A Study of Structural Change during In Vitro Digestion of Heated Soy Protein Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Soy Protein Isolates
2.3. Heat Treatment of SPI
2.4. In Vitro Pepsin Digestion of HSPIs
2.5. The Degree of Hydrolysis Measurement
2.6. Raman Spectroscopy
2.7. Sodium Dodecyl Sulphide-Polyacrylamide Gel Electrophoresis
2.8. Determination of Molecular Weight
2.9. Statistical Analysis
3. Results
3.1. Effect of Heat Treatment on DH of SPIs In Vitro
3.2. Protein Structure Was Determined by Raman Spectra
3.2.1. Secondary Structure
3.2.2. Changes of Protein Tertiary Structure
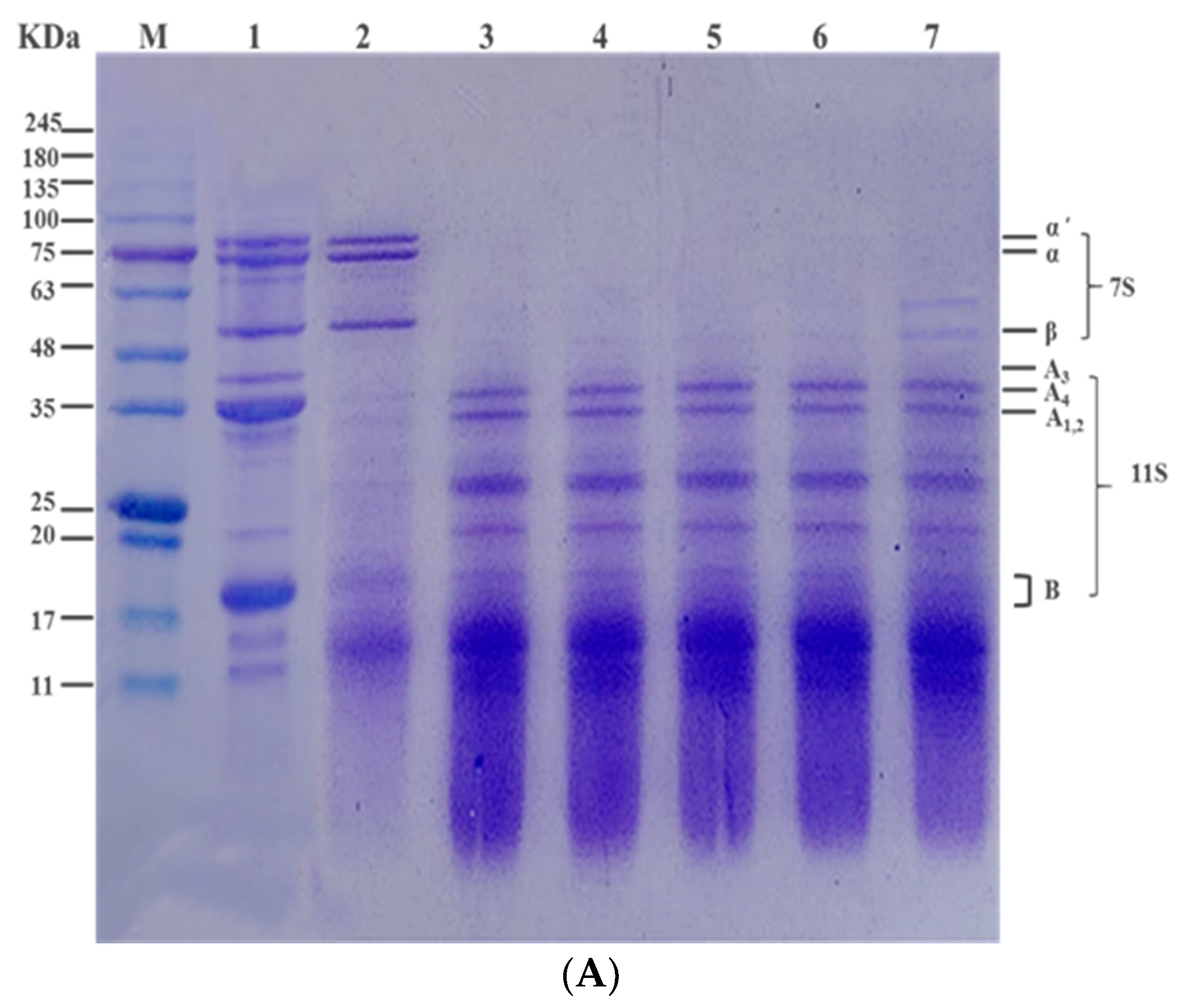
3.3. Effect of Heat Treatment on Subunit Composition of SPI In Vitro by SDS-PAGE Profile
3.4. Molecular Weight Distribution after In Vitro Digestion
3.4.1. Effects of Heating Temperature on Digestion Characteristics of SPI
3.4.2. Effect of Heating Time on the Digestion Characteristics of SPI
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, K. Chemistry and Nutritional Value of Soybean Components. Soybeans Chem. Technol. Util. 1997, 88, 25–113. [Google Scholar]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Fagbenro, O.A. Soybean meal replacement by roquette (Eruca sativa Miller) seed meal as protein feedstuff in diets for African catfish, Clarias gariepinus (Burchell 1822), fingerlings. Aquac. Res. 2004, 35, 917–923. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Yi, J.; Liu, N.; Cao, Y.; Decker, E.A.; McClements, D.J. Impact of Interfacial Composition on Lipid and Protein Co-Oxidation in Oil-in-Water Emulsions Containing Mixed Emulisifers. J. Agric. Food Chem. 2018, 66, 4458–4468. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, C.; Lee, S.J. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 2002, 277, 671–678. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Ma, H.; Zhang, Z.; Gao, W.; Xiao, L. Functional properties of protein isolates from soybeans stored under various conditions. Food Chem. 2008, 111, 29–37. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M. Effects of baking on protein digestibility of organic spelt products determined by two in vitro digestion methods. LWT Food Sci. Technol. 2008, 41, 1282–1288. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Wu, N.-N.; Yang, X.-Q.; He, X.-T.; Wang, L.-J. Improvement of emulsifying properties of Maillard reaction products from β-conglycinin and dextran using controlled enzymatic hydrolysis. Food Hydrocoll. 2012, 28, 301–312. [Google Scholar] [CrossRef]
