Propolis Extract as Antioxidant to Improve Oxidative Stability of Fresh Patties during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Propolis Ethanol Extract Preparation (PEE)
2.3. Phenolic and Antioxidant Activity of PEE
2.3.1. Total Phenolic Content
2.3.2. HPLC-DAD Analysis
2.3.3. Reducing-Power Assay
2.3.4. Free-Radical Scavenging Activity
2.4. Beef and Pork Patties Manufacture
2.5. Phenolic and Antioxidant Activity of Meat Extract
2.6. pH and Color Changes of Patties
2.6.1. pH Measurement
2.6.2. Color Measurement
2.6.3. Metmyoglobin Formation
2.7. Oxidative Stability of Patties
2.7.1. Lipid Oxidation
2.7.2. Protein Oxidation
2.8. Statistical Analysis
3. Results
3.1. Polyphenol Composition and Antioxidant Activity
3.2. Total Antioxidant Activity of Meat Extract
3.3. pH and Oxidation Values of Patties
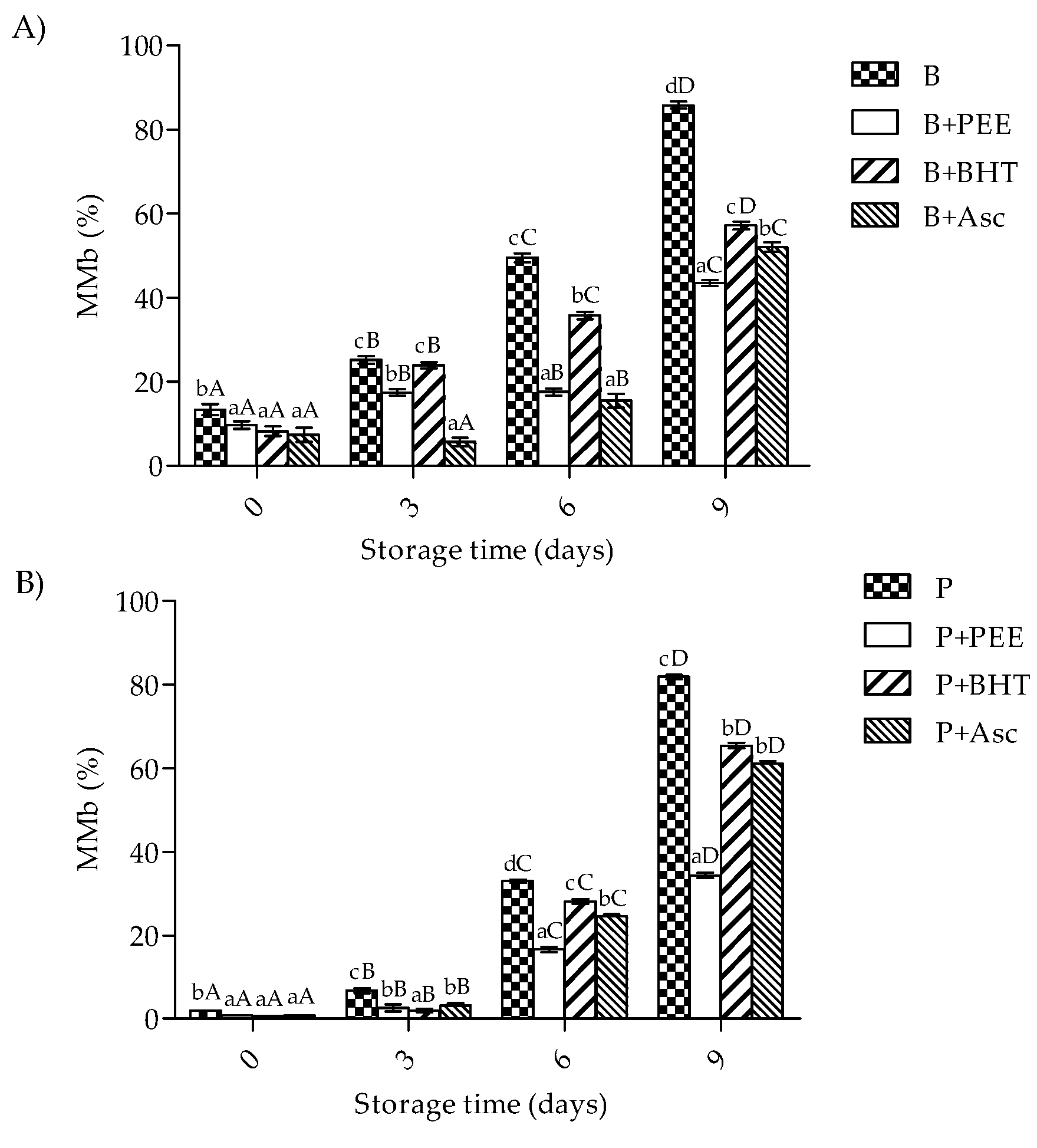
3.4. Metamyoglobin Formation of Patties
3.5. Color Changes of Patties
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pereira, P.M.D.C.C.; Vicente, A.F.D.R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Pașca, C.; Coroian, A.; Socaci, S. Risks and benefits of food additives—Review. UASVM Anim. Sci. Biotechnol. 2018, 75, 71–79. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Mateo, J.; Caro, I.; Ramos, M.-Y.L.; Mendez, N.G.; Cansino, R.G.; Mondragón, E.G.G. Natural antioxidants in fesh and processed meat. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 207–236. [Google Scholar]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.F.J.; Domínguez, R.; Sant’Ana, A.S.A.S.; Khaneghah, A.M.; Gavahian, M.; Gómez, B.; Lorenzo, J.M.J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.M.; Amado, I.R.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) flower as an antioxidant dietary fibre in chicken meat nuggets. Foods 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Pugine, S.M.P.; Lima, C.G.; Lorenzo, J.M.; de Melo, M.P. Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Escalante, A.; Torrescano, G.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Stabilisation of colour and odour of beef patties by using lycopene-rich tomato and peppers as a source of antioxidants. J. Sci. Food Agric. 2003, 83, 187–194. [Google Scholar] [CrossRef]
- Farré, R.; Frasquet, I.; Sánchez, A. El própolis y la salud. Ars Pharm. J. 2004, 45, 23–43. [Google Scholar]
- Cauich-Kumul, R.; Campos, M.R.S. Bioactive compounds. In Bee Propolis: Properties, Chemical Composition, Applications, and Potential Health Effects; Woodhead Publishing: Sawston/Cambridge, UK, 2019; pp. 227–243. [Google Scholar]
