PUT | 8.1 ± 1.18 a | 12.8 ± 1.15 b | 13.8 ± 0.67 b | 12.5 ± 1.95 b | 7.4 ± 0.01 a | 30.9 ± 0.77 c | 23.9 ± 1.56 b | 14.5 ± 0.76 a | 20.0 ± 0.34 b | 15.6 ± 4.03 a |
| CAD | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| SPD | 23.6 ± 1.19 b | 26.6 ± 2.32 c | 22.2 ± 0.66 b | 22.2 ± 0.65 b | 6.2 ± 0.74 a | 84.8 ± 0.68 d | 74.9 ± 2.36 c | 52.3 ± 3.25 b | 46.5 ± 4.93 a,b | 41.4 ± 2.58 a |
| Total | 31.7 ± 2.15 b | 39.4 ± 3.30 d | 36.0 ± 1.33 c,d | 34.7 ± 2.69 b,c | 13.6 ± 0.75 a | 116.8 ± 4.17 d | 98.8 ± 0.93 c | 66.8 ± 3.96 b | 66.5 ± 4.59 b | 57.0 ± 6.43 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.H.; Koh, E.; Choi, B.; Moon, B. Effects of Soaking and Fermentation Time on Biogenic Amines Content of Maesil (Prunus Mume) Extract. Foods 2019, 8, 592. https://doi.org/10.3390/foods8110592
Yoon SH, Koh E, Choi B, Moon B. Effects of Soaking and Fermentation Time on Biogenic Amines Content of Maesil (Prunus Mume) Extract. Foods. 2019; 8(11):592. https://doi.org/10.3390/foods8110592
Chicago/Turabian StyleYoon, So Hee, Eunmi Koh, Bogyoung Choi, and BoKyung Moon. 2019. "Effects of Soaking and Fermentation Time on Biogenic Amines Content of Maesil (Prunus Mume) Extract" Foods 8, no. 11: 592. https://doi.org/10.3390/foods8110592