- Wang, J.M.; Yang, X.Q.; Yin, S.W.; Zhang, Y.; Tang, C.H.; Li, B.S.; Yuan, D.B.; Guo, J. Structural rearrangement of ethanol-denatured soy proteins by high hydrostatic pressure treatment. J. Agric. Food Chem. 2011, 59, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Cheng, Y.Q.; Fan, J.F.; Lu, Z.H.; Yamaki, K.; Li, L.T. Effects of drying method on physicochemical and functional properties of soy protein isolates. J. Food Process. Preserv. 2010, 34, 520–540. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, G.X.; Sun, Z.W.; Zhang, B.; Wang, T. Stability and immunoreactivity of glycinin and β-conglycinin to hydrolysis in vitro. Food Agric. Immunol. 2010, 21, 253–263. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Natarajan, S.; Xu, C.; Bae, H.; Bailey, B.A.; Cregan, P.; Caperna, T.J.; Garrett, W.M.; Luthria, D. Proteomic and genetic analysis of glycinin subunits of sixteen soybean genotypes. Plant Physiol. Biochem. 2007, 45, 436–444. [Google Scholar] [CrossRef]
- Wang, W.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. β-conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit leukemia cells in vitro. J. Agric. Food Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef]
- Kondjoyan, A.; Daudin, J.D.; Sante-Lhoutellier, V. Modelling of pepsin digestibility of myofibrillar proteins and of variations due to heating. Food Chem. 2015, 172, 265–271. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between Secondary Structure and Surface Hydrophobicity of Soybean Protein Isolate Subjected to Heat Treatment. J. Chem. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Chen, F.P.; Li, B.S.; Tang, C.H. Nanocomplexation between Curcumin and Soy Protein Isolate: Influence on Curcumin Stability/Bioaccessibility and in Vitro Protein Digestibility. J. Agric. Food Chem. 2015, 63, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of beta-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Yue, C.; Liu, M.; Zheng, H.; Xia, X.; Yu, G. Improvement of freeze-thaw stability of oil-in-water emulsions prepared with modified soy protein isolates. LWT 2019, 102, 122–130. [Google Scholar] [CrossRef]
- Mirza, J.A.; Park, H.; Park, S.Y.; Ye, S.J. Use of radiochromic film as a high-spatial resolution dosimeter by Raman spectroscopy. Med. Phys. 2016, 43, 4520–4528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, X.; Taha, A.; Wei, Y.; Hu, T.; Fatamorgana, P.B.; Zhang, Z.; Liu, F.; Xu, X.; Pan, S.; et al. Effect of high intensity ultrasound on the structure and physicochemical properties of soy protein isolates produced by different denaturation methods. Food Hydrocoll. 2019, 97, 105216. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yang, B.; Yang, H.; Li, J.; Li, Z.; Jiang, Y. Amino acid composition, molecular weight distribution and antioxidant activity of protein hydrolysates of soy sauce lees. Food Chem. 2011, 124, 551–555. [Google Scholar] [CrossRef]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef]