- Sánchez, R.D.V.; Urrutia, G.R.T.; Escalante, A.S. El propóleos: Conservador potencial para la industria alimentaria. Interciencia 2013, 38, 705–711. [Google Scholar]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Kunrath, C.A.; Savoldi, D.C.; Mileski, J.P.F.; Novello, C.R.; Alfaro, A.D.T.; Marchi, J.F.; Tonial, I.B. Application and evaluation of propolis, the natural antioxidant in Italian-type salami. Braz. J. Food Technol. 2017, 20, e2016035. [Google Scholar] [CrossRef]
- Kročko, M.; Bobko, M.; Bučko, O.; Čanigová, M.; Ducková, V. Sensory quality, colour and oxidative stability of cured cooked ham with propolis extract. Potravinarstvo 2014, 8, 102–106. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Ramírez-Rojo, M.I.; Sánchez-Escalante, A. Propolis extract as an oxidative stabilizer of raw beef and pork patties during chilled storage. In Proceedings of the 61st International Congress of Meat Science and Technology, Clermont-Ferrand, France, 23–28 August 2015. [Google Scholar]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef]
- Dos Reis, A.S.; Diedrich, C.; de Moura, C.; Pereira, D.; de Flório Almeida, J.; da Silva, L.D.; Plata-Oviedo, M.S.V.; Tavares, R.A.W.; Carpes, S.T. Physico-chemical characteristics of microencapsulated propolis co-product extract and its effect on storage stability of burger meat during storage at −15 °C. LWT Food Sci. Technol. 2017, 76, 306–313. [Google Scholar] [CrossRef]
- Bernardi, S.; Favaro-Trindade, C.S.; Trindade, M.A.; Balieiro, J.C.C.; Cavenaghi, A.D.; Contreras-Castillo, C.J. Italian-type salami with propolis as antioxidant. Ital. J. Food Sci. 2013, 25, 433–440. [Google Scholar]
- Hernandez, J.; Goycoolea, F.; Quintero, J.; Acosta, A.; Castañeda, M.; Dominguez, Z.; Robles, R.; Vazquez-Moreno, L.; Velazquez, E.; Astiazaran, H.; et al. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007, 73, 1469–1474. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction-antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Molyneux, P. The use of stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Acedo-Félix, E.; Carvajal-Millán, E.; González-Córdova, A.F.; Vallejo-Galland, B.; Torres-Llanez, M.J.; Sánchez-Escalante, A. Antioxidant and antimicrobial activity of commercial propolis extract in beef patties. J. Food Sci. 2014, 79, C1499–C1504. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011, 87, 46–53. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Gaitherburg, MD, USA, 2005. [Google Scholar]
- Commision International de I´Eclairage (CIE). Recommendations of Uniform Color Spaces-Color Difference Equations Psychometric Color Terms; Commision International de I’Eclairage: Vienna, Austria, 1978. [Google Scholar]
- Stewart, M.R.; Zipser, M.W.; Watts, B.M. The use of reflectance spectrophotometry for the assay of raw meat pigments. J. Food Sci. 1965, 30, 464–469. [Google Scholar] [CrossRef]
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Alpha-tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 62, 5488–5491. [Google Scholar]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Valencia, D.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Galvez-Ruiz, J.C.; Salas-Reyes, M.; Jiménez-Estrada, M.; Velazquez-Contreras, E.; Hernandez, J.; Velazquez, C. Seasonal effect on chemical composition and biological activities of Sonoran propolis. Food Chem. 2012, 131, 645–651. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postepy Hig. Med. Dosw. 2013, 67, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.; Milenković, D.; Đorović, J.; Marković, J.M.D.; Stepanić, V.; Lučić, B.; Amić, D. PM6 and DFT study of free radical scavenging activity of morin. Food Chem. 2012, 134, 1754–1760. [Google Scholar] [CrossRef]
- Geckil, H.; Ates, B.; Durmaz, G.; Erdogan, S.; Yilmaz, I. Antioxidant, free radical scavenging and metal chelating characteristics of propolis. Am. J. Biochem. Biotechnol. 2005, 1, 27–31. [Google Scholar] [CrossRef]
- Lorenzo, J.M.J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- Gheisari, H.R.; Motamedi, H. Chloride salt type/ionic strength and refrigeration effects on antioxidant enzymes and lipid oxidation in cattle, camel and chicken meat. Meat Sci. 2010, 86, 377–383. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Rzeszutek, A.; Triumf, E.C.; Egelandsdal, B. Antioxidant and other quality properties of reindeer muscle from two different Norwegian regions. Meat Sci. 2011, 89, 526–532. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Hawashin, M.D.; Al-Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Babiker, E.E. Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Sci. 2016, 122, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Torrescano, G.; Sánchez-Escalante, A.; Giménez, B.; Roncalés, P.; Beltrán, J.A. Shear values of raw samples of 14 bovine muscles and their relation to muscle collagen characteristics. Meat Sci. 2003, 64, 85–91. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Warner, R.D.; Rosenvold, K. Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: A review. Anim. Prod. Sci. 2014, 54, 375–395. [Google Scholar] [CrossRef] [Green Version]