- Nicorescu, I.; Loisel, C.; Vial, C.; Riaublanc, A.; Djelveh, G.; Cuvelier, G.; Legrand, J. Combined effect of dynamic heat treatment and ionic strength on the properties of whey protein foams–Part II. Food Res. Int. 2008, 41, 980–988. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Wang, R.; Sui, X.; Qi, B.; Han, F.; Li, Y.; Jiang, L. Secondary Structure and Subunit Composition of Soy Protein In Vitro Digested by Pepsin and Its Relation with Digestibility. Biomed Res. Int. 2016, 2016, 5498639. [Google Scholar] [CrossRef] [PubMed]
- Amigo-Benavent, M.; Clemente, A.; Ferranti, P.; Caira, S.; del Castillo, M.D. Digestibility and immunoreactivity of soybean β-conglycinin and its deglycosylated form. Food Chem. 2011, 129, 1598–1605. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman spectroscopy for monitoring protein structure in muscle food systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Cofrades, S.; Jiménez-Colmenero, F. Raman spectroscopic determination of structural changes in meat batters upon soy protein addition and heat treatment. Food Res. Int. 2008, 41, 765–772. [Google Scholar] [CrossRef]
- Petruccelli, S.; Anon, M.C. Thermal Aggregation of Soy Protein Isolates. J. Agric. Food Chem. 1995, 43, 3035–3041. [Google Scholar] [CrossRef]
- Li-Chan, E.; Nakai, S.; Hirotsuka, M. Raman spectroscopy as a probe of protein structure in food systems. Protein Struct. Funct. Relatsh. Foods 1994, 163–197. [Google Scholar] [CrossRef]
- Kang, Z.L.; Chen, F.S.; Ma, H.J. Effect of pre-emulsified soy oil with soy protein isolate in frankfurters: A physical-chemical and Raman spectroscopy study. LWT 2016, 74, 465–471. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y. The applications of Raman spectroscopy in food science. Trends Food Sci. Technol. 1996, 7, 361–370. [Google Scholar] [CrossRef]
- Nawrocka, A.; Miś, A.; Szymańska-Chargot, M. Characteristics of Relationships Between Structure of Gluten Proteins and Dough Rheology–Influence of Dietary Fibres Studied by FT-Raman Spectroscopy. Food Biophys. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- Wu, C.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Release Behavior of Non-Network Proteins and Its Relationship to the Structure of Heat-Induced Soy Protein Gels. J. Agric. Food Chem. 2015, 63, 4211–4219. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.C.; Marigheto, N.A.; Wellner, N.; Fairhurst, S.A.; Jenkins, J.A.; Mann, R.; Belton, P.S. Thermally induced structural changes in glycinin, the 11S globulin of soya bean (Glycine max)-an in situ spectroscopic study. Biochim. Biophys. Acta Proteins Proteom. 2003, 1648, 105–114. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G.; Cappelloni, M. Heat-induced denaturation impairs digestibility of legume (Phaseolus vulgaris L. and Vicia faba L.) 7S and 11S globulins in the small intestine of rat. J. Sci. Food Agric. 2005, 85, 65–72. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, J.; Wang, Y.; Zheng, H.; Tian, Z.; Zhang, Y.; Ou, S.; Hu, S.-Q.; Chen, L. Heat and edible salts induced aggregation of the N-terminal domain of HMW 1Dx5 and its effects on the interfacial properties. Food Hydrocoll. 2018, 82, 388–398. [Google Scholar] [CrossRef]
- Zhongjiang, W.; Xiaoyuan, Z.; Xiaonan, S.; Baokun, Q.; Lianzhou, J.; Xinrui, L.; Nan, J.; Tianci, H.; Xin, Z.; Yang, L. Structural Characteristics of in Vitro Digestion Products of Heat-Treated Soybean Protein. Food Sci. 2017, 38, 20–26. [Google Scholar]
- Chen, X.; Luo, J.; Fu, L.; Cai, D.; Lu, X.; Liang, Z.; Zhu, J.; Li, L. Structural, physicochemical, and digestibility properties of starch-soybean peptide complex subjected to heat moisture treatment. Food Chem. 2019, 297, 124957. [Google Scholar] [CrossRef]




| Heat Treatment | Sencodary Structure (%) | |||||
|---|---|---|---|---|---|---|
| α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) | I850/I830 | I760/I1003 | |
| Untreated | 13.69 ± 0.03 a | 55.68 ± 0.01 e | 23.70 ± 0.10 e | 6.93 ± 0.03 b | 1.02 ± 0.01 a | 0.95 ± 0.04 a |
| 70 °C,15 min | 17.77 ± 0.02 b | 51.41 ± 0.40 a | 25.98 ± 0.01 e | 4.84 ± 0.02 a | 1.01 ± 0.00 a | 0.98 ± 0.03 c |
| 80 °C,15 min | 20.38 ± 0.01 d | 52.48 ± 0.20 b | 22.27 ± 0.02 c | 4.87 ± 0.01 a | 1.01 ± 0.02 a | 0.99 ± 0.02 d |
| 85 °C,15 min | 18.37 ± 0.20 c | 54.78 ± 0.01 d | 19.30 ± 0.20 a | 7.55 ± 0.03 d | 1.02 ± 0.02 a | 0.98 ± 0.03 c |
| 90 °C,15 min | 18.34 ± 0.02 c | 54.42 ± 0.10 c | 19.47 ± 0.02 a | 7.77 ± 0.01 e | 1.02 ± 0.02 a | 0.98 ± 0.01 c |
| 100 °C,15 min | 17.77 ± 0.20 b | 54.53 ± 0.01 cd | 20.45 ± 0.03 b | 7.25 ± 0.10 c | 1.01 ± 0.00 a | 0.97 ± 0.03 b |
| Untreated | 13.69 ± 0.03 A | 55.68 ± 0.01 E | 23.70 ± 0.10 E | 6.93 ± 0.03 B | 1.02 ± 0.01 A | 0.95 ± 0.04 A |
| 85 °C,10 min | 14.19 ± 0.10 B | 55.80 ± 0.10 F | 25.23 ± 0.20 F | 4.78 ± 0.03 A | 1.01 ± 0.02 A | 0.97 ± 0.04 B |
| 85 °C,15 min | 18.37 ± 0.20 E | 54.78 ± 0.01 D | 19.30 ± 0.20 A | 7.55 ± 0.03 C | 1.01 ± 0.02 A | 0.99 ± 0.02 C |
| 85 °C,20 min | 18.72 ± 0.20 F | 52.58 ± 0.01 B | 20.66 ± 0.02 C | 8.04 ± 0.01 D | 1.01 ± 0.03 A | 0.98 ± 0.03 BC |