- Frankel, E.N. Lipid oxidation: Mechanisms, products and biological significance. J. Am. Oil Chem. Soc. 1984, 61, 1908–1917. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Abreu, V.K.G. Lipid peroxidation in meat and meat products. In Lipid Peroxidation; Mansour, M.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Raharjo, S.; Sofos, J.N. Methodology for measuring malonaldehyde as a product of lipid peroxidation in muscle tissues: A review. Meat Sci. 1993, 35, 145–169. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D.J. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007, 75, 256–264. [Google Scholar] [CrossRef]
- Zahid, M.A.; Seo, J.-K.; Parvin, R.; Ko, J.; Yang, H.-S. Comparison of butylated hydroxytoluene, ascorbic acid, and clove extract as antioxidants in fresh beef patties at refrigerated storage. Food Sci. Anim. Resour. 2019, 39, 768–779. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Park, H.K. Accumulation of thiobarbituric acid-reactive substances in cured pork sausages treated with propolis extracts. J. Sci. Food Agric. 2002, 82, 1487–1489. [Google Scholar] [CrossRef]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef]
- Xiong, Y.L. Protein oxidation and implications for muscle foods quality. In Antioxidants in Muscle Foods; Decker, E.A., Faustman, C., Lopez-Bote, C.J., Eds.; Wiley: New York, NY, USA, 2000; pp. 85–111. [Google Scholar]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; Munekata, P.E.S.; de Melo, M.P. Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control 2016, 63, 65–75. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Sant’Ana, A.S.; Carvalho, R.B.; Barba, F.J.; Toldrá, F.; Mora, L.; Trindade, M.A. Main characteristics of peanut skin and its role for the preservation of meat products. Trends Food Sci. Technol. 2018, 77, 1–10. [Google Scholar] [CrossRef]
- Estévez, M.; Heinonen, M. Effect of phenolic compounds on the formation of α-aminoadipic and γ-glutamic semialdehydes from myofibrillar proteins oxidized by copper, iron, and myoglobin. J. Agric. Food Chem. 2010, 58, 4448–4455. [Google Scholar] [CrossRef]
- Heinonen, M.; Rein, D.; Satué-Gracia, M.T.; Huang, S.-W.; German, J.B.; Frankel, E.N. Effect of protein on the antioxidant activity of phenolic compounds in a lecithin−liposome oxidation system. J. Agric. Food Chem. 1998, 46, 917–922. [Google Scholar] [CrossRef]
- Girolami, A.; Napolitano, F.; Faraone, D.; Braghieri, A. Measurement of meat color using a computer vision system. Meat Sci. 2013, 93, 111–118. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Greene, B.E.; Hsin, I.M.; Zipser, M.Y.W. Retardation of oxidative color changes in raw ground beef. J. Food Sci. 1971, 36, 940–942. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Vargas, F.C.F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.; Vázquez, J.; Franco, D. Oxidation stability of pig liver pâté with increasing levels of natural antioxidants (grape and tea). Antioxidants 2015, 4, 102–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |


| (µg/mL) | TPC (mg GAE/g) | RPA (Abs) | FRSA (%) |
|---|---|---|---|
| 500 | 472.3 ± 3.50 f | 0.56 ± 0.03 g | 69.1 ± 0.03 e |
| 250 | 288.1 ± 0.53 e | 0.34 ± 0.01 e | 45.7 ± 0.11 d |
| 100 | 198.5 ± 0.98 d | 0.20 ± 0.01 d | 33.0 ± 0.01 c |
| 50 | 152.5 ± 1.22 c | 0.14 ± 0.01 c | 31.0 ± 0.05 b |
| 25 | 126.6 ± 0.24 b | 0.10 ± 0.01 b | 30.0 ± 0.01 b |
| 12.5 | 122.9 ± 0.53 a | 0.07 ± 0.01 a | 28.7 ± 0.01 a |
| BHT (50 µg/mL) | ND | 0.7 ± 0.01 h | 70.8 ± 0.10 e |
| Asc (25 µg/mL) | ND | 1.0 ± 0.01 i | 73.0 ± 0.10 f |
| # | Compound | Retention Time (min) | PEE (mg/g) a |
|---|---|---|---|
| 1 | Gallic acid | 1.9 | (+) |
| 2 | Cinnamic acid | 3.4 | 2.1 ± 0.2 b |
| 3 | p-coumaric acid | 7.8 | 2.9 ± 0.1 c |
| 4 | Ferulic acid | 8.7 | (−) |
| 5 | Naringenin | 27.3 | 50.2 ± 5.9 j |
| 6 | Quercetin | 30.8 | 6.5 ± 0.2 f |
| 7 | Luteolin | 36.4 | 3.7 ± 0.2 d |
| 8 | Kaempferol | 37.2 | 0.9 ± 0.2 a |
| 9 | Apigenin | 40.6 | 4.4 ± 0.2 e |
| 10 | Pinocembrin | 44.5 | 130.7 ±1.8 k |
| 11 | Pinobanksin 3-acetate | 45.8 | (+) |
| 12 | CAPE | 49.0 | (+) |
| 13 | Chrysin | 51.4 | 12.3 ± 1.0 h |
| 14 | Galangin | 52.4 | 37.0 ± 2.1 i |
| 15 | Acacetin | 57.0 | 8.4 ±0.4 g |
| 16 | Pinostrobin | 62.9 | (+) |
| Item | Beef | Pork | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | B | B+PEE | B+BHT | B+Asc | P | P+PEE | P+BHT | P+Asc | |
| L* | 0 | 44.0 ± 0.9 aA | 45.0 ± 0.6 aA | 45.5 ± 0.6 aA | 44.7 ± 0.6 aA | 54.2 ± 1.3 aA | 56.2 ± 0.8 aA | 56.4 ± 0.9 aA | 55.6 ± 0.9 aA |
| 3 | 43.1 ± 0.8 aA | 44.7 ± 0.7 bA | 45.7 ± 0.6 bA | 44.6 ± 0.4 bA | 53.1 ± 1.5 aA | 57.2 ± 0.8 bA | 56.8 ± 1.0 bA | 56.8 ± 0.7 bA | |
| 6 | 44.1 ± 0.7 aA | 46.3 ± 0.3 cB | 45.4 ± 0.6 bA | 45.3 ± 0.2 bA | 56.1 ± 1.0 aA | 57.0 ± 0.3 aA | 57.5 ± 1.0 aA | 59.7 ± 0.7 bB | |
| 9 | 47.0 ± 0.5 bB | 46.2 ± 0.5 aB | 45.1 ± 0.6 aA | 47.5 ± 0.1 bB | 59.4 ± 1.5 bB | 57.6 ± 1.0 aA | 57.2 ± 0.8 aA | 59.9 ± 0.9 bB | |
| a* | 0 | 23.2 ± 0.6 aD | 22.1 ± 0.4 aC | 23.0 ± 0.4 aD | 23.0 ± 1.0 aC | 20.9 ± 0.5 aC | 20.4 ± 0.6 aC | 20.2 ± 0.5 aC | 19.9 ± 0.6 aC |
| 3 | 20.9 ± 0.6 bC | 18.4 ± 0.4 aB | 20.5 ± 0.4 bC | 22.2 ± 0.8 cC | 20.0 ± 0.4 bC | 17.4 ± 0.6 aB | 19.8 ± 0.5 bC | 19.6 ± 0.6 bC | |
| 6 | 11.3 ± 0.6 aB | 16.0 ± 0.4 bA | 16.9 ± 0.4 bB | 19.5 ± 0.7 cB | 15.3 ± 0.4 aB | 15.8 ± 0.6 aA | 17.0 ± 0.5 bB | 16.1 ± 0.6 bB | |
| 9 | 9.2 ± 0.6 aA | 15.0 ± 0.4 bA | 8.1 ± 0.6 aA | 9.5 ± 0.8 aA | 11.6 ± 0.6 aA | 15.7 ± 0.7 bA | 11.1 ± 0.8 aA | 10.2 ± 0.6 aA | |
| b* | 0 | 18.3 ± 0.5 aC | 20.4 ± 0.5 bB | 18.5 ± 0.5 aB | 18.2 ± 0.3 aC | 17.1 ± 0.6 aAB | 21.8 ± 0.4 bB | 18.8 ± 0.4 aB | 17.6 ± 0.5 aB |