| 85 °C,30 min | 18.31 ± 0.01 D | 50.13±0.01 A | 22.00±0.10 D | 9.56 ± 0.03 F | 1.01 ± 0.03 A | 0.97 ± 0.04 B |
| 85 °C,60 min | 17.35 ± 0.02 C | 54.44 ± 0.30 C | 20.06 ± 0.02 B | 8.15 ± 0.03 E | 1.01 ± 0.03 A | 0.97 ± 0.02 B |
| Molecular Mass Distribution/Da | >100,000(%) | 10,000~100,000(%) | 3000~10,000(%) | <3000(%) |
|---|---|---|---|---|
| Untreated | 54.26 ± 0.10 a | 38.94 ± 0.10 e | 1.31 ± 0.10 a | 5.49 ± 0.20 c |
| 70 °C,15 min | 65.31 ± 0.20 b | 20.55 ± 0.20 d | 7.87 ± 0.20 b | 6.27 ± 0.10 d |
| 80 °C,15 min | 69.57 ± 0.30 d | 18.14 ± 0.10 c | 8.19 ± 0.10 bc | 4.10 ± 0.10 a |
| 85 °C,15 min | 69.02 ± 0.10 e | 18.46 ± 0.20 c | 8.44 ± 0.20 c | 4.08 ± 0.20 a |
| 90 °C,15 min | 68.51 ± 0.40 c | 17.74 ± 0.20 b | 8.26 ± 0.10 c | 5.49 ± 0.20 c |
| 100 °C,15 min | 69.18 ± 0.10 de | 16.06 ± 0.30 a | 9.60 ± 0.30 d | 5.16 ± 0.10 b |
| Untreated | 54.26 ± 0.10 A | 38.94 ± 0.10 F | 1.31 ± 0.10 A | 5.49 ± 0.20 E |
| 85 °C,10 min | 65.62 ± 0.30 B | 23.13 ± 0.10 E | 7.44 ± 0.40 D | 3.81 ± 0.10 B |
| 85 °C,15 min | 68.02 ± 0.10 C | 21.38 ± 0.20 D | 7.44 ± 0.20 D | 3.16 ± 0.10 A |
| 85 °C,20 min | 75.89 ± 0.10 D | 16.47 ± 0.20 C | 4.29 ± 0.10 B | 3.35 ± 0.30 A |
| 85 °C,30 min | 76.47 ± 0.20 E | 14.37 ± 0.30 A | 4.08 ± 0.20 BC | 5.08 ± 0.10 D |
| 85 °C,60 min | 76.21 ± 0.10 DE | 14.82 ± 0.10 B | 4.64 ± 0.30 C | 4.33 ± 0.10 C |
| Molecular Mass Distribution/Da | Different Treatment Temperature | Different Time Processing |
|---|---|---|
| >100,000 Da | 0.88 * | 0.82 * |
| 10,000–100,000 Da | −0.83 * | −0.79 |
| 3000–10,000 Da | 0.80 | 0.36 |
| <3000 Da | −0.54 | −0.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Teng, F.; Zhang, S.; Qi, B.; Wu, C.; Zhou, Y.; Li, L.; Wang, Z.; Li, Y. A Study of Structural Change during In Vitro Digestion of Heated Soy Protein Isolates. Foods 2019, 8, 594. https://doi.org/10.3390/foods8120594
Tian T, Teng F, Zhang S, Qi B, Wu C, Zhou Y, Li L, Wang Z, Li Y. A Study of Structural Change during In Vitro Digestion of Heated Soy Protein Isolates. Foods. 2019; 8(12):594. https://doi.org/10.3390/foods8120594
Chicago/Turabian StyleTian, Tian, Fei Teng, Shuang Zhang, Baokun Qi, Changling Wu, Yan Zhou, Liang Li, Zhongjiang Wang, and Yang Li. 2019. "A Study of Structural Change during In Vitro Digestion of Heated Soy Protein Isolates" Foods 8, no. 12: 594. https://doi.org/10.3390/foods8120594
APA StyleTian, T., Teng, F., Zhang, S., Qi, B., Wu, C., Zhou, Y., Li, L., Wang, Z., & Li, Y. (2019). A Study of Structural Change during In Vitro Digestion of Heated Soy Protein Isolates. Foods, 8(12), 594. https://doi.org/10.3390/foods8120594