| 3 | 16.3 ± 0.5 aB | 17.6 ± 0.4 bA | 17.3 ± 0.5 bB | 18.1 ± 0.6 bC | 17.5 ± 0.7 aB | 19.7 ± 0.4 bA | 19.0 ± 0.4 bB | 17.9 ± 0.5 aB | |
| 6 | 12.6 ± 0.5 aA | 17.4 ± 0.4 cA | 15.0 ± 0.5 bA | 15.3 ± 0.6 bB | 16.1 ± 0.5 aA | 19.9 ± 0.4 cA | 17.4 ± 0.4 bA | 17.7 ± 0.5 bB | |
| 9 | 12.6 ± 0.5 aA | 17.5 ± 0.5 cA | 14.3 ± 0.6 bA | 13.9 ± 0.6 bA | 16.1 ± 0.5 aA | 19.1 ± 0.4 bA | 16.5 ± 0.5 aA | 15.9 ± 0.5 aA | |
| C* | 0 | 30.5 ± 0.6 bD | 28.7 ± 0.4 aC | 30.4 ± 0.5 bD | 30.3 ± 0.9 bC | 27.5 ± 0.3 bC | 26.2 ± 0.4 aC | 27.8 ± 0.6 bC | 27.4 ± 0.1 bD |
| 3 | 27.0 ± 0.6 aC | 26.2 ± 0.5 aB | 26.9 ± 0.5 aC | 28.9 ± 0.9 aC | 27.2 ± 0.6 bC | 27.5 ± 0.6 bB | 26.6 ± 0.6 bC | 25.8 ± 0.5 aC | |
| 6 | 17.0 ± 0.6 aB | 24.1 ± 0.5 bA | 23.3 ± 0.5 bB | 24.8 ± 0.7 bB | 23.1 ± 0.6 aB | 26.0 ± 0.6 bB | 25.0 ± 0.6 bB | 23.8 ± 0.5 aB | |
| 9 | 15.7 ± 0.6 aA | 23.7 ± 0.5 bA | 16.0 ± 0.7 aA | 17.3 ± 0.8 aA | 19.1 ± 1.1 aA | 24.4 ± 0.5 cA | 20.3 ± 0.7 bA | 18.7 ± 0.6 aA | |
| h* | 0 | 38.3 ± 0.5 aA | 41.9 ± 0.5 bA | 38.9 ± 0.4 aA | 37.8 ± 0.5 aA | 41.7 ± 0.7 aA | 43.5 ± 0.5 bA | 42.0 ± 0.6 aA | 41.2 ± 0.8 aA |
| 3 | 38.0 ± 0.5 aA | 43.7 ± 0.5 cB | 40.2 ± 0.4 bB | 37.2 ± 0.5 aA | 42.1 ± 0.5 aA | 47.0 ± 0.6 bB | 43.8 ± 0.6 aA | 43.1 ± 0.7 aA | |
| 6 | 54.8 ± 0.7 dB | 46.5 ± 0.6 cC | 40.5 ± 0.5 bB | 38.2 ± 0.5 aA | 44.6 ± 0.5 aB | 49.2 ± 0.5 cC | 46.1 ± 0.6 bB | 48.5 ± 0.7 cB | |
| 9 | 61.2 ± 0.5 cC | 47.7 ± 0.7 aC | 59.7 ± 0.6 bC | 59.3 ± 0.7 bB | 55.9 ± 0.6 bC | 48.4 ± 0.6 aC | 55.6 ± 0.9 bC | 56.2 ± 1.3 bC | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Torres-Martínez, B.d.M.; Pateiro, M.; Lorenzo, J.M.; Sánchez-Escalante, A. Propolis Extract as Antioxidant to Improve Oxidative Stability of Fresh Patties during Refrigerated Storage. Foods 2019, 8, 614. https://doi.org/10.3390/foods8120614
Vargas-Sánchez RD, Torrescano-Urrutia GR, Torres-Martínez BdM, Pateiro M, Lorenzo JM, Sánchez-Escalante A. Propolis Extract as Antioxidant to Improve Oxidative Stability of Fresh Patties during Refrigerated Storage. Foods. 2019; 8(12):614. https://doi.org/10.3390/foods8120614
Chicago/Turabian StyleVargas-Sánchez, Rey David, Gastón Ramón Torrescano-Urrutia, Brisa del Mar Torres-Martínez, Mirian Pateiro, José Manuel Lorenzo, and Armida Sánchez-Escalante. 2019. "Propolis Extract as Antioxidant to Improve Oxidative Stability of Fresh Patties during Refrigerated Storage" Foods 8, no. 12: 614. https://doi.org/10.3390/foods8120614
APA StyleVargas-Sánchez, R. D., Torrescano-Urrutia, G. R., Torres-Martínez, B. d. M., Pateiro, M., Lorenzo, J. M., & Sánchez-Escalante, A. (2019). Propolis Extract as Antioxidant to Improve Oxidative Stability of Fresh Patties during Refrigerated Storage. Foods, 8(12), 614. https://doi.org/10.3390/foods8120614








